Abstract
Bone marrow plays an important role on the mechanical properties of trabecular bone. Its effect on the mechanical properties of porcine trabecular bone is studied in this paper. Uniaxial compression at a low strain rate (0.01 s−1 to 20% strain) and stress relaxation tests (600 s at 85, 70 and 55% of the max. load) were done on 90 different femur samples. Half the samples were treated to extract the bone marrow. The average pore size of the trabecular network was 0.280 ± 0.056 mm. Higher values of elastic modulus (37%), 0.2% yield stress (48%), maximum stress (39%), strain at maximum stress (54%), and toughness (300%), were found for the samples which had the bone marrow extracted and were saturated with a saline solution. A linear relation between the applied load and the relaxation stress of  was found, which means that the trabecular bone behaves as a linear viscoelastic material. A mathematical approximation of the relaxation response was done using a Kohlrausch-Williams-Watts model for viscoelastic materials. Results show that it is essential to consider the viscoelastic behavior that the marrow has on the mechanical properties of the trabecular bone. The effect that the bone marrow has on the stress relaxation was found to be negligible at low strain rates and in the elastic stage of deformation.
was found, which means that the trabecular bone behaves as a linear viscoelastic material. A mathematical approximation of the relaxation response was done using a Kohlrausch-Williams-Watts model for viscoelastic materials. Results show that it is essential to consider the viscoelastic behavior that the marrow has on the mechanical properties of the trabecular bone. The effect that the bone marrow has on the stress relaxation was found to be negligible at low strain rates and in the elastic stage of deformation.
Export citation and abstract BibTeX RIS
1. Introduction
Understanding the mechanical properties of human bones is extremely important for the biomedical industry, as it plays an important role in injuries and fractures, as well as transplants, prosthetics and insets (Ojanen et al 2015, Ambrose et al 2018). These principles can be further used by other industries, such as automotive and sports, to prevent and treat injuries and to predict the mechanical behavior using FEM (Aula et al 2009, Sandino et al 2015).
Extensive research has been done to determine the mechanical properties of bones (Wu et al 2018, Ambrose et al 2018, Unal et al 2018). However, most studies focus on bovine bones (William, 2018), considering them as a cellular solid, and do not take into account the viscoelastic properties. In vivo, the loads to which a human femoral head and a bovine femoral head are subject to are quite different. Furthermore, porcine tissue is usually considered more similar to human tissue than that of bovines, and since the availability of human cadaver material is very limited, studying human bones is relatively complicated. Moreover, the trabecular network of bovine bones shows a larger pore size, and porcine bones are much more representative of human models and are frequently used as such (Ibrahim et al 2006, Busscher et al 2010), so the advantages of using porcine bone over bovine bone to study mechanical properties are evident. And even though the viscoelastic properties of trabecular bone have been reported for thoracic bone (Halgrin et al 2012), and the mechanics of bone marrow been compared between human, porcine, and bovine bone (Jansen et al 2015), the viscoelastic properties of porcine femur have mostly been overlooked.
The purpose of this work is, therefore, to characterize porcine trabecular bone and to specifically analyze the effect that the bone marrow has on the mechanical properties and in particular the viscoelasticity, considering that it is a composite material made up of a solid (trabecular bone) and a viscous fluid (bone marrow). The bone marrow is embedded in the trabecular bone, which is considered as the main load bearing structure. Trabecular bone is relatively flexible and is able to absorb compression loading by body weight and tensile load during physiological activities. It is considered anisotropic and inhomogeneous and has variations in its structures at different anatomical sites (Mostakhdemin and Goharian 2017).
2. Materials and methods
To analyze the mechanical properties of porcine trabecular bone, uniaxial compression and stress relaxation tests were done. Cubic samples were used due to the reduced volume of the trabecular bone in the femoral head, and to simplify the machining process, which can usually damage the tissue (Jansen et al 2015). The bone marrow plays an important role on the viscoelastic effect (Halgrin et al 2012), so in half of the samples the bone marrow was extracted in order to compare the mechanical properties of the trabecular bone with and without the marrow.
Porcine femur specimens (about 5 months of age) were obtained from a local commercial pork operation. Research on such byproducts is approved by the institution. The femurs, measuring between 20 and 23 cm in length, were cut longitudinally using a blade saw. To reduce dehydration and sample damage, special care was taken that the specimens used had less than 72 h post mortem and were kept at 6 °C (above the freezing temperature of the saline solution) with a humidity around 91% (Laporte et al 2009, Halgrin et al 2012). Trabecular bone was cut off from the femoral neck using an IsoMet 1000® precision circular diamond saw at 900 RPM.
For the mechanical tests, 8 mm cubic samples were cut out. This size guarantees that the sample can be considered as a continuum (Linde 1994). Using a simple visual inspection, the cuts were made considering the principal direction of the trabeculae to be roughly the same direction of the compressive load (figure 1), as a reduction of up to 40% in some mechanical properties has been seen when the applied load and the principal direction are not parallel (Öhman et al 2007). All specimens were cut from roughly the same anatomical site, as it has been shown that it is also important when measuring mechanical properties (Morgan et al 2003)
Figure 1. Preparation of the porcine trabecular bone samples showing (a) the principal direction of the trabeculae in the femoral neck, (b) 8 mm cubic sample with bone marrow, (c) 8 mm cubic sample without bone marrow, the principal direction of the trabeculae roughly corresponds with the (vertical) compressive load.
Download figure:
Standard image High-resolution imageThe applied load during cutting was regulated so that the premature damage of the trabeculae was minimized. Figure 1 shows the area of the sample selection that has the highest volume ratio of the trabeculae and the principal direction of trabeculae, which corresponds to the compressive force applied. However, due to the inhomogeneity of the trabeculae, up to a 30° variation was observed in some of the samples.
Since the mechanical properties of bone depend on the mineral content, freshness, humidity and other factors, and can be affected within minutes of the samples drying out (Pal 2014), samples were kept hydrated (using a 0.9% saline solution) and refrigerated at 6 °C. All tests were performed in less than a week after sample preparation. A total of 90 samples were made, each from a different femur.
To compare the mechanical properties of the trabecular bone with and without bone marrow, half of the samples were treated to extract the marrow. This was done using 30 min water baths at 94 °C to completely extract the fatty tissue, followed by ultrasonic cleaning at 40 °C for 2 h and finally an air jet stream until a colorless porous structure was seen (figure 1(c)). Even though this extraction method could result in specimen alterations, it was necessary to completely remove the large amount of fat that is found in porcine tissue.
To assure that the sample size is adequate for the trabecular bone to be treated as a continuum (Harrigan et al 1988; Linde 1994, Linde and Hvid 1989), a linear intercept analysis was done for the samples without bone marrow. Digital images were taken using a Canon 12 MP camera with macro mode and the average pore size was measured for the plane perpendicular to the compressive load, as shown in figure 2. An arbitrary rotation of 30, 60 and 90 degrees was considered to analyze the possible effect of a preferential direction.
Figure 2. Linear intercept measurements of one of the faces parallel to the compression platens. A more homogeneous pore distribution is observed in this face (as compared to the perpendicular ones shown in figure 1(c).
Download figure:
Standard image High-resolution imageIt has been shown (Cowin 2001) that the effect of temperature during mechanical tests is so small (2 to 4% increase in Young's modulus), that the tests can be done at room temperature without a significant variation in the measured properties (Turner and Burr 1993; Mitton et al 1997, Halgrin et al 2012). Therefore, all tests were done at room temperature (∼26 °C) and samples were kept hydrated using a 0.9% saline solution.
Uniaxial compression tests were done using a Shimadzu AG-X® universal tester with a 5 kN load cell at a constant strain rate of 0.01 s−1 up to a 20% strain. A preload of 5 N was used to ensure that the platens and the sample were parallel to each other. An apparent constant cross sectional area of 64 mm2 was considered.
To study the viscoelastic behavior, stress relaxation tests were carried out at three different values of the maximum load measured in the compression tests (85, 70 and 55%) and a relaxation time of 600 s. Stress relaxation tests are more convenient than creep tests, as the applied load can be more readily controlled in the experiment than an initial strain, as the latter widely depends on microstructural characteristics.
3. Results and discussion
The amounts of cartilage and adipose tissue are limiting factors for the preparation of the samples, as the amount of trabecular bone is barely enough to obtain 8 mm cubic samples. Even though 90 samples were tested, 30 of them failed prematurely. This was mostly due to the abundant adipose tissue found around the trabecular bone. Figure 3 shows a premature sample failure with a highly fatty tissue flowing out and inhomogeneous deformation. This premature failure was mostly seen in the longest femur specimens, which suggests that the size and diet of the pigs has a direct influence on the mechanical properties of their bones, as it has been shown that increasing dietary protein results in a decrease of adipose tissue fat deposition and carcass backfat thickness in growing pigs (Zhao et al 2010).
Figure 3. One of the samples that showed a premature failure due to the abundant adipose tissue.
Download figure:
Standard image High-resolution imageAn average pore size of 0.280 ± 0.056 mm was found with the linear intercept method. According to Linde (Linde 1994, , Linde and Hvid 1989, Linde and Sorensen 1993) the minimum length required for the specimen to be considered a continuum would be 2.8 mm, or 10 times the inter-trabecular distance. No significant preferential orientation was found when rotating the sample 30, 60 and 90 degrees.
The selected constant cross section results in an underestimation of the strength, but measuring the real cross-sectional area would be impractical, especially considering that it is widely accepted that the mechanical tests should be done right after the sample preparation. Furthermore, the variation in size and distribution of the pores means that even the most advanced measuring techniques would not be enough to accurately predict their effect during the experimental setup. However, one of the most important microstructural parameters that directly impacts the mechanical properties is the volume fraction of trabecular bone. The assumption of a constant cross section implies that the volume fraction of trabecular bone is 1, as no pores would be considered. A higher volume fraction theoretically results in a higher ultimate tensile strength (Prot et al 2015). Even if the microstructural analysis were considered for future work, the additional time it would take to analyze each sample would likely result in a bigger dispersion of the data from the demineralization, dehydration and sample alterations. The anisotropy and heterogeneity of the trabeculae also have a significant effect on the continuum properties (Guedes et al 2006). The volume fraction of the interstitial fluid and its contribution to the mechanical response is therefore difficult to quantify, as it highly depends on the experimental setup and considerations. However, the density of each sample could be related to the relatively high dispersion of data in the stress-strain curves, and should be taken into consideration for future studies.
There are three distinct phases of the (engineering) stress-strain curve for low strain rates (Halgrin et al 2012). First, a linear behavior characteristic of the elastic deformation is observed, until the maximum stress is reached. Then a quick drop in stress is observed as a consequence of the initial collapse of the trabecular network, reaching a quasistatic stress plateau which corresponds to the progressive failure of the trabecular network. Finally, after about a 60% strain, the sample is completely compacted and the stress increases rapidly. However, the structural damage at this point is excessive and the measured values have no real significance. For this reason, in this study the tests were halted after a 20% strain, corresponding to the quasistatic stress plateau stage, as shown in figure 4.
Figure 4. Representative stress-strain curves for samples with and without bone marrow.
Download figure:
Standard image High-resolution imageThe compression test results are summarized in table 1, and are congruent with Halgrin et al (Halgrin et al 2012). The 0.2% offset yield stress criterion was used, and even if it is mostly used for metallic materials and not for biological tissue, it can be considered the stress at which damage starts to occur in the material. Toughness was calculated using the area under the stress-strain curve up to the maximum stress.
Table 1. Measured mechanical properties.
| Young's modulus [MPa] | 0.2%Yield stress [MPa] | Max stress [MPa] | Strain at max stress [%] | Toughness [kJ/m3] | |
|---|---|---|---|---|---|
| With bone marrow | 226.9 ± 102.9 | 4.59 ± 1.58 | 5.72 ± 1.93 | 3.8 ± 0.8 | 95.4 ± 41.4 |
| Without bone marrow | 331.9 ± 131.4 | 6.81 ± 2.07 | 8.52 ± 2.32 | 6.6 ± 2.4 | 348.8 ± 120.1 |
Higher values of elastic modulus (37%), 0.2% yield stress (48%), maximum stress (39%), strain at maximum stress (54%), and toughness (300%), were found for the samples which had the bone marrow extracted and were saturated with a saline solution. A statistical analysis was made and the data are reported as mean ± standard deviation values. The mean measured stress-strain curves are shown in figure 5. The higher values of the elastic modulus for the samples without bone marrow may be attributed to the effects of the removal during sample preparation, as it has been found that porcine bone marrow is a material with dominant elastic contributions to its mechanical response. However it must be considered that the marrow tissue is heterogeneous and that there is not one, but a range of appropriate moduli values for it (Jansen et al 2015). Different values of Young's moduli can be found in the literature, ranging from 0.73 to 135.6 kPa (Jansen et al 2015).
Figure 5. Average measured stress-strain curves for samples with and without bone marrow.
Download figure:
Standard image High-resolution imageThe stress-strain curves for the samples without bone marrow show a considerable increase in strain after the maximum stress has been reached. This means that the structure is actually resisting the load without fracturing, which results in considerably higher toughness. This suggests that the samples without bone marrow have a higher stiffness and, as the interstitial flow through the trabecular structure is less viscous, it can more readily flow out through the structure without causing damage. Therefore, during the deformation, the compression of the bone marrow in the vertical direction would cause the (incompressible, more viscous, non-Newtonian) fluid to bulge in the horizontal direction, therefore bending the trabecula and reducing the apparent strength (Ruiz et al 2011). This does not happen in the samples without the bone marrow, where the (less viscous) saline solution does not pose an immediate additional lateral stress on the trabeculae.
Another possible explanation is that, without the bone marrow, the load is mostly distributed along the bone tissue, not through the marrow, and is instead distributed through the saline solution contained in the pores. The viscosity of the bone marrow is 3 to 5 times higher than that of water (Hall 2015). Since the viscosity is higher, it presents a higher resistance to fluid flow, resulting in higher stress concentration along the trabeculae and an earlier failure of the trabecular structure, as Halgrin et al (Halgrin et al 2012) showed using a finite element model.
It is important to consider that the fluid is not confined, as would be the case in vivo, where the cortical bone impedes the flow of bone marrow out of the structure. This most likely would result in higher hydrostatic pressure inside the structure and lower values of the measured mechanical properties. Moreover, the intertrabecular regions are not always connected, and so removing the bone marrow would promote hinging and bending of the trabecula (Ruiz et al 2011). When the marrow is inside the pores of the trabecular structure, the limited flow causes the internal pressure to rise, resulting in higher localized stress and a premature failure of the trabeculae.
For the stress relaxation tests, the maximum load values (summarized in table 2) of the compression tests were considered and the corresponding loads of 85, 70 and 55% were used. The stress relaxation curves are shown in figure 6. The relaxation time can be defined as the time it takes for the applied load to become stable, and after which no other significant changes in the stress response are seen. In other studies (Guedes et al 2006; Quaglini et al 2009) the relaxation times have ranged from 100 to 1000 s. The stress relaxation tests show that after approximately 600 s (relaxation time), the stress has dropped down to about 80% of its original value. After the relaxation time of 600 s, the applied load becomes constant and no other significant changes in the stress response are seen. A slightly higher percentage of stress reduction is observed for the samples without bone marrow, which could be attributed to the viscoelastic effect of the saline solution. This result is consistent with other studies reported in the literature (Halgrin et al 2012), and could be further studied using FEM.
Table 2. Maximum load, maximum stress and percentages of maximum load used for the stress relaxation tests (equivalent stress).
| With bone marrow | Without bone marrow | |||||
|---|---|---|---|---|---|---|
| Max average load [N], (stress [MPa]) | 366, (5.72) | 545, (8.52) | ||||
| Percentage of max load [%] | Load, (stress) | Relaxation stress [MPa] | % of stress reduction | Load, (stress) | Relaxation stress [MPa] | % of stress reduction |
| 85 | 310, (4.8) | 2.40 ± 0.10 | 23.63 | 460, (7.2) | 3.60 ± 0.33 | 24.52 |
| 70 | 250, (3.9) | 3.11 ± 0.08 | 22.25 | 380, (5.9) | 4.46 ± 0.22 | 22.76 |
| 55 | 200, (3.1) | 3.75 ± 0.11 | 22.78 | 300, (4.7) | 5.22 ± 0.04 | 27.28 |
| Average | 22.89 | 24.85 | ||||
Figure 6. Stress relaxation curves for samples without and with bone marrow, showing the three different loads used.
Download figure:
Standard image High-resolution imageIn order to compare the relaxation response between the different applied loads, a normalized stress ( ) was calculated and is plotted in figure 7. This normalized stress was used to correlate the initial applied load with the relaxation stress. A linear relationship between the applied load and the relaxation stress of
) was calculated and is plotted in figure 7. This normalized stress was used to correlate the initial applied load with the relaxation stress. A linear relationship between the applied load and the relaxation stress of  (R2 = 0.995) was found, which means that the trabecular bone behaves as a linear viscoelastic material. The normalized stress was also used to obtain a mathematical approximation of the relaxation response using a Kohlrausch-Williams-Watts (KWW) model for viscoelastic materials (Iyo et al 2003).
(R2 = 0.995) was found, which means that the trabecular bone behaves as a linear viscoelastic material. The normalized stress was also used to obtain a mathematical approximation of the relaxation response using a Kohlrausch-Williams-Watts (KWW) model for viscoelastic materials (Iyo et al 2003).
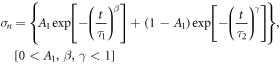
Where A1, τ1, β, τ2, and γ are the adjusted parameters shown in table 3.
Figure 7. Normalized stress relaxation curves for samples with and without bone marrow.
Download figure:
Standard image High-resolution imageTable 3. Adjusted parameters for the KWW model.
| Parameters | Iyo et al (2003) | Adjusted, with bone marrow | Ajusted, without bone marrow |
|---|---|---|---|
| A1 | 0.11 | 0.127 | 0.134 |
| τ1 | 50 | 18.695 | 18.695 |
| β | 0.26 | 0.505 | 0.493 |
| τ2 | 6400000 | 103926 | 61850 |
| γ | 0.37 | 0.414 | 0.399 |
KWW adjusted model for samples with bone marrow:
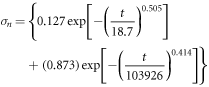
KWW adjusted model for samples without bone marrow:

These stress relaxation functions closely correlate with the experimental results. Figure 8 shows the average experimental normalized stress relaxation and the plotted adjusted models. It is evident that the samples without bone marrow show higher stress relaxation. The most notable difference between the two adjusted models is the value of  This suggests that initially the stress relaxation is roughly the same, but after the first couple of seconds (about 10% reduction of the normalized stress), the behavior of the samples without bone marrow show a higher decrease in normalized stress. This indicates the importance of the viscosity of the intertrabecular fluid and confirms the hypothesis that the trabecular bone should be considered as a viscoelastic material to obtain a better approximation of its real behavior.
This suggests that initially the stress relaxation is roughly the same, but after the first couple of seconds (about 10% reduction of the normalized stress), the behavior of the samples without bone marrow show a higher decrease in normalized stress. This indicates the importance of the viscosity of the intertrabecular fluid and confirms the hypothesis that the trabecular bone should be considered as a viscoelastic material to obtain a better approximation of its real behavior.
Figure 8. Normalized stress relaxation curves showing the experimental results and the KWW adjusted model.
Download figure:
Standard image High-resolution image4. Conclusions
The bone marrow plays an important role on the mechanical properties of porcine bone at low strain rates. All the compression test measured properties were found to have higher values for the samples that had the bone marrow extracted and replaced with a saline solution. An increase of 37% for the elasticity modulus, 48% for the 0.2% yield stress, 39% for the maximum stress, 54% for the strain at maximum stress, and of 300% for the toughness was observed. These results indicate that without the bone marrow the trabecular structure shows a higher stiffness and with the lower viscosity of the saline solution compared to the bone marrow the fluid flows more freely out of the unrestrained structure without causing premature damage. This results in the apparently higher mechanical properties. It is therefore of great importance to consider the viscous properties that the marrow has on the mechanical properties of the trabecular bone, keeping in mind that the experimental conditions of the mechanical tests are significantly different than the ones that take place in vivo and that the bone marrow may be too complex to be considered a simple viscoelastic material. The stress relaxation tests showed that the trabecular bone has a linear viscoelastic behavior. A slightly higher (2%) relaxation stress was found after 600 s for the samples without bone marrow. It can then be concluded that, at low strain rates and in the elastic stage of deformation, the effect that the bone marrow has is negligible. However, after the initial 10% drop in normalized stress, a higher stress relaxation was found for the samples without bone marrow.
Acknowledgments
The authors would like to thank E Ramos, J Romero, I Cueva, L Morales and G Alvarez for their technical assistance and gratefully acknowledge the financial support provided by the National Autonomous University of Mexico [grant DGAPA PAPIIT IN115415].
: Declarations of interest
None.










