Abstract
The changes in intermetallic phases of Mg-6%Al-5%Pb (Mg-Al-Pb) alloy prepared by powder metallurgy and their effects on electrochemical properties were studied. Experimental results showed that coarse Mg17Al12 phases (with size ranges from 20 μm to 70 μm) and fine Mg2Pb phases (with the size of 100 nm scale) formed in the mixed Al, Pb and Mg powder during the hot press sintering procedure. Fine Mg2Pb phases exhibit strong diffusional ability to diffuse into Mg17Al12 phases. Extruding further promotes the diffusion of Mg2Pb phases into Mg17Al12 phases. Solution treatment produced a mixed microstructure which is Mg17Al12 phase containing Mg2Pb phases inside and made other Mg2Pb phases homogeneously distributed in Mg matrix. Mg17Al12 phases and the gathered Mg2Pb phases will accumulate the Mg(OH)2 corrosion products, which impedes the subsequent discharge behavior. Homogeneously distributed Mg2Pb phases will fall off during discharge, which makes the discharge curve more stable. The mixed microstructure has positive effect on corrosion resistance and discharge potential. Therefore, solution-treated alloy shows the highest negative potential and the smoothest curve during the galvanostatic discharge test. Compared with the commercial AZ61 sheet, solution-treated alloy exhibit much higher anode properties in Mg/PbCl2 battery test. Thus, the solution-treated alloy could serve as an excellent anode in seawater-activated batteries.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Seawater activated batteries are the potential power source to be used in underwater equipment such as sonobuoys, underwater defense devices, air-sea rescue equipment and meteorological radiosondes [1]. Seawater activated battery contains two significant parts, i.e., active metals as anode and metal chlorides as cathode [2]. When the battery works, the seawater will be poured into the battery system as the electrolyte and the metal anode will be activated to deliver electrons for power production [3]. During the discharge process, metal anode continuously dissolves into the electrolyte in the form of metallic cations, e.g., Mg2+ cations and those electrons are sent through an external circuit to power underwater device [4]. Anode property is an important factor to seawater activated battery. Magnesium alloys have been considered as promising anode materials for seawater activated battery due to their wide voltage range, high energy density, negative discharge potential and light weight [5–9].
Among magnesium seawater activated anodes, Mg-6%Al-5%Pb (Mg-Al-Pb) alloy have gained an increasing attention [10]. The addition of Al can improve the surface activity and the corrosion resistance of magnesium alloys [11–14]. Pb provides a high hydrogen evolution overpotential to inhibit the self-corrosion hydrogen evolution reaction and improves the current efficiency of the anode. Al together with Pb in Mg-Al-Pb alloy have a synergistic effect on the discharge process [10]. However, the density of Pb (11.3 g cm−3) is so high that it will be microsegregated from melting magnesium (1.7 g cm−3). This will cause a component segregation of alloy element Pb in ingots. Such a microsegregation is usually eliminated by heating at high temperature for a long time [10], which could increase the production cost of magnesium anode. As for the influence of intermetallic phases on electrochemical properties of magnesium anode, previous research mainly focused on the phases in single form [15]. It is hard to produce a microstructure mixed containing several different phases by melting and casting which may further increase the electrochemical properties [16, 17].
Powder metallurgy is a technology that uses metal powders as the raw materials to manufacture metal materials, composites and various types of products through forming and sintering [14]. It can directly prepare alloys with uniform composition without losing of raw materials [18], which could save costs and has a profit to uniform corrosion of magnesium alloys. And powder metallurgy has the potential to produce a new microstructure mixed with a variety of second phases, which may further increase the electrochemical properties. At present, there are almost no researches focus on magnesium alloy anodes by powder metallurgy. It is of great significance to explore the microstructure and electrochemical properties of magnesium alloys prepared by powder metallurgy. In this work, Mg-Al-Pb alloy samples were prepared by hot press sintering. Then, the samples were extruded, solution-treated, rolled and annealed in order to obtain microstructures mixed with a variety of second phases. The changes in the microstructures and discharge performance of Mg-Al-Pb alloys in different states and the relationship between them are studied and analyzed to express the electrochemical properties and discharge behavior.
Table 1. Average particle size and purity of raw material powder.
| Powder | Average particle size μm−1 | Purity |
|---|---|---|
| Mg | 70 | 99. 9% |
| Al | 25 | 99. 9% |
| Pb | 50 | 99. 9% |
2. Materials and methods
2.1. Raw materials and preparation
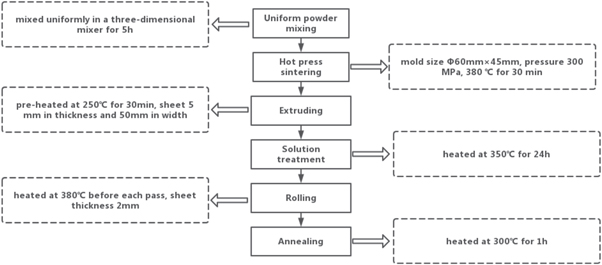
Figure 1 shows the experimental preparation flowchart. Mg powders, Al powders and Pb powders were mixed uniformly in a three-dimensional mixer for 5 h. The mixed powder was then placed in a prepared mold (Φ60 mm × 45 mm) with argon protection. Firstly, in the hot pressing sintering procedure, the mixed powder was held at 380 °C for 30 min under a pressure of 300 MPa. Secondly, the sintered Mg-Al-Pb alloy was heated to 250 °C for 30 min and then extruded into sheets 5 mm in thickness and 50 mm in width. The extruded Mg-Al-Pb sheets were solution-treated at 350 °C for 24 h. Subsequently, the solution-treated Mg-Al-Pb alloys were hot-rolled to sheets with a thickness of 2 mm. During each hot-rolling pass, the Mg-Al-Pb alloy was held at 380 °C for 30 min to maintain the deformability. Finally, the as-rolled Mg-Al-Pb alloy sheets were annealed at 300 °C for 1 h. The chemical composition of the sintered Mg-Al-Pb alloy are shown in table 2.
Figure 1. The experimental preparation flowchart.
Download figure:
Standard image High-resolution imageTable 2. The analyzed chemical compositions of the Mg-Al-Pb alloy(wt%).
| Al | Pb | Cr | Cu | Ni | Mg | |
|---|---|---|---|---|---|---|
| Mg-Al-Pb | 5. 9300 | 4. 8700 | 0. 0014 | 0. 0012 | 0. 0012 | Bal |
2.2. Characterization of microstructures
The microstructure of Mg-Al-Pb alloys was observed by optical microscopy (OM, DM4 M) and scanning electron microscopy (SEM,Quanta-200) with energy-dispersive x-ray spectroscopy (EDS). The crystalline phases were identified by x-ray diffraction (XRD, D Max 2550).
2.3. Electrochemical tests
The electrochemical performances of the Mg-Al-Pb alloy were measured by a CHI660E electrochemical workstation using a three-electrode system. The working electrode was Mg-Al-Pb alloy, with a platinum plate serving as the counter electrode and a saturated calomel electrode as the reference electrode. For the potentiodynamic polarization curves, the scanning voltage ranges from −2. 4 V to −0. 8 V to ensure that corrosion potentials of each state of Mg alloys are included, and the scanning rate is 1 mV s−1. In hydrogen evolution corrosion test, the anode samples with a diameter of 10 mm and thickness of 2 mm for immersion tests were ground with 1000 grit paper and cleaned with absolute ethanol. Galvanostatic discharge curves were obtained at current densities of 4. 5 mA cm−2 and 45 mA cm−2 for 1 200 s. Electrochemical impedance spectra (EIS) were measured after 20 min immersion in electrolyte, and the perturbation amplitude was 5 mV.
Finally, the prototype battery was assembled by using the solution-treated alloy as the anode and PbCl2 as the cathode. The battery for comparison used a commercial AZ61 sheet as the anode. The anode sheets were 75 mm × 60 mm × 2 mm in size and the current density was 45 mA cm−2 which mean that the working current of the prototype battery was 2 A. The whole discharge process was evaluated by a battery testing system called Neware Technology Limited (Battery charge and discharge detection equipment manufacturer). The electrochemical tests were carried out using a 3.5 wt% NaCl solution at (25 ± 0.5) °C. Three parallel experiments were conducted to minimize the error in each electrochemical test.
3. Results and discussion
3.1. The changes in the microstructures of Mg-Al-Pb alloys in different states
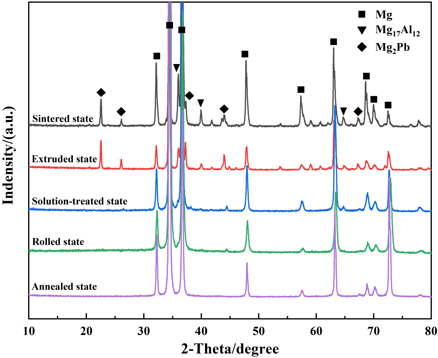
Figure 2 shows the XRD results for the studied Mg-Al-Pb alloys under different states. It is clear that second phases (Mg17 Al12 and Mg2 Pb) formed during the hot press sintering. The extrusion procedure did not cause the dissolution or precipitation of the second phases. In the solution-treated alloy, the amount and strength of the peaks of the Mg17 Al12 phases and Mg2Pb phases significantly decrease. This indicates that solution treatment promoted the dissolution of Mg17 Al12 phases and Mg2 Pb phases. In the rolled state and annealed state, the Mg17 Al12 and Mg2 Pb phases did not precipitate from the Mg matrix.
Figure 2. XRD results of Mg-Al-Pb alloys in different states.
Download figure:
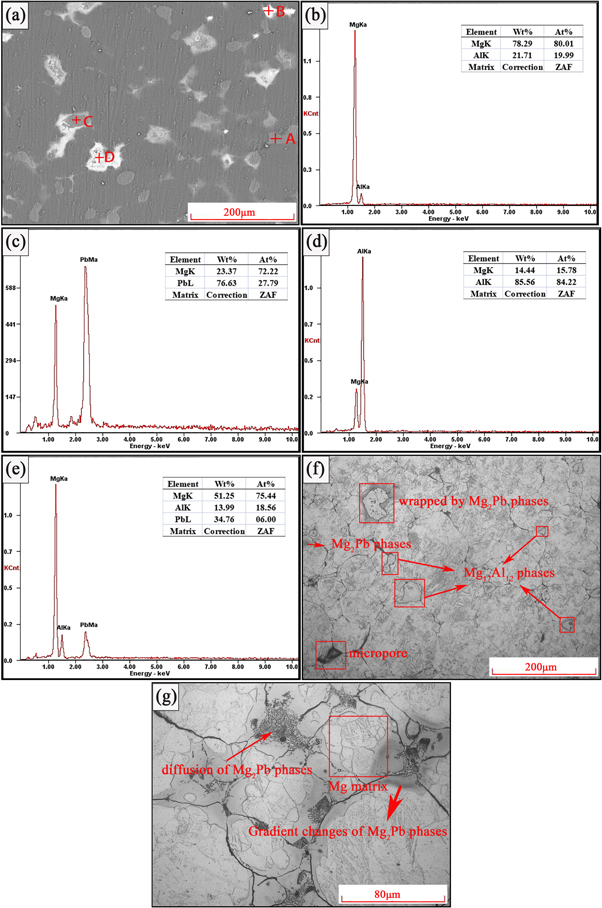
Standard image High-resolution imageTo further confirm the specific microstructures, optical micrographs and SEM images of Mg-Al-Pb alloys in different states were obtained. Figure 3 shows the microstructures of the sintered Mg-Al-Pb alloy. As indicated by SEM images and the EDS results, the pure Mg17 Al12 phases were found embedded in matrix in the form of single block (point A). The size of Mg2 Pb phases was much smaller, these intermetallic phases could gather together (point B) or diffused into Mg17 Al12 phases (point C). When too many Mg2 Pb phases diffuse into one Mg17 Al12 phase, a mixture (point D) of Mg17 Al12 phases and Mg2 Pb phases was formed. In figure 3(f), it was observed that sintered alloy composed of intermetallic phases, irregular coarse grains and some micropores existing around grain boundaries. The volume shrinkage of micropores was generated by different diffusion rates of metal elements during sintering [19]. The sizes of Mg17 Al12 phases were about 20 μm to 70 μm. In figure 3(g), the grain size of Mg matrix (about 20 μm) was much smaller than Mg17 Al12 phases. Gradient changes of Mg2 Pb phases could be found in figure 3(g), which indicates that Pb powder produced fine Mg2 Pb phases (with the size of 100 nm scale) with Mg powder. These fine Mg2 Pb phases have strong diffusion ability to diffuse into Mg17 Al12 phases, which eventually resulted in the formation of the microstructure corresponding to point D.
Figure 3. Microstructure of the sintered Mg-Al-Pb alloy. (a) SEM image, EDS results of point A(b), point B (c), point C (d)and point D(e), (f) and (g) showing the optical micrographs.
Download figure:
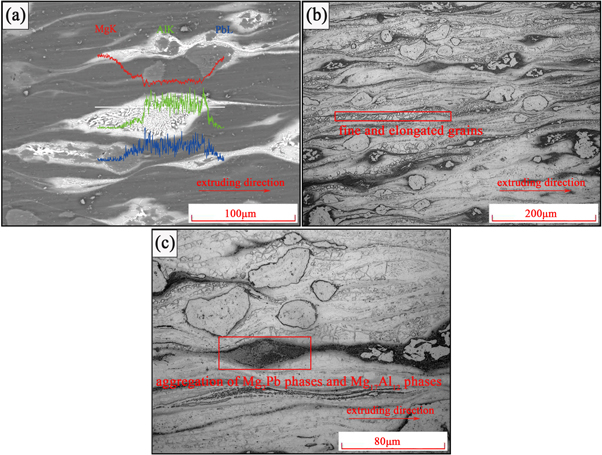
Standard image High-resolution imageAs showed in figure 4(a), the Mg2 Pb aggregation stretched into a line shape distributing along the extrusion direction, and some Mg17 Al12 phases crushed into small pieces after extrusion. In addition, extrusion produced a special microstructure in figure 4(a). According to the line swept result, the contents of Al and Pb increased in this microstructure, which means that it was an aggregation of Mg17 Al12 phases and Mg2 Pb phases. The extrusion stretched the Mg2 Pb aggregation to contact Mg17 Al12 phases, which caused more Mg17 Al12 phases that were wrapped by Mg2 Pb phases appearing in the extruded Mg-Al-Pb alloy. During the extrusion, these Mg2 Pb phases diffused into Mg17 Al12 phases thus resulting in the stress concentration during extrusion and made those Mg17 Al12 phases crushed. Figure 4(b) showed that the extruded alloy exhibited significantly fine and elongated grains, which were caused by partial dynamic recrystallization during the hot extrusion [20]. According to figure 4(c), Mg17 Al12 phases, which were not affected by Mg2 Pb phases, did not crushed during extrusion. It also can be found in figure 4(c) that the size of crushed Mg17 Al12 phase was affected by the amount of Mg2 Pb phases.Excessive diffusion of Mg2 Pb phases could result in the crushing of Mg17 Al12 phase, tailoring fine parts with similar size to those Mg2 Pb phases. The interdiffusion happened between the fine Mg17 Al12 phases and Mg2 Pb phases during extrusion, which result the formation of the aggregation of Mg17 Al12 phases and Mg2 Pb phases in figures 4(a) and (c).
Figure 4. Microstructures of the extruded Mg-Al-Pb alloy. (a) Image showing the SEM image, (b) and (c) showing the optical micrographs.
Download figure:
Standard image High-resolution imageThe volume fractions of Mg17 Al12 phases and Mg2 Pb phases in sintered alloy (figure 3(f)) and extruded alloy (figure 4(b)) were determined by ImageJ software. The results show that the volume fraction of Mg17 Al12 phases in sintered alloy is about 2.4%, while the volume fraction of Mg2 Pb phases is about 0.8%. In extruded alloy, the volume fraction of Mg17 Al12 phases in sintered alloy is about 8.3% and the volume fraction of Mg2 Pb phases is about 14.1%. This indicates that extruding procedure promoted the precipitation of Mg17 Al12 phases and Mg2 Pb phases.
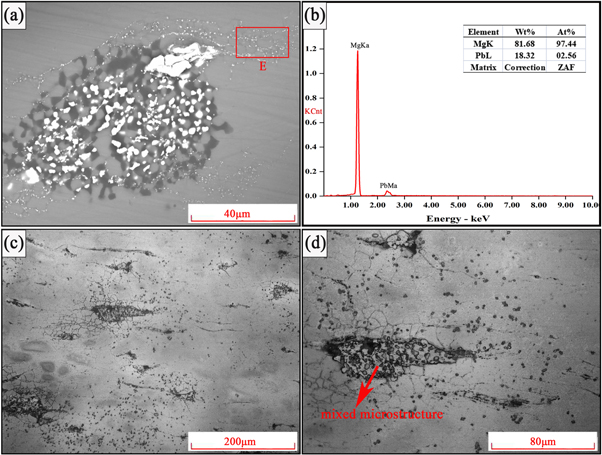
As depicted in figure 5(a), the Mg17 Al12 phases and Mg2 Pb phases formed a mixed microstructure (with size of 50 μm) after solution treatment (figure 5(c)). This mixed microstructure was Mg17 Al12 phase containing Mg2 Pb phases inside. Moreover, some white spots were found to be distributed homogeneously in the Mg matrix. The EDS result of area E in figure 5(b) shows that these spots are fine Mg2 Pb phases. This indicates that solution treatment made Mg2 Pb phases homogeneously distributed in the Mg matrix. From figure 5(c) and figure 5(d), the grain size of Mg matrix increased after solution treatment. The single form of Mg17 Al12 phases and most of Mg2 Pb phases dissolved into the Mg matrix after solution treatment.
Figure 5. Microstructure of solution-treated Mg-Al-Pb alloy. (a) image showing the SEM image, (b) image showing EDS result of area E, (c) and (d) showing the optical micrographs.
Download figure:
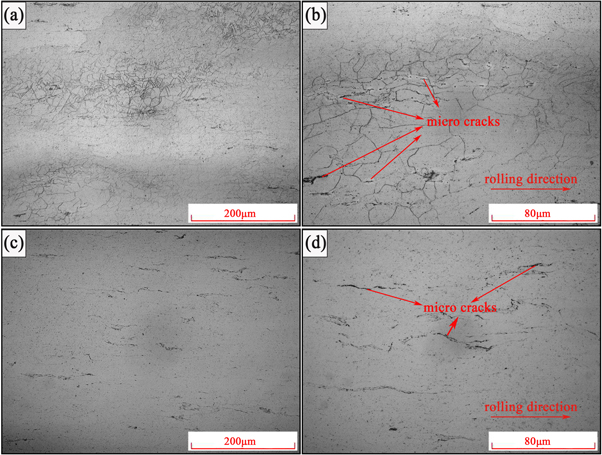
Standard image High-resolution imageFigure 6 shows the optical micrographs of the rolled Mg-Al-Pb alloy and annealed Mg-Al-Pb alloy. Twin crystals were found in rolled Mg-Al-Pb alloy (figure 6(a)). Under the combined action of heat treatment (380 °C) and rolling, the grain size of rolled Mg-Al-Pb alloy slightly increased and remaining intermetallic phases were dissolved. Figure 6(b) showed that rolling procedure generated microcracks along the rolling direction. Figure 6(d) indicated that the amount of the crack reduced after annealing. From figures 6(c) and (d), twin crystals disappeared and grain size obviously increased by annealing treatment.
Figure 6. Microstructures of rolled Mg-Al-Pb alloy and annealed Mg-Al-Pb alloy. (a) and (b) showing the optical micrographs of rolled Mg-Al-Pb alloy, (c) and (d) showing the optical micrographs of annealed Mg-Al-Pb alloy.
Download figure:
Standard image High-resolution image3.2. Potentiodynamic polarization test and hydrogen evolution corrosion test of Mg-Al-Pb alloys
Figure 7(a) gives the potentiodynamic polarization curves of the Mg-Al-Pb alloys, and the corresponding parameters are summarized in table 2. Polarization tests could reflect the corrosion behavior [21]. The corrosion potential (Ecorr ) and corrosion current (Jcorr ) are common electrochemical parameters that reflect the behavior of corrosion occurrence [22]. Table 3 shows that the sintered alloy exhibit the highest Jcorr (773.3 μA cm−2) among the Mg-Al-Pb alloys. It is believed to be caused by the excessive intermetallic phases that acted as the cathode during the micro-galvanic corrosion [23–26]. Compared with sintered alloy, extruded alloy with more intermetallic phases showed more negative Ecorr . While the Jcorr (300.3 μA cm−2) of extruded alloy was lower as the finer grain could create more grain boundaries which acted as corrosion barriers thus enhancing corrosion resistance [27, 28]. The Ecorr of solution-treated alloy was more positive than those of sintered alloy and extruded alloy, but the Jcorr was much lower. It was was attributed to the significant reduction of the intermetallic phases. However, rolled alloy showed more negative Ecorr (−1.521 V) and higher Jcorr (84.0 μA cm−2) than the solution-treated alloy. This indicated that the mixed microstructure in solution-treated alloy could enhance the corrosion resistance. The Jcorr of annealed alloy increased sharply since the annealing treatment obviously increased the grain size which decreased the corrosion resistance of grain.
Figure 7. Polarization curves (a) and hydrogen evolution (b) of Mg-Al-Pb alloys.
Download figure:
Standard image High-resolution imageTable 3. Parameters of Mg-Al-Pb alloys obtained from polarization curves.
| Ecorr /V(versus SCE) | Jcorr /(μA cm−2) | |
|---|---|---|
| Sintered alloy | −1.552 | 773.3 |
| Extruded alloy | −1.561 | 300.3 |
| Solution-treated alloy | −1.496 | 75.9 |
| Rolled alloy | −1.521 | 84.0 |
| Annealed alloy | −1.514 | 674.7 |
The hydrogen evolution corrosion test can reveal the anode efficiency of different anodes [29]. In figure 7(b), hydrogen evolution rate after 10 h of immersion in 3.5 wt% NaCl solution increased in the order of solution-treated alloy < rolled alloy < extruded alloy < annealed alloy < sintered alloy, which is consistent with the order of Jcorr of potentiodynamic polarization test. This indicated that the anode efficiency of solution-treated alloy is the highest.
3.3. Galvanostatic discharge test of Mg-Al-Pb alloys
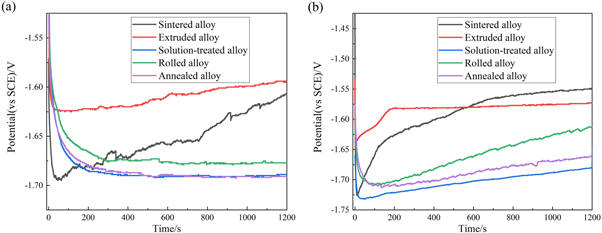
Figure 8 shows the galvanostatic discharge curves of the Mg-Al-Pb alloys at the current densities of 4.5 mA cm−2 and 45 mA cm−2. The average discharge potentials of different anodes are shown in table 4. The activation times (time used for discharge potential to reach the most negative) of the sintered and the extruded Mg-Al-Pb alloys are shorter than those of the solution-treated, rolled and annealed Mg-Al-Pb alloys. This could be explained by the improved discharge activity by joint effect of the Mg17 Al12 and Mg2 Pb phases, which is consistent with the results of the potentiodynamic polarization test. However, the discharge potential suddenly shifted to more positive direction at much higher rates, which caused the average discharge potentials of these two Mg-Al-Pb alloys to be more positive than those of the solution-treated, rolled and annealed Mg-Al-Pb alloys. The average discharge potential of extruded alloy was more positive than that of sintered alloy (table 4), indicating that extrusion decreased the discharge property.
Figure 8. Galvanostatic potential-time curves of Mg-Al-Pb alloys at (a) 4.5 mA cm−2 and (b) 45 mA cm−2 for 1200 s.
Download figure:
Standard image High-resolution imageTable 4. Average discharge potentials of different anodes obtained from the potential-time curves at current densities of 4.5 mA cm−2 and 45 mA cm−2.
| Average discharge potential (V versus SCE) | ||
|---|---|---|
| Magnesium anode | 4. 5mA cm−2 | 45mA cm−2 |
| Sintered alloy | −1.653 | −1.590 |
| Extruded alloy | −1.610 | −1.583 |
| Solution-treated alloy | −1.684 | −1.704 |
| Rolled alloy | −1.670 | −1.661 |
| Annealed alloy | −1.684 | −1.687 |
At low current density (figure 8(a)), no obvious difference could be found in the curves of the solution treated the as-rolled and the annealed alloys, since most of intermetallic phases in those alloys had dissolved. As seen from figure 8(b), the discharge curve of Mg-Al-Pb alloy is more stable after solution treatment. Previous discussions indicate that solution-treated alloy contains more second phases than rolled alloy and annealed alloy. Thus, the activation time of the solution-treated alloy is relatively shorter than that of the rolled alloy and annealed alloy at a current density of 45 mA cm−2. According to table 4, the average discharge potential of solution-treated Mg-Al-Pb alloys is the most negative at both low and high current densities, showing the highest discharge properties. This means that the mixed microstructure in solution-treated alloy could further increase the discharge potential.
To further understand the evolution of the microstructure of the studied Mg-Al-Pb alloys during discharge and explain the difference in the electrochemical properties of Mg-Al-Pb alloys, figure 9 characterizes the corrosion morphologies after discharging at 45 mA cm−2 for 10 s. Many bubble-shape corrosion products can be found in figures 9(a) and (b). The distribution of bubble corrosion products is very close to that of the intermetallic phases. Thus, the bubble corrosion products were from the microbattery. The process of chemical reaction is shown as follows:



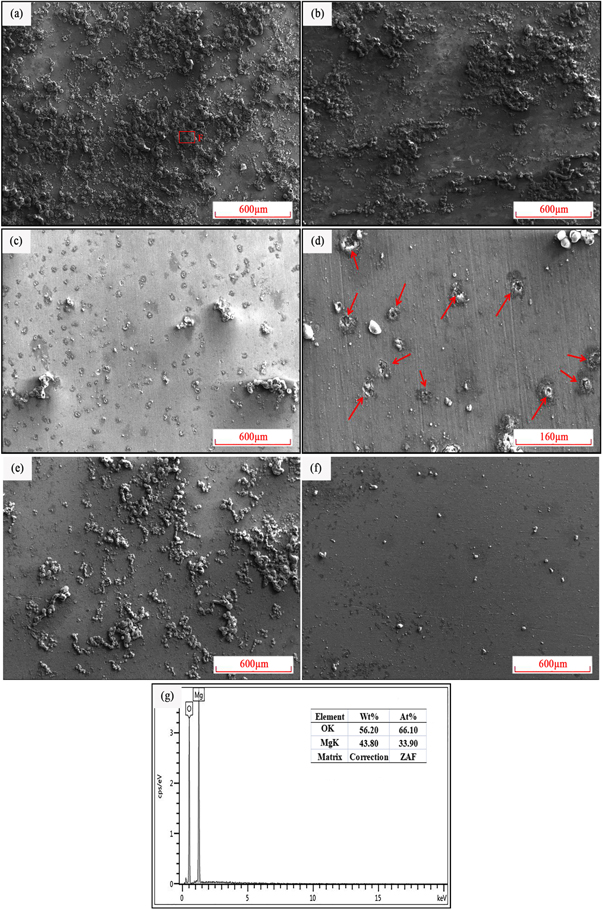
Figure 9. Corrosion morphologies of different states after discharging at 45 mA cm−2 for 10 s:(a) sintered alloy, (b) extruded alloy, (c) and (d) solution-treated alloy, (e) rolled alloy, (f) annealed alloy, (g) EDS result of area F.
Download figure:
Standard image High-resolution imageAs shown in the EDS result (figure 9(g)), the atomic ratio of Mg:O is 1:2. Combining equation (3), it is obvious that the bubble products are Mg(OH)2 . This indicates that coarse Mg17 Al12 phases and the gathered Mg2 Pb phases will result in preferential corrosion of the surrounding Mg matrix and corrosion products will accumulate on those phases. The accumulation of Mg(OH)2 impedes the subsequent discharge behavior and rapidly shifts the discharge potential toward the positive direction. Grain refinement in extruded alloy slowed down the accumulation of corrosion products. Therefore, the amount of Mg(OH)2 corrosion products in the extruded alloy (figure 9(b)) is less than that in the sintered alloy (figure 9(a)). Fewer corrosion products also mean fewer obstacles to the discharge behavior, which made the discharge potential of the extruded alloy shift to the positive direction at a lower speed than that of the sintered alloy (figure 8).
In figure 9(c), the amount of Mg(OH)2 further decreases, and some black dots can be found in the solution-treated alloy. Figure 9(d) gives the enlarged images of the red box area in figure 9(c),the black dots (marked by red arrows) in this figure can be identified as corrosion holes. These corrosion holes are from the homogeneously distributed Mg2 Pb phases. The homogeneously distributed Mg2 Pb phases will fall off with the dissolution of Mg to form the corrosion holes in figure 9(d). As mentioned earlier, the gathered Mg2 Pb phases will accelerate the discharge corrosion of surrounding Mg matrix and then accumulate corrosion products to impede the discharge. The homogeneously distributed Mg2 Pb phases can also promote the discharge corrosion of surrounding Mg matrix, while these fine Mg2 Pb phases will fall off before the accumulation of Mg(OH)2 . With the dissolution of Mg matrix and Mg2 Pb phases shedding, other fine Mg2 Pb phases and surrounding Mg matrix will contact the electrolyte and participate in discharge, which indicates that those homogeneously distributed Mg2 Pb phases could continuously promote the corrosion of Mg matrix thus making discharge curve more stable. As shown in figure 9(e), the amount of bubble Mg(OH)2 increases in the rolled alloy. These newly increased corrosion products are mainly from the dislocations that are introduced by the rolling procedure. These dislocations act as nucleation sites to create Mg(OH)2 corrosion products and reduce the active electrode area [30]. Figure 9(f) proved that annealing could eliminate dislocations, which is consistent with the optical micrographs.
In conclusion, the discharge curve of the solution-treated alloy is the most stable and the discharge potential is the most negative among all states of Mg-Al-Pb alloys due to the joint effect of the mixed microstructure and homogeneously distributed Mg2 Pb phases. Thus, the solution-treated alloy exhibits the best comprehensive discharge properties at the galvanostatic discharge of 1200 s.
3.4. Electrochemical impedance spectra (EIS) of Mg-Al-Pb alloys
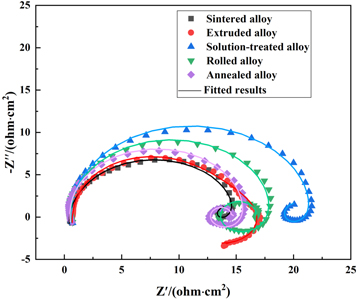
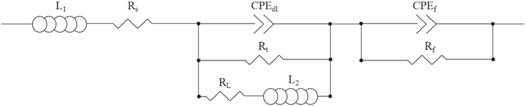
The electrochemical impedance spectra of the Mg-Al-Pb alloys are shown in figure 10. The Nyquist plots of all Mg-Al-Pb alloys were obtained after soaking in 3.5% NaCl electrolyte for 20 min to obtain a stable open circuit potential. Figure 11 exhibits the equivalent circuit of the Mg-Al-Pb alloys. L1 is related to hydrogen evolution corrosion [31]. Rs is the solution resistance. Rt represents the charge transfer resistance of the Mg-Al-Pb alloys, and CPEdl is the double-layer capacitance of the alloy surface [31, 32]. RL and L2 are the parameters related to localized corrosion [33]. Rf and CPEf represent the constant phase angle element and the resistance of corrosion products, respectively [34].
Figure 10. Electrochemical impedance spectra (EIS) in Nyquist plots of Mg-Al-Pb alloys prepared by powder metallurgy.
Download figure:
Standard image High-resolution imageFigure 11. Equivalent circuit of Mg-Al-Pb alloys fabricated by powder metallurgy.
Download figure:
Standard image High-resolution imageThe equivalent parameters of the studied Mg-Al-Pb alloys under different states are listed in table 5. The Rt of Mg-Al-Pb alloys is arranged as follows: solution treated (21.1 Ω cm2) > rolled (17.6 Ω cm2) > annealed (15.1 Ω cm2) > sintered (14.7 Ω cm2) > extruded (9.9 Ω cm2). The Rt of Mg-Al-Pb alloys was arranged as the same as their Ecorr (table 3). Homogeneously distributed Mg2 Pb phases improved the uniform corrosion of the solution-treated alloy, which made the RL of the solution-treated alloy (158.5 Ω cm2) the highest in Mg-Al-Pb alloys. A smaller Rf means a lower resistance of the corrosion product film, the annealed alloy without Mg17Al12 phases and dislocations has the lowest Rf (1.8 Ω cm2).
Table 5. Equivalent parameters of the studied Mg-Al-Pb alloys under different states.
| Magnesium anode | Sintered | Extruded | Solution-treated | Rolled | Annealed |
|---|---|---|---|---|---|
| L1 (×10−6H cm2) | 1.36 | 1.04 | 1.32 | 1.19 | 1.17 |
| Rs (Ω cm2) | 0.412 | 0.670 | 0.551 | 0.545 | 0.505 |
| CPEdl (×10−5Ω−1 cm−2 s) | 3.25 | 13.91 | 0.96 | 0.78 | 0.68 |
| n1 | 0.94 | 0.94 | 0.96 | 0.93 | 0.97 |
| Rt (Ω cm2) | 14.7 | 9.9 | 21.1 | 17.6 | 15.1 |
| RL (Ω cm2) | 57.2 | 4.2 | 158.5 | 48.3 | 61.0 |
| L2 (H cm2) | 0.34 | 5.44 | 1.07 | 0.25 | 0.44 |
| CPEf (Ω−1 cm−2 s) | 0.018 | 0.0045 | 0.012 | 0.012 | 0.012 |
| n2 | 0.80 | 0.74 | 0.95 | 0.98 | 0.92 |
| Rf (Ω cm2) | 4.6 | 7.2 | 2.5 | 3.2 | 1.8 |
| x2 | 2.74 × 10−3 | 2.91 × 10−3 | 1.37 × 10−3 | 1.44 × 10−3 | 1.75 × 10−3 |
3.5. Mg/PbCl2 battery test
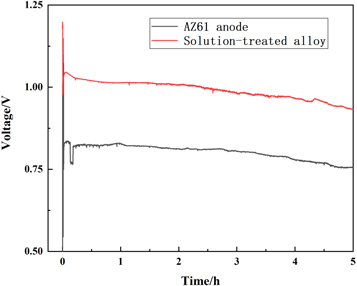
The above electrochemical tests show that solution-treated alloy has the best comprehensive discharge properties among Mg-Al-Pb alloy that were prepared by powder metallurgy. To further comprehend the discharge behavior of solution-treated Mg-Al-Pb alloy compared with other commercial magnesium anode materials, Mg/PbCl2 battery tests were performed at the current density of 45 mA cm−2 (2 A of discharge current). Figure 12 shows the Voltage-Time curves of the Mg/PbCl2 battery using the solution-treated alloy and a commercial AZ61 sheet as anodes.
Figure 12. Voltage-Time curves of Mg/PbCl2 battery at the current density of 45 mA cm−2.
Download figure:
Standard image High-resolution imageThe battery based on the solution-treated alloy anode shows higher voltage and its curve is as stable as that of the battery based on commercial AZ61 anode. This indicates that solution-treated alloy shows much better anode properties than the commercial AZ61 sheet. Thus, the solution-treated Mg-Al-Pb alloy has the potential to serve as an excellent anode in seawater-activated batteries.
4. Conclusions
In this work, Mg-Al-Pb alloys were prepared by the powder metallurgy method. The electrochemical properties of the studied alloys vary under different thermal-mechanical treatments. The evolution of the microstructure and electrochemical performance of the Mg-Al-Pb alloys under different states are studied and the main results are summarized as follows:
Coarse Mg17 Al12 phases (with size ranges from 20 μm to 70 μm) and fine Mg2 Pb phases (with the size of 100 nm) formed in the mixed Al, Pb and Mg powder during the hot press sintering procedure. Mg2 Pb phases have a strong diffusibility to diffuse into Mg17 Al12 phases. Extruding procedure promotes the precipitation of Mg17 Al12 phases and Mg2 Pb phases and the diffusion of Mg2 Pb phases into Mg17 Al12 phases. Solution treatment reduces the amount of intermetallic phase and produced a mixed microstructure which is Mg17 Al12 phase containing Mg2 Pb phases inside. And the remaining Mg2 Pb phases were homogeneously distributed in the Mg matrix. The rolling procedure dissolved the remaining internetallic phases and generated some micro cracks. Annealing treatment sharply increased the grain size.
Mg17 Al12 phases and Mg2 Pb phases promote localized corrosion of the Mg matrix to give Mg-Al-Pb alloys higher discharge activity. However, Mg17 Al12 phases and gathered Mg2 Pb phases also result in the accumulation of Mg(OH)2 corrosion products, which impede the subsequent discharge behavior. The homogeneously distributed Mg2 Pb phases will fall off with dissolution of the Mg matrix, which will not impede the discharge behavior and made the discharge curve more stable. The mixed mcrostructure could make the discharge potential more negative and increase the corrosion resistance. Under the combined action of the mixed microstructure and homogeneously distributed Mg2 Pb phases, solution-treated alloy exhibit the best comprehensive discharge properties. The Mg/PbCl2 battery test indicates that solution-treated alloy shows much higher anode properties than the commercial AZ61 sheet. This means that the solution-treated alloy could serve as an excellent anode in seawater-activated batteries.
Acknowledgments
The authors acknowledge the Graduate Scientific Research Innovation Project of Central South University (2021XQLHO08); R & D of Key Technology of Light Metal Air Battery, Transformation and Industrialization of Scientific and Technological Achievements of Hunan Province (2020GK2071); R & D of Key Technology and Materials of Magnesium Air Battery, Transformation of Scientific and Technological Achievements of Chang Sha City (Kh2005186); Technical Area Fund of Foundation Strengthening (2021-JCJQ-JJ-0432).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).