Abstract
The consumption of too much fluoride ions through drinking water can seriously harm human health. Thus fluoride ions need to be removed by the novel and efficient nanomaterials materials synthesized via eco-friendly method. The pure and iron-doped hydroxyapatites were synthesized using a simple co-precipitation technique for the removal of fluoride from water. The synthesized materials were characterized by advanced technical tools. The point of zero charge of the materials was determined by the salt addition method. Crystallite size and degree of crystallinity were observed to decrease with the substitution of calcium. However, the surface area and pore volume were found to have enhanced with modification of iron in the apatite. Batch adsorption experimental data were well fitted to pseudo-second order and Langmuir models, which implied that the sorption process is chemisorption through a monolayer on a homogenous surface. The maximum sorption capacities of HA and Fe-HA were found to be 40.46 and 83.86 mg g−1, respectively. The thermodynamic data revealed that the adsorption process is endothermic and spontaneous. The regeneration and reuse analysis insured that the materials have good potential for reuse. The adsorption mechanism was inferred as chemisorption through electrostatic interaction and ion exchange. The modification of hydroxyapatite using iron considered as a competent sorbent for the removal of fluoride ions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
A lack of clean and safe water exists globally as a result of pollution-related issues (Bello et al 2017). Fluoride is one of the major pollutants naturally from the soil, which causes adverse effects on human health if there is long-term consumption of water with its concentration above WHO recommended value (0.5–1.5 mg l−1). Dental and skeletal fluorosis is one of the most apparent health risks common in different countries (Tomar et al 2015, Demelash et al 2019, Fernando et al 2019). Therefore, defluoridation using the various techniques such as precipitation, ion exchange, adsorption, membrane and electrochemical methods is in progress (He et al 2020, Alhassan et al 2021, Huang et al 2022).The adsorption process is more attractive to researchers than other techniques in terms of cost, efficiency, flexibility, simple and ease of operation (Yang et al 2019, Özsin et al 2019a). Currently, various materials have been used for defluoridation, such as zeolites supported aluminium hydroxide (Dessalegne et al 2017), aluminium hydroxide coated activated carbon (Amalraj and Pius 2017), diatomite modified with aluminium hydroxide (Akafu et al 2019), magnesium and iron complex hydroxides (Ogata et al 2020), aluminium fumarate metal-organic framework (Dechnik et al 2016), carbon nanotubes stabilized in chitosan sponge (Affonso et al 2020) and magnetic nanomaterials (Scheverin et al 2021). However, most of these materials are expensive, and some have low adsorption capacities towards fluoride ions. The other drawback of some sorbents is the separation of the adsorbent from the matrix after completion of the adsorption process (Mehta et al 2015, Suriyaraj and Selvakumar 2016, Scheverin et al 2021). Therefore, researchers have to focus on the search for innovative materials with high binding capacities, locally available, biodegradable, environmentally friendly, easily separable and able to remove contaminates at low cost and energy.
Hydroxyapatite, Ca10(PO4)6(OH)2, with resemblance to the mineral components of the bone and teeth, is one of the widely used biomaterials for bone grafting, orthopedic, orthodontic, drug delivery and other biomedical applications (Sheikh et al 2018, Tsai et al 2018, Targonska et al 2020). Recently, the use of hydroxyapatite bio-ceramic as an adsorbent has become an important area of research due to its renewability, degradability, environment-friendly nature, low cost, high stability under reducing and oxidizing conditions, favorable surface characteristics, acidity and basicity, hydrophilicity, porosity and biocompatibility nature (Adeogun et al 2018, Hamzah et al 2019). Various research groups have modified hydroxyapatite differently to enhance the defluoridation activity, such as composites with different biopolymers (Yu et al 2013, Pandi and Viswanathan 2014, Fernando et al 2021), decoration with carbon nanotubes (Tang et al 2018), composite with silicates (Fernando et al 2019), combining organic metal frameworks incorporated with biopolymer beads (Jeyaseelan and Viswanathan 2022), modification with aluminum hydroxide (He et al 2017) and doping with divalent and trivalent metal ions (Nie et al 2012, Mondal et al 2016, Chen et al 2018). The cation doping on the hydroxyapatite changes its degree of crystallinity, crystallite size, surface charge distribution, surface area, morphology and other properties (Alshemary et al 2015, Chen et al 2018, Tite et al 2018, Neacsu et al 2019). Significantly, the change in surface area, surface charge and size of the hydroxyapatite have an essential effect on its adsorption performance towards various pollutants. For instance, the incorporating transition metals like silver and yttrium in the HA lattice structure, was reported to have increased the dye adsorption capacity (Srilakshmi and Saraf 2016, Vasugi et al 2017). The presence of silver, iron, aluminum, copper, strontium, cerium, and titanium was observed to enhance Cd (II) and U (VI) removal tendency of hydroxyapatite (Guo et al 2019, Skwarek et al 2019, Chen et al 2022, Zhu et al 2022). Iron (III) substituted hydroxyapatite exhibited enhanced tetracycline removal capacity compared to that of the pure hydroxyapatite (Li et al 2017). Similarly fluoride removal efficiency of hydroxyapatite can be enhanced by cation doping (Chen et al 2018). In their study, the authors compared the fluoride adsorption capacity of hydroxyapatite doped with the Mg2+, Al3+ and La3+ and the maximum fluoride adsorption capacities were found to be 18.018, 23.598 and 46.729 mg g−1 at 328 K, respectively. They come to the conclusion that whereas hydroxyapatite doped with trivalent metal ions exhibited a high inclination to remove fluoride, the cation size had little bearing.
This work was aimed to synthesize iron doped hydroxyapatite nanomaterials with different amounts of dopant using an eco-friendly method and study in detail the adsorption behaviours towards fluoride ions. The presence of iron in the hydroxyapatite structure was found to decrease the degree of crystallinity and crystallite size and changes the surface charge (Kanchana et al 2014, Trinkunaite-Felsen et al 2015, Sheikh et al 2018). On the other hand, due to the paramagnetic nature of iron doped hydroxyapatite (Iannotti et al 2017, Morsi and Abd Elhamid 2019), the adsorbent can be easily removed from the aqueous solution using external magnet. According to the previous reports (Sujana and Anand 2010, Corral-Capulin et al 2019, Ogata et al 2020), iron-containing adsorbent was found to interact strongly with the fluoride ion. In this work, pure hydroxyapatite was prepared and used for fluoride adsorption for the comparison purpose. The work also included optimizing different adsorption parameters such as pH, contact time, initial fluoride concentration, dose and ionic strength. The kinetics of adsorption, isotherm, thermodynamics, desorption and reusability studies were also included. Lastly, the adsorption mechanism was proposed based on the findings of FT-IR result before and after adsorption, from kinetics, isotherm and pH effect.
2. Materials and methods
2.1. Chemicals
Analytical grade chemicals such as CaNO3.4H2O (99%), (NH4)2HPO4 (99%) and Fe(NO3)3.9H2O (98%) were used, without any further treatment, as a source of calcium, phosphate and iron respectively. Furthermore, NaOH (98%) and NaF (99.5%) were used to adjustment the pH and prepare stock solution of fluoride ion respectively.
2.2. Synthesis of the adsorbents
The pure hydroxyapatite and iron doped hydroxyapatite with various dopant amount (x = 0.1, 0.3, 0.5 and 0.7), were synthesized using the co-precipitation technique (Paduraru et al 2021) based on the molar ratio of Ca/P and (Ca + Fe)/P equals to 1.67. At first 0.3 mol l−1 of phosphate, 0.5 mol l−1 of calcium and 0.5 mol l−1 of calcium plus iron (different concentration of calcium and iron) solutions with equal volumes were prepared separately. Then the solution of the metal source from the burette was added dropwise to the phosphate source in the beaker with continuous heating to a temperature of 80 °C and with constant stirring at 800 rpm. The pH of the solution was maintained at ten throughout the synthesis using 0.4 M NaOH solution. The precipitate was allowed to settle for 15 h and washed using de-ionized water. Then it was oven dried at 110 °C for 24 h and set for characterization and the adsorption study. The synthesized materials were labelled as HA for pure and 1%Fe-HA, 3%Fe-HA, 5%Fe-HA and 7%Fe-HA for iron-doped hydroxyapatite nanomaterials. The chemical reactions for these synthesis are based on the chemical equations (1) and (2) (1 for pure hydroxyapatite and 2 for iron doped hydroxyapatite):
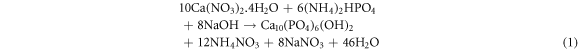
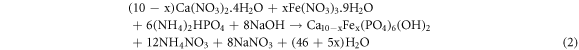
2.3. Characterization of the synthesized materials
The phase purity, crystal structure and crystallinity of the synthesized materials were identified using x-ray Diffractometer (XRD-7000) with CuKα (λ = 1.5406 Å) radiation in the range of 2θ from 10°–80° with continuous mode. The presence of functional groups was determined by FT-IR spectrum (65 PerkinElmer) from 4000–400 cm−1 wavenumber range. Surface area, pore volume and pore diameters of the synthesized materials were determined by BET technique using Quantachrome Instruments, version 5.21 on the bases of nitrogen adsorption at 77.35 K after degassing of the samples at 200 ◦C for 2.3 h under vacuum. Similarly, the morphology of the materials was analysed by field emission scanning electron microscope (FESEM), and elemental composition was evaluated using energy dispersive spectroscopy (EDS). The point of zero charge (pHPZC), of the materials was determined by the pH drift (salt addition) method (Mahmood et al 2011, Yang et al 2016). During the experiment, 20 ml of 0.1 M NaNO3 was taken in different reaction vessel, and the initial pH was adjusted from 2–11 using 0.1 M HCl and 0.1 M NaOH. Then 0.1 g of the adsorbent was added in the separate vessels, and the mixture was agitated in an orbital shaker for 24 h at room temperature; finally, pH of the suspension was measured after settling.
2.4. Selection of adsorbent and batch adsorption experiment
Before the optimization of the adsorption parameters, the adsorption performances of the synthesized materials were evaluated using 0.5 g l−1 of the synthesized materials and 10 mg/l fluoride solution at room temperature (25 ± 2 °C) by shacking the mixture at 150 rpm until equilibrium was attained (Tang et al 2018). Then the suspension was filtered or separated by the external magnet (for Fe-HA), and the residue of fluoride ion was determined using ion selective electrode (Ion meter, S-613F Peak Instrument) after the calibration according to the manufacturer. Then based on the percentage removal result, 1%Fe-HA was selected for further studies, and adsorption parameters were optimized by using 1%Fe-HA and pure HA. The effect of pH (3–11), contact time (1–240 min), initial concentration (5–30 mg l−1), adsorbent amount (0.05–0.5 g) and temperature (25 °C–60 °C) were evaluated during the experiment. The effect of ionic strength was analysed using different salts having concentration range of 0–5 mM. Similarly, the regeneration and reuse analysis were performed by washing the fluoride loaded adsorbent using 1.0 M NaOH solution at the optimum conditions for adsorption. Then, the adsorbent was separated by using centrifugation and/ external magnet (for Fe-HA). The separated material was washed using de-ionized water until neutral pH and oven dried; after that the adsorbent was loaded using fresh solution of fluoride ion for reuse study (Indah et al 2018). The regeneration and reuse analysis were done for five consecutive cycles. All the adsorption experiments were conducted in triplicate, and the mean ± standard deviation was reported. The amount of fluoride ion adsorbed at equilibrium, (qe) and the percentage removal (%) were calculated using the equations (3) and (4) (Nayak et al 2017).
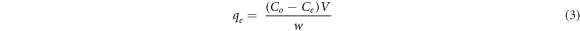
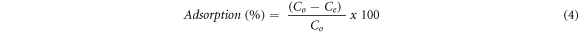
3. Result and discussion
3.1. Characterization
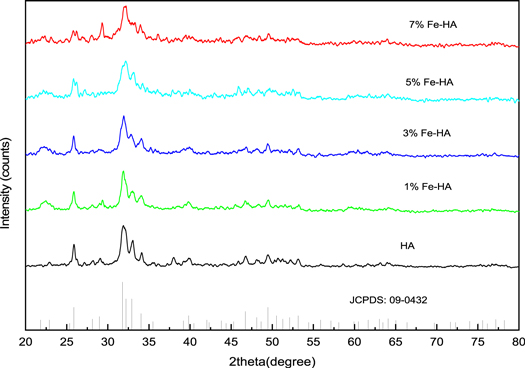
The x-ray diffraction patterns of the pure and iron doped hydroxyapatite synthesized materials are shown in figure 1. All the diffraction peaks were in good agreement with the JCPDS database of hydroxyapatite (PDF No: 09–0432) with hexagonal structure and a space group of P63/m. The significant peaks for pure and iron-doped hydroxyapatites were observed at around 2θ of: 25.9, 31.8, 32.2, 32.9, 34.1, 39.9, 46.7 and 49.5°. These peaks correspond to the crystal planes of (002), (211), (112), (300), (202), (310), (222) and (213) respectively. No secondary phase was observed in the diffraction patterns except the intense peak at 29.3 for 7%Fe-HA, which can be assigned to calcite impurity (JCPDS No.88–1807) or it may be the crystal growth of (210) plane of hydroxyapatite. In the iron-doped hydroxyapatite, the (002) plane shifted to the lower 2θ values and that of (300) plane shifted to the higher 2θ values. As the amount of iron in HA increases, the intensity of all the peaks was observed to have decreased. The decrease in intensity of the peak indicates the decreasing of crystallinity since the smaller size of iron ion inhibits the crystal growth of hydroxyapatite (Trinkunaite-Felsen et al 2015, Sheikh et al 2018). On the other hand, the decrease in intensity and shifting of the peaks could be the substitution of phosphate ion by carbonate ion (B-type substitution). The presence of the carbonate in the apatite structure causes the shifting of (002) plane to the left and that of (300) plane to the right. The degree of crystallinity was also observed to decrease in the hydroxyapatite structure (Yusufoglu and Akinc 2008, Zhao et al 2016, Safarzadeh et al 2020).
Figure 1. XRD patterns of the synthesized materials.
Download figure:
Standard image High-resolution imageThe crystallite size (D), lattice parameters (α and c), degree of crystallinity,  and unit cell volume
and unit cell volume  were estimated using equations (5)–(8) respectively(Kaygili et al
2021) .
were estimated using equations (5)–(8) respectively(Kaygili et al
2021) .
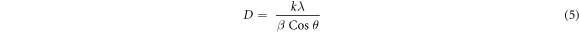
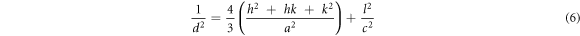
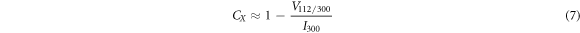
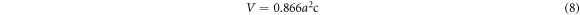
Where D is the mean crystalline size, k is the shape constant (0.9), λ is the wavelength of the radiation (0.15406 nm), β is the full width at half maxima (FWHM) of the peak intensity, θ is the diffraction angle, d is the distance between adjacent planes in the set of Miller indices (h k l),  is the intensity of the hollow between (112) and (300) peaks and
is the intensity of the hollow between (112) and (300) peaks and  is the intensity of (300) peak.
is the intensity of (300) peak.
The incorporation of iron into the lattice of calcium apatite has changed the lattice parameters and unit cell volume (table 1). The substitution of larger size Ca2+ (0.099 nm) ion for a smaller size ion, Fe3+ (0.064 nm), has resulted in the increment in the c-axis parameter. This increment may be due to the substitution of PO4 3– (larger size) by CO3 2– ion (smaller size) (Zhao et al 2016, Ahmed et al 2017), since B-type carbonate substitution causes peak broadening and decreasing of the crystallinity of hydroxyapatite.
Table 1. The values of crystallite size and lattice parameters of the synthesized materials.
| Sample | D (nm) | Lattice parameters (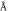 ) ) | c/a | Degree of crystallinity 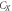 (%) (%) | Unit cell volume 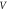 (Å
3) (Å
3) | |
|---|---|---|---|---|---|---|
| a | c | |||||
| HA | 22.96 | 9.4500 | 6.864 | 0.7225 | 66.03 | 530.834 |
| 1%Fe-HA | 15.21 | 9.4223 | 6.874 | 0.7295 | 52.52 | 528.495 |
| 3%Fe-HA | 17.64 | 9.4189 | 6.880 | 0.7304 | 36.40 | 528.575 |
| 5%Fe-HA | 19.82 | 9.4189 | 6.890 | 0.7315 | 19.99 | 529.343 |
| 7%Fe-HA | 21.17 | 9.3912 | 6.896 | 0.7343 | 21.63 | 526.693 |
The FT-IR spectra of pure and iron doped hydroxyapatites are given in figure 2. In the spectra, the characteristic bands of hydroxyl, adsorbed water and phosphate functional groups were observed. For the pure hydroxyapatite, the peak at 3693 cm−1 is due to the O-H stretching. Both the broad peak at 3427 cm−1 and sharp peak at 1631 cm−1 , corresponds to stretching and bending vibrations of H2O, respectively (Krukowski et al 2018, Zeng et al 2019). Similarly, the phosphate group shows asymmetric stretching at 1031 cm−1 and bending vibration at 602 cm−1, 563 cm−1 and 451 cm−1 (He et al 2017, Krukowski et al 2018). The peaks at 1411 cm−1 and 869 cm−1 indicates B-type carbonate substituted hydroxyapatite (carbonate that comes from absorption of CO2 from the atmosphere) (Krukowski et al 2018, Zeng et al 2019).
Figure 2. FT-IR spectra of (a) HA before adsorption, (b) HA after adsorption, (c) Fe-HA before adsorption and (d) Fe-HA after adsorption.
Download figure:
Standard image High-resolution imageIn the case of iron-doped hydroxyapatite, the intensity of OH– stretching at 3693 cm−1 was found to decrease as the substitution of calcium ion (Ca2+) with iron ion (Fe3+), charge imbalance will be created on the surface of HA, so that the OH– ion is changed in to O2– ion to balance the charge (Ramakrishnan et al 2016, Priyadarshini et al 2017). The absorption bands of adsorbed water were observed at 3433 cm−1 and 1632 cm−1, that of phosphate were appeared at 1028 cm−1, 569 cm−1, 531 cm−1 and 480 cm−1. Similarly, carbonate shows peaks at 1391 cm−1 and 826 cm−1. In this case, additional peaks were observed at 447 cm−1 and 416 cm−1, which may be due to more polarization of the phosphate group, or it may show the linkage of iron with oxygen in the lattice since Fe—O bond have vibration peaks in this range (Sujana and Anand 2010, Mercado et al 2014, Jayarathna et al 2015). The carbonate peak at 1391 cm−1 becomes broader and intense, this may be an indication of the presence of more carbonate ion in the modified hydroxyapatite material. These change in peak positions, shape and the presence of additional absorption bands on hydroxyapatite structure may indicate the structural distortions (Manatunga et al 2018). The absences of OH– and PO4 3– bands at around 634 cm−1 and 963 cm−1, respectively, in both pure and iron-doped hydroxyapatite materials indicates the less crystallinities of the products (Valizadeh et al 2014, Selvakumar et al 2016, Manatunga et al 2018), which is confirmed by XRD.
The FESEM images of pure and iron doped hydroxyapatites are given in figure 3. The result showed that metal doping changes the morphology of the hydroxyapatite material. The pure hydroxyapatite exhibited a rod and plate-like structure. Iron-doped HA has a rod/needle-like morphology. Different researchers obtained similar morphologies on pure and doped hydroxyapatite (Yuan et al 2018, Wei et al 2019, Goldberg et al 2020). The result also indicates that pure hydroxyapatite has a more highly ordered arrangement than that of iron-doped HA. The particles also have different sizes. The pure hydroxyapatite has a size distribution in the range of 12.00–29.65 nm, having an average value of 17.70 nm and that of iron doped was in the range of 9.43–20.64 nm with a mean value of 13.81 nm, which agree with the XRD results.
Figure 3. FE-SEM images of pure and iron doped hydroxyapatite.
Download figure:
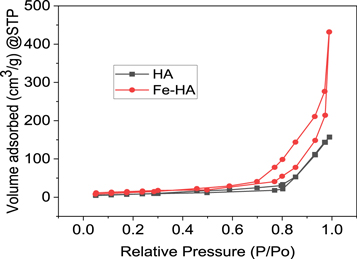
Standard image High-resolution imageThe nitrogen adsorption-desorption isotherm given in figure 4 indicates that the adsorbent is classified under type V with a hysteresis loop of type-A cylindrical porous material (Labani et al 2013). The BET analysis indicates that the surface area and pore volume of iron-doped hydroxyapatite were greater than those of the pure hydroxyapatite, and more amount of gas was adsorbed by iron-doped hydroxyapatite. The surface area, pore volume and average pore diameter of ion doped HA were found to be 117.618 m2 g−1, 1.67 cm3 g−1 and 24.3 nm, respectively. In the case of pure hydroxyapatite, the respective values were found to be 49.771 m2 g−1, 0.57 cm3 g−1 and 23.01 nm. The elemental composition analysis using EDS confirms the presence of calcium, phosphorous and oxygen in the pure hydroxyapatite. Moreover, in the modified sample, iron was also detected in addition to calcium, phosphorous and oxygen. The molar ratio of Ca /P was 1.64, lower than 1.67, which indicates that iron ion was successfully substituted in to the apatite structure.
Figure 4. The N2 adsorption-desorption isotherm of HA and Fe-HA.
Download figure:
Standard image High-resolution image3.2. Adsorption study
3.2.1. Point of zero charge of the adsorbents and pH effect on adsorption
The point of zero charge (pzc) is the point at which initial and final pH becomes equal (Mahmood et al 2011). Moreover, it is determined by plotting the change in pH (the difference in initial and final) versus initial pH (pHi). The point of zero charge of the synthesized pure and iron-doped hydroxyapatite were found to be 7.6 and 8.2, respectively (figure 5(a)). This indicates that the surface charge of the materials is positive below the corresponding values of pzc and negatively charged above the values. The high pzc value of iron-doped hydroxyapatite indicates that the surface of Fe-HA has extra positive charge. The solution pH is one factor affecting the removal of fluoride since a change in pH of the solution could also changes the surface properties and dissolution behavior of materials (Wang et al 2017, Fernando et al 2019, Fernando et al 2021). At pH less than 3, both pure and iron-doped hydroxyapatite were not stable, so that pH influence was optimized by varying it from 3–11 using 0.1 N HCl and 0.1 N NaOH solutions. The fluoride removal tendency of the pure hydroxyapatite was found to increase with increase in pH and attained maximum value of 78.46% at pH = 6 and then sharply decreased in the alkaline media to 45.31% at pH = 11 (figure 5(b)). The decrease in fluoride removal in the acidic solution may be due to apatite dissolution, according to the reaction given in different reports (Dorozhkin 2012, Nayak et al 2017, Wimalasiri et al 2021). However, in the case of alkaline pH, the electrostatic repulsion between fluoride and OH− ions decreases the removal of percentage (Tang et al 2018, Fernando et al 2021). In the case of iron-doped hydroxyapatite, the adsorption efficiency was high in acidic media and then dropped to a lower value in the basic media. This indicated that the surface charge of iron-doped hydroxyapatite is positive in acidic media and becomes negative in the alkaline condition. This result agrees with previous reports on tri-valet metal ion-doped hydroxyapatites (Nie et al 2012, Chen et al 2018). During the pH change from 3 to 11, the percentage removal was observed to change from 89.8%–71.5% for iron-doped hydroxyapatite. The result also showed that iron-doped hydroxyapatite was less affected by pH change when compared with the pure hydroxyapatite.
Figure 5. Point of zero charge of HA and Fe-HA (a) and the effect of pH on adsorption (b) using 10 mg l−1 F−1- and 0.5 g l−1.
Download figure:
Standard image High-resolution image3.2.2. Kinetics of adsorption
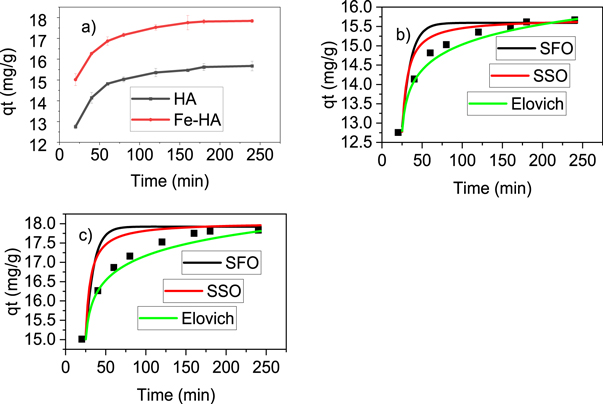
The variation of fluoride removal capacity of HA and Fe-HA was determined separately by varying the time of interaction from 10–240 min using 10 mg l−1 of F–, pH 6.5 and 0.5 g L−1 of adsorbents. The result is shown in figure 6(a). Similarly, the non-linear fitting plots for pure and iron-doped hydroxyapatites are given in figures 6(b) and (c) respectively.
Figure 6. The effect of contact time (a), kinetic models for HA (b) and kinetic models for Fe-HA (c) using pH 6.5, 10 mg l−1 F−1−, dose 0.5 g L−1 and stirring speed 150 rpm.
Download figure:
Standard image High-resolution imageThe rate of adsorption processes of pure and iron-doped hydroxyapatites (figure 6(a)) varied very fast in the first 60 min and then slowly increased up to 180 min which was considered as the equilibrium time. In order to further understand the rate and mechanism of adsorption, the experimental data were non-linearly fitted with pseudo-first order, pseudo-second order and Elovich models based on equations (9), (10) and (11), respectively (Nayak et al 2017).
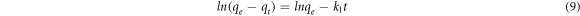
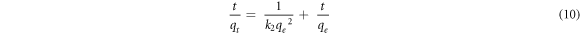
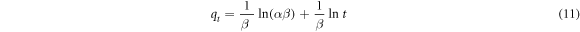
Where,  and
and  are the amounts (mg g−1) of fluoride ion adsorbed at time t and equilibrium respectively
are the amounts (mg g−1) of fluoride ion adsorbed at time t and equilibrium respectively  (1/min)
(1/min)  the pseudo-first order rate constant,
the pseudo-first order rate constant,  (g/(mg.min)) the pseudo-second order rate constant, α (mg/(g.min)) the initial sorption rate constant and β (mg g−1) is the extent of surface coverage during chemisorption.
(g/(mg.min)) the pseudo-second order rate constant, α (mg/(g.min)) the initial sorption rate constant and β (mg g−1) is the extent of surface coverage during chemisorption.
Based on the comparison of Adj. R2, rate constants (k1, k2 and  ) and the closeness of the experimental value to the calculated values of the equilibrium adsorption capacity (qe) given in table 2; the kinetics result fits more with pseudo-second order model. This indicated that the adsorption process is chemisorption through ion exchange or electrostatic interaction (Nayak et al
2017, Wang et al
2017, Chen et al
2018), is also confirmed by the high values of Adj.R2 and the constants in the Elovich model.
) and the closeness of the experimental value to the calculated values of the equilibrium adsorption capacity (qe) given in table 2; the kinetics result fits more with pseudo-second order model. This indicated that the adsorption process is chemisorption through ion exchange or electrostatic interaction (Nayak et al
2017, Wang et al
2017, Chen et al
2018), is also confirmed by the high values of Adj.R2 and the constants in the Elovich model.
Table 2. Calculated and experimental values of different kinetic parameters.
| Kinetic models | Parameters | HA | Fe-HA |
|---|---|---|---|
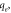 exp. (mg g−1) exp. (mg g−1) | 15.62 | 17.81 | |
| SFO |
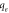 (mg g−1) (mg g−1) | 15.15 | 17.42 |
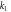 (1/min) (1/min) | 0.086 | 0.094 | |
| R2 | 0.988 | 0.993 | |
| SSO |
 (mg g−1) (mg g−1) | 15.91 | 18.16 |
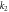 (g/(mg.min)) (g/(mg.min)) | 0.012 | 0.013 | |
| R2 | 0.999 | 0.999 | |
| Elovich |
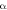 (mg/(g.min)) (mg/(g.min)) | 0.396 | 0.445 |
| β (mg g−1) | 0.854 | 0.883 | |
| R2 | 0.998 | 0.998 |
3.2.3. Initial concentration effect and isotherm studies
The effect of the amount of fluoride ion in the solution was optimized using fluoride ion concentration from 5–30 mg l−1 at pH 6.5, contact time of 3 h and adsorbent dose of 0.5 g L−1. It was perceived that initially, the removal percentage of the adsorbents (HA and Fe-HA) rose with increasing initial fluoride amount until 10 mg l−1 and then it slightly decreased. However, the adsorption capacity increased even beyond 10 mg l−1. The high fluoride ion uptake of the materials at the lower concentration indicates that there is limited amount of energetic sites of the materials (Nayak et al 2017). In order to understanding the adsorption mechanisms well, the obtained results were fitted non-linearly to Langmuir, Freundlich, and Temkin isotherm models. Langmuir isotherm of monolayer sorption onto homogenous surface (Girish 2017), is expressed by equation (12):
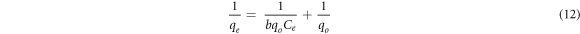
Where  the amount of fluoride ion adsorbed at equilibrium (mg g−1), and
the amount of fluoride ion adsorbed at equilibrium (mg g−1), and  is the equilibrium fluoride ion concentration (mg l−1), b (l mg−1) is a constant related to binding energy, and
is the equilibrium fluoride ion concentration (mg l−1), b (l mg−1) is a constant related to binding energy, and  is monolayer adsorption capacity in (mg g−1). Another important parameter (r) is needed to describe the feasibility of adsorption:
is monolayer adsorption capacity in (mg g−1). Another important parameter (r) is needed to describe the feasibility of adsorption:  where
where  is the initial concentration of F− ion (mg l−1) and r is the Langmuir isotherm constant.
is the initial concentration of F− ion (mg l−1) and r is the Langmuir isotherm constant.
The Freundlich isotherm model of multilayer sorption on heterogeneous surfaces (Girish 2017), is given by the equation:
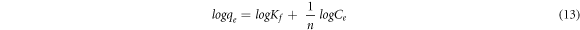
Where Kf ((mg g−1) (l mg−1)^1/n) a constant is related with the adsorption capacity and n is an empirical parameter indicating the extent of adsorption.
Similarly, the Temkin isotherm that considers the induced heterogeneity due to the indirect adsorbate/adsorbent interaction (Ayawei et al 2017, Özsin et al 2019b), is expressed using:
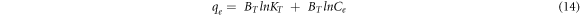
Where,  (l g−1) is the Temkin isotherm binding constant, and
(l g−1) is the Temkin isotherm binding constant, and  (J/mg) is the constant related to the heat of sorption.
(J/mg) is the constant related to the heat of sorption.
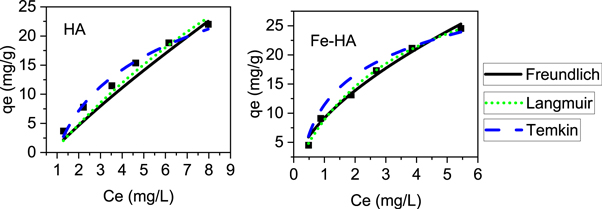
The plots of the isotherm models and the isotherm constants are presented in figure 7 and table 3 respectively. The data indicates that Langmuir model describes better the experimental result, it confirms that adsorption is more of a monolayer on homogeneous surface (Chen et al 2018). The value of r in any of the tested concentration for both pure and iron-doped hydroxyapatite is the range of 0 < r < 1, which indicates that the adsorption is favourable.
Figure 7. Isotherm models @ pH of 6.5, 3 h contact time, 0.5 g l−1 of adsorbents and 25 ± 2 °C temperature.
Download figure:
Standard image High-resolution imageTable 3. Calculated Values of isotherm constants of the different models.
| Adsorbent | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
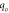
| b | R2 | n |
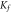
| R2 |

|
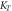
| R2 | |
| HA | 40.46 | 0.046 | 0.987 | 1.18 | 3.96 | 0.976 | 95.3 | 1.02 | 0.971 |
| Fe-HA | 83.86 | 0.280 | 0.988 | 1.67 | 9.16 | 0.977 | 305.54 | 3.36 | 0.970 |
The defluoridation capacity of Fe-HA and HA were 83.86 and 40.46 mg g−1, respectively. The high adsorption capacity of Fe-HA is associated with the strong interaction of trivalent metal ion doped hydroxyapatite with fluoride ion (Chen et al 2018), high fluoride ion removal efficiency of iron-containing material (Sujana and Anand 2010, Corral-Capulin et al 2019, Ogata et al 2020, Scheverin et al 2021), the less size, high surface area, high pore volume and less crystalline nature of it as observed in BET, FE-SEM and XRD.
3.2.4. Adsorbent amount effect
The effect of adsorbent amount was studied by taking 10 mg l−1 of fluoride concentration, pH 6.5, contact time 3 h, agitation speed 150 rpm and varying the adsorbent doses from 1.0–10.0 g l−1. Figure 8 showed that the adsorption capacity of pure and iron-doped hydroxyapatite decreased sharply with increasing the adsorbents amount up to 4 g L−1, and after that, it became near to constant. This is because, at the lowest amount of adsorbent, the number of active sites for the adsorbent is insufficient to remove the fluoride ions. However, when the adsorbent amount increases, the number of active sites also increases, causing increment in the removal tendency. The decreasing tendency of defluoridation capacity by further increasing of adsorbent amount is due to adsorbent-adsorbent interaction (Nayak et al 2017), or it may be associated with the repulsion between the fluoride ions in solution and those that are attached on the surface of the materials (Roy and Das 2016). It was observed that the fluoride residual concentration was within the WHO recommended limit (1.5 mg l−1) for Fe-HA in all the amounts of adsorbent used.
Figure 8. The effect of adsorbent doses: @ pH of 6.5, 3 h contact time and 10 mg l−1 of F− ion.
Download figure:
Standard image High-resolution image3.2.5. Thermodynamic studies
The temperature effect on adsorption of fluoride ion was analysed in the range of 298–333 K using 10 mg l−1 of F– and 4 g l−1 doses. The result indicates that adsorption capacity of pure and iron-doped hydroxyapatite was observed to increase with increase in temperature. This is because as the temperature increases, the free volume of the pores of the adsorbent increases as a consequence to the movement of the solute (Nayak et al 2017). In order to determine the viability and spontaneous nature of the adsorption process, thermodynamic parameters were determined using equations (15)–(17) (Fernando et al 2019).
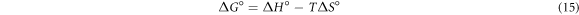
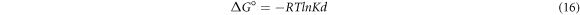
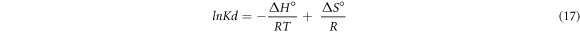
Where, R is universal gas constant, T is absolute temperature,  is the distribution coefficient of F−,
is the distribution coefficient of F−,  is the F− concentration on the adsorbent at the equilibrium and
is the F− concentration on the adsorbent at the equilibrium and  is the concentration of F− in the solution at the equilibrium.
is the concentration of F− in the solution at the equilibrium.
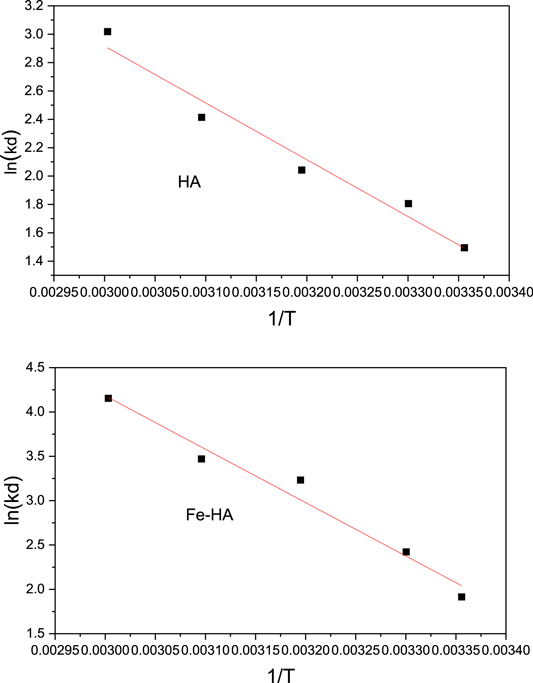
The plots of  versus 1/T and thermodynamics parameters for the pure and iron-doped hydroxyapatite are depicted in figure 9 and table 4 respectively. The negative values of Gibbs free energy change (ΔG°) for both materials in all the temperatures indicates that the adsorption process is spontaneous. Similarly, the positive values of enthalpy change (ΔH°) showed that the adsorption process is endothermic since energy is needed to break the high interaction between the fluoride ion and water molecule in the solution. In addition, the positive entropy change (ΔS°) for pure and iron-doped hydroxyapatite indicates that the adsorption process is endothermic and random (Chen et al
2018, Fernando et al
2019). Furthermore, the lower values of ΔH° and ΔS° for iron-doped hydroxyapatite indicates that fluoride ion was more readily adsorbed on the surface of the iron-doped hydroxyapatite than that of the pure hydroxyapatite.
versus 1/T and thermodynamics parameters for the pure and iron-doped hydroxyapatite are depicted in figure 9 and table 4 respectively. The negative values of Gibbs free energy change (ΔG°) for both materials in all the temperatures indicates that the adsorption process is spontaneous. Similarly, the positive values of enthalpy change (ΔH°) showed that the adsorption process is endothermic since energy is needed to break the high interaction between the fluoride ion and water molecule in the solution. In addition, the positive entropy change (ΔS°) for pure and iron-doped hydroxyapatite indicates that the adsorption process is endothermic and random (Chen et al
2018, Fernando et al
2019). Furthermore, the lower values of ΔH° and ΔS° for iron-doped hydroxyapatite indicates that fluoride ion was more readily adsorbed on the surface of the iron-doped hydroxyapatite than that of the pure hydroxyapatite.
Figure 9. The van't Hoff plot of F ion adsorption on HA and Fe-HA.
Download figure:
Standard image High-resolution imageTable 4. Calculated Values of thermodynamics parameters.
| Adsorbents | ΔG° (kJ mol.−1) | ΔH° (kJ mol.−1) | ΔS0 (kJ mol.−1) | R2 | ||||
|---|---|---|---|---|---|---|---|---|
| 298 | 303 | 313 | 323 | 333 | ||||
| HA | −4.434 | −5.705 | −7.095 | −8.395 | −9.078 | 34.593 | 0.132 | 0.904 |
| Fe-HA | −7.255 | −8.634 | −9.235 | −9.745 | −10.917 | 17.235 | 0.083 | 0.954 |
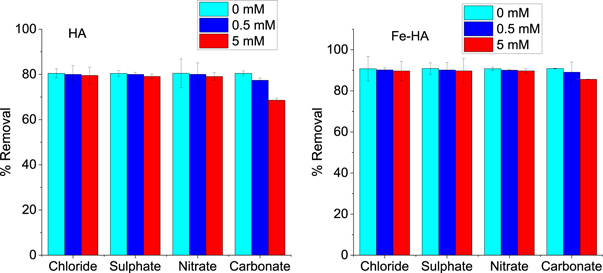
3.2.6. Effect of ionic strength
The effect of ionic strength was evaluated by taking 0–5 mM of sodium salts of chloride, nitrate, sulphate and carbonate ions with 20 mg l−1 of fluoride and 4 g l−1 of the adsorbents. The result (figure10 ) ilustrated that the ionic strengt of the ions such as chloride, sulphate and nitrate have a negligiable effect on the adsorption of fluoride ion on both pure and iron-doped hydroxyapatite. However, the precence of carbonate ion at different ionc strengths has an effect on the fluoride removal. The reduction in fluoride removal of the adsorbents in the presence of carbonate ion indicates that the competation of this ion is dependant mainly on the size and charge (Mondal et al 2016). Moreover, this effect is more pronounced in pure hydroxyapatite than in iron-doped hydroxyapatite, which implies that iron-modified hydroxyapatite has a more active site for fluoride interaction than pure hydroxyapatite.
Figure 10. The effect of ionic strength on removal of fluoride ion.
Download figure:
Standard image High-resolution image3.2.7. Regeneration and reusability study
Since fluoride ion was poorly adsorbed in alkaline conditions for the two adsorbents, 1.0 M NaOH solutions was used as a regenerator. The regeneration analysis was performed using 10 mg l−1 F−1−, 4 g l−1 adsorbents, 3 h contact time, and an agitation speed of 150 rpm at room temperature (25 ± 2 °C). Five consecutive adsorption - desorption cycles were performed to investigate the regeneration and recyclability of the pure and iron-doped hydroxyapatite. The results (figure 11) indicates that the removal percentage of the materials in the successive cycle was changed insignificantly assuring that the materials are suitable for regeneration and reuse.
Figure 11. The reusability tests of pure and iron doped hydroxyapatite using 10 mg l−1 F−1-, 3 h contact time and 4 g l−1 adsorbent.
Download figure:
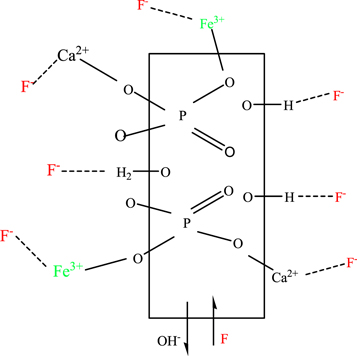
Standard image High-resolution image3.2.8. Adsorption mechanism analysis
Adsorption mechanism prediction is highly significant for the study of materials characteristics (He et al 2017). To understand fluoride uptake through the pure and iron-doped hydroxyapatites, FT-IR analysis before and after adsorption, the adsorption tendency of fluoride ion at different pH range, adsorption models and thermodynamics data were considered. The probable dominant mechanisms of adsorption could be ion exchange and electrostatic interaction/complexation on the surfaces of the adsorbent materials, as shown in figure 12. FT-IR spectra of HA after fluoride adsorption (figure 2(b)) showed a decrease in the intensity of the O-H stretching at 3693 cm−1 which is due to partial replacement of OH− ions by fluoride ions (He et al 2017, Chen et al 2018, Nagaraj et al 2018). Similarly, the slight shift of the O-H stretching from 3427 cm−1 to 3416 cm−1 may indicate the formation of H.....F bond. A new band at 695 cm−1 (less intense) may be associated with forming the H....F bond or it may be the electrostatic interaction between calcium ion and fluoride ion. For iron doped hydroxyapatite (figure 2(d)), the O-H peak was shifted from 3433 cm−1 to 3331 cm−1 with decreasing intensity and increasing broadness. It may be due to the formation of F-HA through the linkage of OH2 +...F− (Nayak et al 2017). In addition, a new intense and sharp peak at 557 cm−1 may be associated with the linkage of negative fluoride ions with the positive metal ions through complex formation. In this case, the carbonate ions also participated in the fluoride adsorption, as it is observed that the two carbonate peaks shifted from 826 cm−1 to 869 cm−1 (with decreasing intensity) and from 1391 cm−1 to 1352 cm−1 (with decreasing in intensity and broadness). Compared to the pure and hydroxyapatite, the high adsorption capacity of iron-doped hydroxyapatite indicates that the interaction of fluoride ion with Fe-HA is a complex process.
Figure 12. The possible fluoride ion adsorption mechanism on pure and iron doped HA.
Download figure:
Standard image High-resolution image3.2.9. Comparative analysis
The adsorption capacity of the pure and iron-doped hydroxyapatites was compared with the previously reported hydroxyapatite-based adsorbents (table 5). Iron-doped hydroxyapatite exhibited a high adsorption capacity at low temperature, neutral pH and moderate contact time than most of the adsorbents used previously except Al-HA nanowire (He et al 2017), which uses a high amount of initial fluoride ion (200 mg l−1) and high temperature.
Table 5. Comparison of the adsorption performance of the synthesized materials with previously reported adsorbents.
| Adsorbents | Co (mg l−1) | Dose (g l−1) | pH | Time | Temp (°C) | qe (mg g−1) | Reference |
|---|---|---|---|---|---|---|---|
| Al-HA nanowire | 200 | 0.5 | 7 | 3 h | 54 | 107.41 | (He et al 2017) |
| Al-doped HA | 5–50 | 0.5 | 7 | 5 h | 25 | 32.57 | (Nie et al 2012) |
| Cellulose-HA nanocomosite | 10 | 1 | 6.5 | 6 | 25 | 4.2 | (Yu et al 2013) |
| Nano-hydroxyapatite/chitosan composite | 9–15 | 5 | 7 | 30 min | 50 | 2.32 | (Sundaram et al 2008) |
| HA-CNTs | — | 0.5 | 7 | 2 h | 45 | 16.78 | (Tang et al 2018) |
| Montmorillonite-HA nanocomposite | 1–30 | — | — | 1 h | 16.71 | (Fernando et al 2019) | |
| HA-modified activated alumina | 10 | — | 7 | 8 h | — | 14.4 | (Tomar et al 2015) |
| Al-doped HA | 5–10 | 0.1 | 5.7 | 25 min | 55 | 46.729 | (Chen et al 2018) |
| Nanocrystalline- HA | 5–40 | 3 | 6 | 1 h | 25 | 22.3 | (Nayak et al 2017) |
| Porous nanohydroxyapatite | 1–200 | 2 | 6.8 | <2 min | Microwave | 54.0 | (Wimalasiri et al 2021) |
| HA | 5–30 | 0.5 | 6.5 | 3 h | 25 | 40.46 | This work |
| Fe-doped HA | 5–30 | 0.5 | 6.5 | 3 h | 25 | 83.86 | This work |
4. Conclusion
The co-precipitation method was used in this research to synthesis the pure and iron-doped hydroxyapatites. XRD analysis revealed that the formed products have a hexagonal structure with a space group of P63/m. The FE-SEM result showed rod/needle-like morphology. The elemental composition analysis of the HA and Fe-HA by EDS confirmed the presence of all the respective elements of HA and Fe-HA. The surface area and porosity of the materials were investigated from N2 adsorption-desorption isotherm using BET analysis. The surface area was changed from 49.771 m2 g−1 (for the pure) to 117.618 m2 g−1 (iron doped). The point of zero charge of HA and Fe-HA estimated by pH drift method, are 7.6 and 8.2 respectively. Various adsorption parameters such as contact time, initial fluoride concentration, and adsorbent dose were analysed at room temperature and pH of 6.5, and the optimum values obtained were, 3 h, 10 mg l−1 and 4 g l−1 respectively. The presence of carbonate ions at different ionic strength reduces the adsorption capacity of both pure and iron-doped hydroxyapatites. The experimental data well fitted to pseudo-second order and Langmuir models, indicating that the adsorption process is more governed by chemisorption on monolayer surface. The maximum adsorption capacities of HA and Fe-HA were found to be 40.46 and 83.86 mg g−1, respectively. The high adsorption capacity of iron-doped hydroxyapatite is due to the strong interaction of trivalent metal ion-doped hydroxyapatite with fluoride ion and the high efficiency of iron-containing materials for the removal of fluoride. In addition, the smaller size, the high surface area, high pore volume and less crystalline nature of Fe-HA can contribute to the high adsorption tendency. The thermodynamic data indicates that the adsorption process is endothermic and spontaneous. In addition, the regeneration and reusability studies indicates that the synthesized materials are easily regenerated and reused. Based on FT-IR results before and after adsorption, pH effect on adsorption, kinetic, isotherm and thermodynamics data, the mechanism of adsorption was proposed as ion exchange between OH− and F− and electrostatic interaction (hydrogen bonding and/complexation) between fluoride and cations on the surface of the materials. Due to its biocompatibility, high adsorption capacity, easily separable nature as well as reusability, iron-modified hydroxyapatite can be considered as economical and practical for the defluoridation purpose.
Acknowledgments
The authors would like to thank Adama Science and Technology University for financial and materials support. And our thanks goes to Universiti Malaysia for the support of sample characterization.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Competing interests
The authors declare that there is no conflict of interest.