Abstract
Blends of PLLA and PCL yielded by solvent casting usually exhibit phase separation and crystallization behavior which have a strong impact on their suitability for certain biomedical applications such as degradable coatings or drug carriers. Therefore, it is important to understand the underlying mechanisms. In this study, high-molecular biodegradable semi-crystalline poly(L-lactide) (PLLA) (Mw = 320 kDa) was blended with low-molecular biodegradable semi-crystalline poly(ε-caprolactone) (PCL) (Mw = 40 kDa) in various combinations (10, 50 and 90 wt.% PCL) by solvent casting. The yielded blends were subjected to annealing at 40 °C, 80 °C and 200 °C and cooled down slowly to maintain thermodynamic equilibrium. Scanning electron microscopy, atomic force microscopy, Raman images and differential scanning calorimetry were used to investigate the structure, morphology and thermal properties of the solvent cast PLLA/PCL blends. It was shown that the physico-chemical properties of PLLA/PCL blends prepared by solvent casting differ substantially compared to those accessed by melt manufacturing processes. In summary, the blends showed a complex phase separation behavior, which is completely dependent on the method of preparation and the adjusted temperature during production process.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
PLLA and PCL are both widespread and highly relevant materials in biomedical engineering, either individually as well as in combination as blends. In fact, however, blend formation leads to additional matrix effects which alter the material properties such as mechanical resilience, drug release behavior or degradation rate. This impact not only depends on the polymer combination to form a blend but furthermore on the underlying manufacturing process. Polymer based materials can be processed thermally, e.g. by extrusion or melt electro spinning. However, for medical applications, the focus recently shifted to solvent cast processes such as dip coating, airbrush and spin coating. Contrary to thermal processing, the solvent cast processes require additional post-treatment of the materials, which can be either washing or annealing or both, to eliminate solvent residues [1, 2]. However, solvent cast processes are usually more versatile and easy to handle compared to thermal extrusion and allow for a wider variety of substrates or temperature susceptible additives such as drug molecules since no adverse effects from high temperature treatment are involved. Each step of the process influences the physico-chemical properties, which also means that the final characteristic properties of the desired material can already be specifically controlled during production, e.g. by annealing protocol, choice of solvent and washing procedure [3–5].
Solvent cast processes allow for the generation of polymer blends which may exhibit improved adaptive properties for certain fields of application. For example, elastic components can be combined with stiffer ones by blending to obtain ductile yet resilient materials [6, 7]. In this background, the preferred and main biodegradable polymers used for blend generation are aliphatic polyesters, such as poly(L-lactide) (PLLA) and poly(ε-caprolactone) (PCL). It is well known that PLLA exhibits a relatively brittle fracture, because glass transition temperature is above room temperature [8]. On the other hand, PCL, a ductile biodegradable thermoplastic polymer with a much lower glass transition temperature, has been shown to be a promising blend partner for PLLA to obtain a more ductile material. López-Rodriguez et al observed that PLLA and PCL are immiscible, but that a certain adhesion at the PLLA/PCL interface can be achieved if the main phase is PCL [6, 9]. Todo et al also demonstrated that even a PCL of 5% can significantly change the mechanical properties [10]. Both research groups used PLLA with a lower molecular weight (Mw < 200 kDa) and prepared blends by melt extrusion. Studies with high-molecular PLLA blended with low-molecular PCL are not further known in the literature.
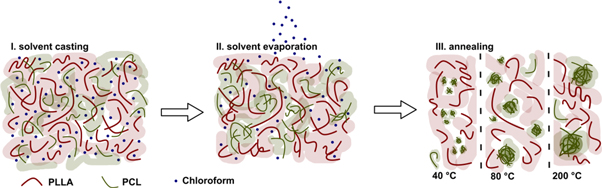
Broz et al concluded that the immiscibility of PLLA and PCL causes phase separation in PLLA/PCL blend, see figure 1, leading to a suppression of an improved toughness, especially with increasing PCL content.
Figure 1. Schematic view of the phase separation of PLLA and PCL blends, prepared by solvent cast processes.
Download figure:
Standard image High-resolution imageNotably, these effects significantly vary depending on the production protocol in a way that melt processes lead to different equilibration states on a molecular level compared to solvent cast processes making blend manufacturing a complex matter. Nevertheless, blending can radically alter the resultant properties, which depend sensitively on the mechanical properties of the components as well as the blend microstructure and the interface between the phases [9].
Flory Huggins model is an important theory regarding interactions in polymer solutions and miscibility of polymer blends [11–14]. This lattice model describes the thermodynamics of polymer solutions and polymer blends. For our PLLA/PCL blend system, low miscibility leads to a complex situation with various phases and mixed crystals, even more when solvents are taken into account [15, 16]. Therefore, theoretical considerations such as the determination of parameters such as χ are not trivial. Pillin et al determined the interaction parameter via thermal analysis using several approximations, revealing that miscibility also yields some changes in crystallization and melting temperatures with the decrease of the integrated enthalpies [13]. Therefore, we used thermal analysis via DSC measurements to describe PLLA/PCL blends. A more basic question concerns the phase morphology and the nature of the interphase. Uncontrolled morphology and a weak interphase lead to poor mechanical properties and therefore to ineligibility [6]. Aim of our work was the investigation of PLLA/PCL blends in respect to the processibility of these polymers and their suitability for biomedical applications such as implant coatings or drug delivery systems.
Another effect that influences the mechanical properties of PLLA/PCL was reported by Takayama et al. They observed that annealing of the blends is linked to an increase in crystallinity, which effectively improves the strength of the blends [1].
A stable and controlled application of PLLA/PCL blends requires detailed knowledge of their phase separation behavior and morphology. A deeper understanding of the thermal and mechanical properties of the combined polymers is required. In this work, we investigated the properties of solvent cast films in comparison to melting cast films. However, to eliminate solvent effects during annealing, washing processes were performed to completely remove the solvent from the blends. This was established in in-house studies and resulted in residual solvent contents below the ICH limits for medical devices [4, 5]. Afterwards, the blends were thermally treated to investigate the influence of temperature on the physicochemical properties of the blends previously produced from solvents.
Therefore, we have investigated high-molecular biodegradable semi-crystalline PLLA (Mw = 320 kDa), low-molecular PCL (Mw = 40 kDa) and their blends (10, 50 and 90 wt.% PCL contents) prepared by solvent casting. The samples were subjected to annealing at 40 °C, 80 °C and 200 °C, respectively. Both polymers have been widely used in a variety of medical applications and, to our knowledge, there has not been a systematic investigation of the morphology and thermal properties after a different annealing process of high-molecular PLLA combined with low-molecular PCL as solvent cast blends. Scanning electron microscopy (SEM), atomic force microscopy (AFM), Raman images and differential scanning calorimetry (DSC) were used to investigate the structure, morphology and thermal properties of the solvent cast PLLA/PCL blends.
Materials and methods
Materials
Poly(L-lactide) (PLLA, intrinsic viscosity in chloroform: 3.8 dl g−1, Mw = 320 kDa) was purchased from Evonik Industries AG (Darmstadt, Germany). Poly-ε-caprolactone (PCL, intrinsic viscosity in chloroform: 0.8 dl g−1, Mw = 40 kDa) was supplied by LACTEL Absorbable Polymers (Birmingham, AL, USA). Chloroform was purchased from Baker (Fisher Scientific GmbH, Austria, Vienna). All chemicals were used as received without further purification.
Blend preparation
For cast film production, polymer solutions (1 g Polymer in 25 ml chloroform) of PLLA, PCL and their blends in different ratios (90/10, 50/50, 10/90 w/w) were poured into glass petri dishes (Ø = 9 cm). After evaporation of the solvent under ambient conditions in a fume hood, films were washed for 2 days in methanol (LiChrosolv, Merck KGaA) with daily exchange, two days in distilled water (MilliQ Ultrapure, R = 18.2 MΩ.cm at 25 °C) with daily exchange, and dried for 7 days in a vacuum oven (Memmert GmbH + Co.KG) at 40 °C and 40 mbar. With this preparation method film samples with a thickness of ∼100 μm were achieved. For annealing experiments, samples of PLLA, PCL and their blends were placed in a chamber furnace and kept at different temperatures (40 °C, 80 °C and 200 °C) for 60 min at ambient atmosphere (KLS 05/13 Thermconcept). An annealing temperature of 40 °C was selected that corresponds to a temperature lower than the melting temperature (Tm) of PCL and PLLA. 80 °C were used due to the temperature lower than Tm of PLLA but higher than Tm of PCL. Furthermore 200 °C was selected, since this temperature exceeded the melting temperature for both polymers. Subsequently, all samples were cooled down with 1 K min−1 in the furnace, see figure 2. The PLLA/PCL blend samples were kept in the laboratory refrigerator at 8 °C until the investigations were completed.
Figure 2. Temperature time profile for sample preparation; PLLA, PCL and their blends were heated up with q = 10 K min−1 to different temperatures (black line 40 °C, blue line 80 °C and red line 200 °C) and hold isothermally for 60 min. Then, all samples were cooled down with a cooling rate of q = 1 K min−1 to crystallize the samples.
Download figure:
Standard image High-resolution imageMorphology analysis
SEM
Morphology of the polymer films was examined with scanning electron microscopy SEM QUANTA FEG 250 (FEI Company, Germany) with an accelerating voltage of 10 kV and a proprietary spot size value of 3.0. The samples were fixed onto aluminum trays with conductive tape and sputter coated with gold. The images were taken at magnification x500.
AFM
Atomic force microscopy was performed with a NanoWizard II from JPK Instruments AG (Berlin, Germany). Commercial Si cantilevers (CSC37) were applied for the contact mode experiments. Height and deflection images with a resolution of 512 × 512 pixels in a 100 μm × 100 μm area were simultaneously observed with a scan rate of 0.6 Hz under ambient conditions (IGain: 150 Hz, PGain: 0.0048, Setpoint: 0.6 V). Images were taken at five different measuring points for each sample. In each image the heights were determined by Peak to valley roughness (Rt) at five different positions.
Raman imaging
Test samples were investigated with regard to polymer phase separation at the surface by means of Raman imaging. Therefore, a WITec alpha 300 confocal Raman microscope from WITec GmbH (Ulm, Germany) with 10× magnification and an input laser wavelength of 532 nm was used (excitation energy = 17.6 mW). For Raman area scans, a 500 μm × 500 μm field with 50 dots per row and column was used. Signals that are specific for the distinct polymers (PLLA: 878 rel. cm−1, PCL: 1727 rel. cm−1) were chosen for visualizing phase separation phenomena. According to the manufacturer's specification the typical resolution of the Raman system is 300 nm in xy direction and <1000 μm in z direction.
Mechanical characterization
Uniaxial tensile testing was performed with a Zwicki ZN 2.5 (Zwick, Ulm, Germany). Tests were conducted with a 50 N load cell and a crosshead speed of 25 mm min−1. During the procedure samples were kept at room temperature. Dumbbell-shaped tensile test specimens with effective dimensions of 12 mm × 2 mm × 0.1 mm were prepared with a blanking tool according to DIN EN ISO 527-2 1BB standards [17]. An image with the detailed dumbbell-shaped size is given in the supplementary data available online at stacks.iop.org/MRX/7/095302/mmedia. The tensile force as a function of elongation was measured. Based on these data the elastic modulus (E) was calculated in the linear elastic region. Furthermore, the tensile strength (σmax) and elongation at break (εB) were determined. All results were averaged over n = 6 samples.
Thermal analysis
The thermal behavior of the polymer films was investigated in a DSC 1 system (Mettler Toledo, Schwerzenbach, Switzerland) using the conventional calibration methods with highly pure standards. The specimens were heated at a rate of 10 K min−1 operating under nitrogen at atmospheric pressure. The samples were heated from −90 °C up to 200 °C. The sample weights were in the range of 3–6 mg. Samples were analyzed with respect to glass transition (Tg), melting temperature (Tm), and degree of crystallinity (χ). The Tg were determined by the midpoint of the inflection of the measured heating curve. The heats of fusion ΔH and crystallization χC were quantitatively evaluated by means of equation (1)
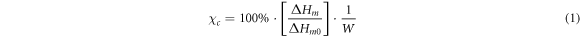
where ΔHm0 is the enthalpy value of a pure crystalline material, ΔHm is the enthalpy corresponding to the fusion process and W the amount of each homopolymer in the blend. The reference value taken for ΔHm0 of PLLA was χ100 = 93.7 J g−1 [18] and for PCL χ100 = 135.44 J g−1 [19]. All results were averaged over n = 6 samples.
Results and discussion
Influence of annealing temperature on the morphology measured by SEM
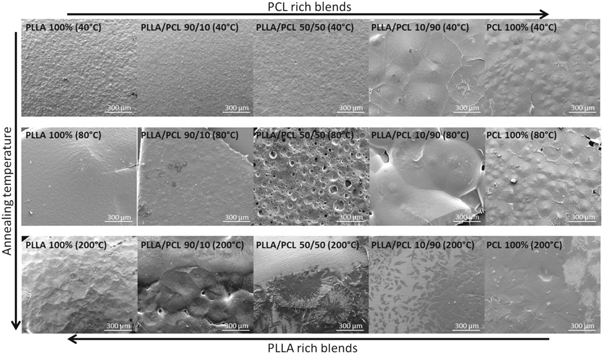
Figure 3 shows SEM documentation of the polymer morphology with increasing PCL content after 40 °C, 80 °C and 200 °C annealing temperature. Pure PLLA appears to have one uniform phase with spherulite crystals of about 20 μm at an annealing temperature of T = 40 °C (PLLAT40) and T = 80 °C (PLLAT80). In comparison to this, the radius of such spherulites of PLLA annealed at T = 200 °C (PLLAT200) increased dramatically to about 100 μm. These results are comparable with the literature, which show that PLLA spherulites increase with increasing temperature [7, 20, 21]. The smaller crystal size of PLLA film obtained from solution compared to PLLA film from melt may be explained by the surrounding solvent and evaporation processes during thermal annealing. PLLA/PCL 90/10 blends show significant increase in crystal size with increasing annealing temperature. However, two separated phases could not be clearly observed.
Figure 3. SEM images of various PLLA, PCL and their blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures (40 °C, 80 °C and 200 °C).
Download figure:
Standard image High-resolution imageInterestingly, the surface morphology of PLLA/PCL 50/50 differs with different annealing conditions, see figure 3. From an initial smooth surface at T = 40 °C (PLLA/PCL 50/50T40), the surface at T = 80 °C (PLLA/PCL 50/50T80) changes to a perforated structure, which develops into two phases at T = 200 °C (PLLA/PCL 50/50T200), a smooth and a spherulite-seeded structure. It is known from literature that a mixing gap of PLLA/PCL occurs from 80 °C and higher. When reaching mixing gap, PLLA spherulites are formed and small PCL crystals are deposited on the surface of the spherulite, thereby enhancing PLLA crystal growth [9]. This can also be found considering the solubility parameters of the two polymers which show a difference of approximately 0.9. Coleman et al describe a relatively weak favorable intermolecular interaction, if the difference between the solubility parameters of these polymer pairs is Δδ = 1.0 (cal cm−3)0.5, respectively [16]. They conclude in their work, that for high molecular polymers Δδ should be much smaller than 1 to ensure that the components are miscible. The fact that the value of PLLA and PCL are δPLLA = 10.1 (cal cm−3)0.5 and δPCL = 9.2 (cal cm−3)0.5 and therefore Δδ = 0.9 suggests that the blend has only weak intermolecular interactions.
PLLA/PCL 10/90 shows large spherulites of about 300 μm at an annealing temperature of T = 40 °C (PLLA/PCL 10/90T40) and T = 80 °C (PLLA/PCL 10/90T80). The crystal shape of PLLA/PCL 10/90 at T = 200 °C (PLLA/PCL 10/90T200) changes from circular to oval and the diameter decreased dramatically to about 50 μm.
Pure PCL exhibits remarkably smaller spherulites compared to PLLA/PCL 10/90 with diameters of about 200 μm at annealing temperatures of T = 40 °C (PCLT40) and T = 80 °C (PCLT80). The radii of spherulites of PCL at T = 200 °C (PCLT200) increased dramatically to about 300 μm and the crystal height seems to regrade to a smooth surface.
Influence of annealing temperature on the morphology measured by AFM
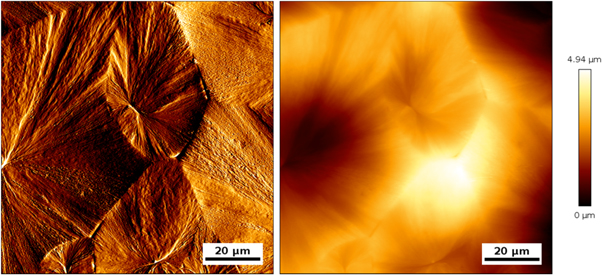
Figure 4 shows a typical error, used for topography interpretation and height image of PLLAT200 (image size 100 μm × 100 μm), which are used for the roughness evaluation Rt, see table 1. PLLA that was annealed at 200 °C forms spherulites with different sizes and heights.
Figure 4. Representative AFM images of 100 × 100 μm2 area. Contact mode error signal (left) and topography image (right) of PLLA, annealed at T = 200 °C for 60 min are shown.
Download figure:
Standard image High-resolution imageTable 1. Mechanical properties (elastic modulus (E), tensile strength (σmax), elongation at break (εB)), determined from uniaxial tensile tests, roughness (Rt), determined from AFM images and spherulite sizes, obtained from all morphology methods, of PLLA/PCL blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures. Due to low melting temperature PCL could not be observed (n.a. = not available).
| T = 40 °C | |||||
|---|---|---|---|---|---|
| Composition (PLLA/PCL) | E (MPa) | σmax (MPa) | εB (%) | Rt (μm) | ØS (μm) |
| 100/0 | 2090 ± 80 | 75.9 ± 1.3 | 60 ± 18 | 2.68 ± 0.14 | 10–20 |
| 90/10 | 998 ± 17 | 38.1 ± 1.8 | 130 ± 18 | 3.40 ± 0.11 | n.a. |
| 50/50 | 696 ± 15 | 24.5 ± 0.3 | 14.9 ± 2.9 | 4.0 ± 0.6 | 50 |
| 10/90 | 419 ± 9 | 19.8 ± 0.9 | 12.4 ± 0.9 | 5.7 ± 0.5 | 200–400 |
| 0/100 | 302 ± 16 | 15.2 ± 0.4 | 14.3 ± 1.7 | 5.2 ± 0.4 | 50–200 |
| T = 80 °C | |||||
| Composition (PLLA/PCL) | E (MPa) | σmax (MPa) | εB (%) | Rt (μm) | ØS (μm) |
| 100/0 | 2015 ± 22 | 75.5 ± 1.9 | 18 ± 10 | 2.35 ± 0.15 | 10–30 |
| 90/10 | 943 ± 19 | 34.3 ± 1.4 | 79 ± 30 | 4.54 ± 0.21 | n.a. |
| 50/50 | 850 ± 23 | 28.05 ± 0.02 | 7.86 ± 0.11 | 16.2 ± 0.9 | 50–100 |
| 10/90 | 230 ± 27 | 8.6 ± 1.0 | 10.2 ± 2.6 | 5.7 ± 1.4 | 300–600 |
| 0/100 | n.a. | n.a. | n.a. | 5.12 ± 0.12 | 100–200 |
| T = 200 °C | |||||
| Composition (PLLA/PCL) | E (MPa) | σmax (MPa) | εB (%) | Rt (μm) | ØS (μm) |
| 100/0 | 2250 ± 70 | 73.1 ± 2.9 | 3.90 ± 0.08 | 4.2 ± 0.8 | 100–200 |
| 90/10 | 810 ± 110 | 27.5 ± 0.8 | 9.7 ± 1.2 | 5.3 ± 1.2 | 200–400 |
| 50/50 | 861 ± 12 | 29.2 ± 0.9 | 5.58 ± 0.23 | 7.9 ± 0.5 | 300–500 |
| 10/90 | 160 ± 27 | 4.2 ± 1.2 | 24 ± 6 | 5.7 ± 1.0 | 50–500 |
| 0/100 | n.a. | n.a. | n.a. | 3.5 ± 0.4 | 300–500 |
Figure 5 shows error signal images of the tested polymer samples with a picture size of 100 μm × 100 μm. The top row represents the polymers and their blends at an annealing temperature of T = 40 °C. In the middle, the samples are shown at T = 80 °C and below at T = 200 °C. Pure PLLA, annealed at 40 °C, crystallizes into small conical spherulites between 10 and 30 μm in size. The size of the crystals increases drastically at T = 200 °C. Based on the height profiles, an increase from 2.68 ± 0.14 μm to 4.2 ± 0.8 μm is determined. PLLA/PCL 90/10, annealed at 40 °C, exhibits a smoother substructure, which is pervaded with approximately 5 μm pores and suggestively of small PCL inclusions. Chloroform diffuses rapidly through the PCL, leading to higher chloroform concentrations in PCL areas, which leads to pore formation during evaporation. The pore size increases at an annealing temperature of 200 °C. Dell'Erba et al discovered that low interfacial tensions are obtained in binary PLLA/PCL blends due to the similar chemical nature of the blends components, which allows interpolymer polar interactions across phase boundaries [22]. This can be also observed at PLLA/PCL 50/50. In PLLA/PCL 50/50, annealed at 40 °C, lamellar structures in circular arrangements of about 60 μm in size are embedded in a smooth surface. In addition, approximately pores with a diameter of 5 μm can be observed. With PLLA/PCL 50/50T80, the surface becomes very porous exhibiting pore diameters up to 50 μm. The height difference increases drastically to Rt = 16.2 ± 0.9 μm at T = 80 °C. Similar SEM images and pore sizes have been observed after solvent etching of PLLA/PCL blends [23]. This may be due to the melting of PCL in contrast to PLLA at the thermal processing step at 80 °C due to its lower melting temperature. Hence, PCL is liquefied, similar to the etching process where PCL is solved. As a result of this liquefying process a highly porous structure mainly built by PLLA would be expected. Such pores are not observed at T = 40 °C, where PCL remains solid and at T = 200 °C, where both polymers are melted, which results in a smooth surface without pores. For the PLLA/PCL 10/90T80 also a smooth surface is expected, due to the low amount of PLLA, witch rather forms particles than a connected structure. At T = 200 °C height difference drops down to 7.9 ± 0.5 μm. Clearly, two types of structures are visible: First, a smooth surface combined with a lamellar structure which corresponds to PCL crystals was observed in accordance to [22]. However, PLLA/PCL 10/90 exhibits a rough surface that can be interpreted as partial segments of spherulites. In part, the crystal boundaries are still visible. The height values (seen in table 1) show the same results of approx. 5.7 μm at all annealing temperatures. A similar picture is visible with pure PCL. When applying an annealing temperature of 200 °C, the structure changed. Plate-shaped structures were formed on a generally rough base structure. The AFM results obtained are consistent with the SEM images.
Figure 5. AFM contact mode error signal images of various PLLA, PCL and their blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures (40 °C, 80 °C and 200 °C).
Download figure:
Standard image High-resolution imageInfluence of annealing temperature on the morphology measured using Raman images
Confocal Raman imaging was used in order to detect the phase separation in the different polymeric blends. In figure 6 the Raman images received by mapping the PLLA/PCL samples and their blends are shown. The corresponding Raman spectra are given in the supplementary data. In particular, the yellow area is related to the intensity of PLLA signal from the band centered at 878 rel. cm−1. The red area is related to the intensity of the PCL signal, centered in the band at 1727 rel. cm−1. The dark regions of the Raman images correspond to regions out of focus plane. From this analysis it becomes clear that phase separated morphology exists for all the three different blends, and it is possible to detect both, the PLLA-rich phase and the PCL-rich phase. For PLLA/PCL 90/10 it can be supposed that PLLA forms a continuous phase with dispersed spheres of PCL with different sizes (red regions of figure 6). Most interestingly, for PLLA/PCL 50/50 T40 and PLLA/PCL 50/50T80, two well differentiated areas of each homopolymer are detected, indicating the existence of large domains of each homopolymer. It is known from the literature that if polymer components are being phase separated, the interface width (w) can be related to the Flory-Huggins parameters (χ) by a general relation in the form w ∼ (χ−χcrit)−0.5 [14]. That means for our PLLA/PCL system a narrow interfacial width indicates relatively low interface interactions, which is consistent with the reported results of incompatibility between PCL and PLA [24]. López et al noted that in phase-separated PLA/PCL blends sharp interfaces are existing in the microstructure, whereas Broz et al found poor adhesion at the phase boundary interface and confirmed immiscibility and phase separation through NMR [6, 9]. It was hypothesized that to improve the mechanical properties of the blend, the samples should be annealed in the single-phase region of the LCST phase-diagram to enhance interfacial adhesion. It was concluded that interfacial adhesion may occur when the majority phase is PCL [25]. This could be also seen at T = 200 °C, but it is a more dominant PLLA phase in that case. Finally, PLLA/PCL 10/90T200 presents the PCL continuous region with small irregular PLLA domains of different sizes. At T = 40 °C, red regions indicating PCL phases are overlayed with yellow phases of PLLA, hence the areas appear orange. Thus, Raman maps are well related with the morphology previously received from the SEM and AFM images.
Figure 6. Raman images of various PLLA, PCL and their blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures (40 °C, 80 °C and 200 °C).
Download figure:
Standard image High-resolution imageInfluence of annealing temperature on the mechanical properties
The mechanical properties of the PLLA/PCL blends are plotted in figure 7. In this graphic, pure PLLA shows a stiff and brittle behavior, but after annealing to a temperature of 40 °C PLLA shows an elongation at break of 60 ± 18%. This is probably due to the chain alignment during solvent evaporation [26]. Annealing above melting point (200 °C) reduces the elongation at break of pure PLLA down to a value of approximal 3%, which is known from literature [27].
Figure 7. Elongation at break of solvent cast PLLA/PCL blends treated at different annealing temperatures as a function of PCL content, n=6. Tensile strength of solvent cast PLLA/PCL blends as a function of PCL content are given in the supplementary data.
Download figure:
Standard image High-resolution imagePCL shows, in contrast to the literature, a brittle behavior. This can be associated with its high crystallinity, as described later in this work. Due to the washing and annealing processes, PCL reached such a high degree of crystallization and as a result the mechanical properties varied. Comparing the elongation values of PCL at 80 °C and 200 °C [6], it is evident that the crystallization values are significantly lower and consequently the elongation at break increases. Annealing of pure PCL above the melting temperature led to the degradation of the samples, which is why no mechanical properties could be determined. Addition of 10% PCL to PLLA and annealing to 40 °C results in a substantial increase in elongation at break, decreasing again with further addition of PCL (figure 7). From the literature, it is known from blending PLLA with PEG, that there is probably still an adhesion between PLLA and PEG based on the crystallization kinetics [27]. After annealing at 80 °C the 90/10 PLLA/PCL blend reveals the same effect, which indicates that the temperature has no significant effect on the PLLA/PCL boundary interfaces. Table 1 shows all mechanical properties of PLLA/PCL obtained by solvent casting, followed by an annealing process. The corresponding stress strain curves are given in the supplementary data. With further addition of PCL tensile strength (table 1) and elastic modulus decreases, which is comparable with the reported results [6, 9]. In summary, blending can substantially change the mechanical properties by influencing factors such as crystallization kinetics. To address this issue, thermal properties of the blends were investigated.
Influence of annealing temperature on the thermal properties
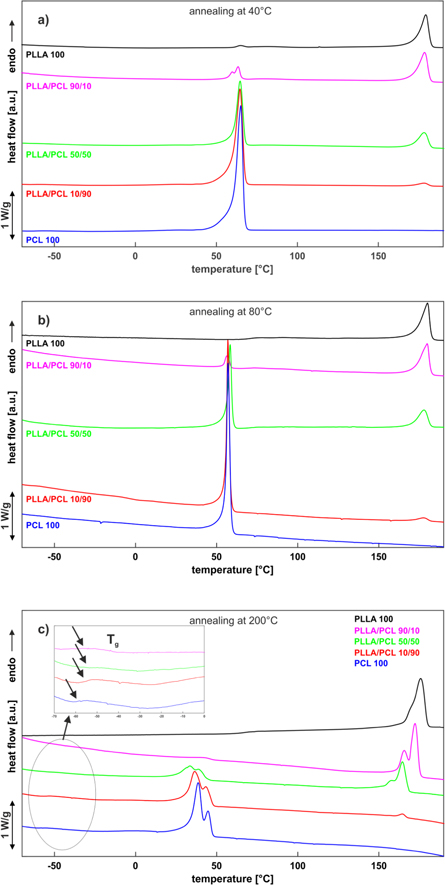
As typical examples, figure 8 shows the DSC traces of PLLA, PCL, and their blends in different ratios (90/10, 50/50, 10/90 w/w) of the cast film samples recorded by the first heating scan. The DSC spectrum of pure PLLA film exhibits a glass transition temperature at approximal 67 °C, see table 2 for all annealing temperatures and are consistent compared to literature values [26]. No exothermic peak, but an endothermic melting peak at 179 °C for an annealing temperature of 40 °C and 80 °C is observed. At 200 °C the melting peak of pure PLLA decreases to 173.4 ± 1.4 °C. With increasing annealing temperature two PLLA melting peaks are formed, see figure 8(c). The DSC spectrum of pure PCL film exhibits a glass transition temperature at −64.66 ± 0.03 °C for an annealing temperature of 40 °C (figure 8(a)) and increases with increasing annealing temperature up to −61,6 ± 1,9 °C, see table 2. Pure PCL and all blends show a different melting behavior at 200 °C compared to annealing temperatures of 40 °C and 80 °C. For an annealing temperature of 200 °C, two melting peaks are formed for PCL (figure 8(c)). The appearance of two PCL and two PLLA melting peaks might be an indication of formation of mixed crystalline structures. There are some explanations for the PCL and PLLA double melting peaks phenomenon in the literature. Finotti et al state that the lower peak temperature can be associated with the partial migration of PLLA molecules to the PCL phase. The low molecular weight allows the diffusion of PLLA from the PLLA/PCL interface to the interior of the PCL rich-phase, decreasing the size and degree of perfection of the crystalline lamellae, hence lowering the TM of the PCL. They also described the second melting peak at 56 °C as typical of pure PCL [28]. Regarding PLLA, Zhou et al stated the possibility to crystallize into two different crystal structures, the α-form, melting at higher temperature and the β-form, melting at lower temperature [29]. They also pointed out that the presence of the two endothermic peaks can also be explained by the growth of small crystals, which at low heating rates melt and recrystallize before melting again. Shan et al suggested the higher temperature peak may be a result of the melting of the thicker lamellae, possibly generated during the production process. In contrast, the lower temperature peak is attributed to the crystallization of the sample during the annealing process [30].
Figure 8. DSC curves of PLLA, PCL and their blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures (a = 40 °C, b = 80 °C and c = 200 °C).
Download figure:
Standard image High-resolution imageTable 2. DSC results (glass transition (Tg), melting temperature (Tm) and degree of crystallinity (χ)) of PLLA/PCL blends in different ratios (90/10, 50/50, 10/90 w/w) treated at different annealing temperatures (n = 6 for each group, n.a. = not available).
| T = 40 °C | PLLA | PCL | ||||
|---|---|---|---|---|---|---|
| Composition (PLLA/PCL) | Tg (°C) | χ (%) | Tm (°C) | Tg (°C) | χ (%) | Tm (°C) |
| 100/0 | 67.25 ± 0.08 | 39.3 ± 1.7 | 179.51 ± 0.24 | |||
| 90/10 | 75.4 ± 0.5 | 43.3 ± 0.7 | 180.14 ± 0.11 | n.a. | 38 ± 3 | 60.64 ± 0.08 |
| 50/50 | 74.65 ± 0.18 | 42.5 ± 0.9 | 178.61 ± 0.28 | −62.95 ± 0.14 | 70.7 ± 1.2 | 65.04 ± 0.11 |
| 10/90 | n.a. | 37.7 ± 1.8 | 178.42 ± 0.10 | −60.5 ± 0.8 | 75.9 ± 2.0 | 64.48 ± 0.11 |
| 0/100 | −64.66 ± 0.03 | 79.66 ± 0.15 | 65.5 ± 0.5 | |||
| PLLA | PCL | |||||
| T = 80 °C | ||||||
| Composition (PLLA/PCL) | Tg (°C) | χ (%) | TM (°C) | Tg (°C) | χ (%) | TM (°C) |
| 100/0 | 68.2 ± 0.6 | 45.0 ± 1.7 | 179.80 ± 0.15 | |||
| 90/10 | 71.6 ± 0.5 | 41.2 ± 1.2 | 179.56 ± 0.14 | n.a. | 28.1 ± 0.9 | 55.7 ± 0.3 |
| 50/50 | 71.7 ± 0.4 | 40.0 ± 1.2 | 178.18 ± 0.06 | −63.9 ± 0.9 | 49.4 ± 1.9 | 57.74 ± 0.12 |
| 10/90 | n.a. | 40 ± 5 | 178.49 ± 0.12 | −64.1 ± 0.8 | 55 ± 3 | 57.40 ± 0.09 |
| 0/100 | −63.00 ± 0.23 | 62.81 ± 0.12 | 57.28 ± 0.05 | |||
| PLLA | PCL | |||||
| T = 200 °C | ||||||
| Composition (PLLA/PCL) | Tg (°C) | χ (%) | TM (°C) | Tg (°C) | χ (%) | TM (°C) |
| 100/0 | 66.1 ± 0.8 | 62.8 ± 0.9 | 173.4 ± 1.4 | |||
| 90/10 | 66.5 ± 0.7 | 61 ± 3 | 173.7 ± 1.4 | −63.59 ± 0.20 | 21 ± 6 | 45.8 ± 2.8 |
| 50/50 | n.a. | 62 ± 4 | 169.8 ± 2.6 | −63.1 ± 1.0 | 45 ± 3 | 46 ± 3 |
| 10/90 | n.a. | 43 ± 8 | 174.89 ± 0.23 | −62.7 ± 0.4 | 63.3 ± 0.5 | 55.6 ± 0.6 |
| 0/100 | −61.6 ± 1.9 | 70.6 ± 0.7 | 54.64 ± 0.11 | |||
Table 2 shows the peak temperatures Tm and the Tg of PLLA and PCL as determined from the DSC curves as well as the percentage PLLA and PCL crystallinity, χPLLA (%) and χPCL (%) values as determined according to equation (1). Heat of fusion (ΔH) of PLLA, PCL and their blends are given in the supplementary data. Tg is not discussed here due to insufficient heating rate (10 K min−1) and some overlay characteristics.
Thus, it can be concluded that blending PLLA with PCL changes the inherent crystallization behaviors of both polymers in combination. χPLLA at T40 is about 39.3 ± 1.7% and increases with increasing annealing temperature up to 62.8 ± 0.9%. The crystallinity of PLLAT80 in the blend decreases at PLLA/PCL 90/10 and increases with higher PCL content. This can be explained in a way that PCL separates from the PLLA crystal due to the PLLA/PCL mixing gap, which is known from the literature [31]. This approaches the original crystallinity of PLLA 100. At T200 χPLLA in the 10/90 blend decreases from 62 ± 4% to 43 ± 8%. Other studies report an increase in the crystallinity of PLLA in the presence of PCL obtained from melt processes, probably due to the increase in nucleation rate [22, 32, 33]. However, this effect could not be observed in this work which may be explained by the fact that a solvent casting process was chosen in this paper. This has a different effect on the crystallization mechanism of PLLA and underlines the impact of the manufacturing process of such blend materials. The trend of increasing crystallinity with increasing PCL content in the blend can be seen in the case of all applied annealing temperatures, in a way that the high temperature thermal treatment at 200 °C approximates melt cast conditions.
Annealing at 200 °C is comparable to the thermally induced equilibration from melting process, which is widely applied for the investigation of crystalline polymers. Notably, it can be seen in figure 9 that the degree of crystallinity of PCL gradually decreases with increasing PLLA content, while the crystallinity of PLLA increases slowly with increasing PCL content (see table 2). Liu et al showed that a higher crystallization rate coefficient (CRC) of the blend in comparison to pure PLLA causes faster crystallization [34]. Therefore, χPLLA increases with increasing PCL content. Clearly, high PCL content substantially decreases the crystallinity of PLLA/PCL blends. This means that the large nucleus formation induced by PCL molecules inhibit the mobility of PLLA chains. As a result, chain rearrangement is limited, so low χPLLA are noted. Our assumption is that similar processes on the surface of the PLLA nuclei lead to a decrease in the crystallinity of the PCL when raising the PLLA concentration. Due to the increase in the annealing temperature, the crystal sizes of both polymers may decrease, resulting in a decrease in the melting temperature.
Figure 9. Crystallization behavior of PCL and their blends in different ratios (10/90, 50/50, 90/10 w/w) treated at different annealing temperatures, n = 6 Crystallization behavior of PLLA and their blends in different ratios (10/90, 50/50, 90/10 w/w) are given in the supplementary data.
Download figure:
Standard image High-resolution imageIn general, solvent cast processes followed by low thermal annealing lead to substantially different properties of the yielded films in comparison to melt cast process data from the literature [22, 35, 36]. In addition to thermal equilibration, solvent evaporation plays an important role and can lead to different crystal growth rates compared to the cooling rate.
Conclusion
Polymer blends, in our case PLLA/PCL, may form complex structures and morphologies, leading to drastically altered properties of the resulting material. The interplay of the polymers depends on the material composition, the choice of preparation protocol, as well as on postprocessing procedures, such as washing or thermal annealing. Our aim was the investigation regarding the applicability of high-molecular PLLA blended with low-molecular PCL, e.g. as implant coating or drug delivery matrix. In this context, the thermal and mechanical properties are of great interest to ensure the formation of a functional biomaterial. With respect to this, we investigated how annealing temperature during postprocessing influences the properties and therefore the suitability of solvent cast PLLA/PCL blends for biomedical products.
In general, the applied solvent cast process used included evaporation under reduced pressure and a washing process to completely remove residual solvent from the polymer, which impacted the morphological and thermal properties of the material. Furthermore, during thermal analysis, to investigate crystallization and phase separation processes, the materials were cooled down with a very low cooling rate after annealing process in order to avoid cold crystallization of the material and to maintain thermodynamic equilibrium. Interestingly, it could be demonstrated that annealing processes strongly influence surface morphology, mechanical and thermal characteristic of PLLA, PCL and their blends. Blending led to an improvement of the mechanical performance of polymers, such as increase in ductility and therefore elongation at break or mechanical resilience overall. PLLA/PCL 90:10 has shown different stress strain behavior when tempered at 40 °C compared to 200 °C and correlated with changes in crystallinity, where χPLLA increased from 43 to 61%. This allows the modification of material properties without further additives such as plasticizers, only by changing the process protocols.
Furthermore, solution cast versus melt cast processes have been investigated. It was demonstrated that the thermal properties changed with the annealing temperature.
Our results shown that the method of preparation has great influence on the resulting polymer film and should be considered when developing polymer based implant materials.
In summary, we have demonstrated the influence of different annealing temperatures on the morphological, mechanical and thermal properties of PLLA/PCL blends. Thus, it is important to carefully choose and to maintain temperatures throughout the production path of such coatings, in particular regarding applications in the field of medical engineering, since production parameters not only affects the mechanical properties of the blends, but can also show an effect on drug release and polymer degradation. In order to verify this, further studies are necessary, especially regarding storage stability, degradation and drug release.
Acknowledgments
The authors thank Andrea Rohde, Katja Hahn, Caroline Dudda and Nathalie Böttcher for their expert technical assistance. Furthermore, partial financial support by the Federal Ministry of Education and Research (BMBF) within RESPONSE 'Partnership for Innovation in Implant Technology' and by the European Social Fund (ESF) within the excellence research program of the state Mecklenburg-Vorpommern Card-ii-Omics is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.