Abstract
Bio-molecule capped α-MnO2 nanoparticles have been successfully synthesized from the reduction of KMnO4 via a facile green synthesis route using an aqueous leaf extract of Bryophyllum pinnatum as a reducing and capping agent. The synthesized α-MnO2 nanoparticles were characterized by x-ray diffraction (XRD), Fourier transform—infrared (FT-IR), energy dispersive x-ray (EDX), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), thermogravimetric (TG) and differential scanning calorimetry (DSC) techniques. Experimental results clearly demonstrate the successful synthesis of bio-molecule capped crystalline tetragonal α-MnO2 nanoparticles with the size of 4–18 nm. The magnetic property of the product was evaluated using a vibrating sample magnetometer (VSM) and the result reveals that the presently synthesized bio-molecule capped α-MnO2 nanoparticles exhibit ferromagnetic property at room temperature.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Nano-manganese oxides have been the focus of various researchers because of their prospective properties and enormous applications in high –Tc semiconductors, high density magnetic storage, electrochemical storage material, solar energy transformation, giant magnetoresistance, ion-exchange material, catalysis, and molecular adsorption [1–6]. These wide range of applications and distinctive properties have attracted broad attention to the polymorphic forms of MnO2 (α-, β-, and γ- type). The specific properties of α-MnO2 allow it as a technologically attractive material for various industrial applications [7]. For instance, α-MnO2 has been intensively used as an electrode material for lithium batteries [8], as a molecular sieve [9], etc The properties of nanomaterials strongly depend on their size, shape, and morphology, which play the key factor to ensure their potentiality and applications [10]. Thus, controlled synthesis of α-MnO2 nanoparticles has attracted much attention to the current researchers of materials science.
The crystal structure of hollandite minerals was first determined by Bystrom et al [11] in 1950 using x-ray diffraction data taken with a Weissenberg camera and they found the mixture of tetragonal (I4/m) and distorted monoclinic (I2/m) phase. Manganese dioxides with controlled morphologies and crystalline structures have been fabricated using various methods of synthesis that include refluxing, soft chemical processes, hydrothermal, sol–gel, solid state reactions, and precipitation [12]. Particularly, Rossouw et al [13] prepared polycrystalline α-MnO2 which was free from any large stabilizing cations. Kijima et al [14, 15] successfully synthesized a high-purity and well-crystallized α-MnO2 using precipitation method by ozone oxidation. Previously, several methods have been used to synthesize α-MnO2 in different shapes like nanorods [16, 17], nanowires [18], whiskers [19], and dandelion-like three dimensional structures [20]. The chemicals and reagents used in these synthesis processes increase unwanted environmental toxicity and biological hazards.
Consequently, establishment of a suitable route for the synthesis of α-MnO2 nanoparticles without using non-toxic reagents is of great interest. Green synthesis methods using leaf extract as the source of both the reducing and capping agents might be a potential candidate for the synthesis of α-MnO2 nanoparticles. Reports on the green synthesis of MnO2 nanoparticles using leaf/plant/peel extracts of Yucca gloriosa [21], Matricaria chamomilla L. [22], Brassica oleracea [23], and lemon peel [24] are available. In the present study, it was aimed to establish an environmentally friendly route for the synthesis of bio-molecule capped α-MnO2 nanoparticles using Bryophyllum pinnatum aqueous leaf extract as a reducing and capping agent and subsequently evaluate their physicochemical property. Bryophyllum pinnatum, commonly known 'resurrection plant', is a crassulescent herb of about 1 metre in height, with opposite, glabrous leaves (with 3–5 deeply crenulated, fleshy leaflets). The fleshy leaves of Bryophyllum pinnatum are frequently used as herbal remedy for an array of human disorders including hypertension, diabetes mellitus, bruises, wounds, boils, abscesses, insect bites, arthritis, rheumatism, joint pains, headaches, and body pains [25].
Materials and methods
Materials
Bryophyllum pinnatum leaves were collected locally from Dhaka, Bangladesh. KMnO4 was purchased from Merck, India. De-ionized water was used throughout the experiments whenever it is needed. All other reagents and chemicals used in this study were of analytical grade and used without further purification.
Synthesis of α-MnO2 nanoparticles
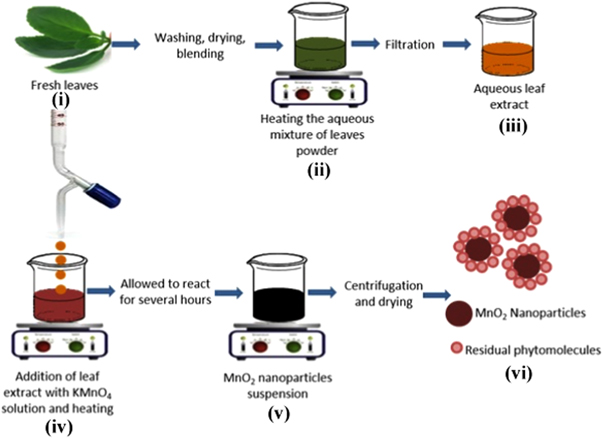
Fresh and healthy leaves of Bryophyllum pinnatum were washed with tap water followed with de-ionized water in order to remove the dust particles. The cleaned leaves were then dried at room temperature and grinded to get the finest leaf powder. 10 g of the dried leaf powder was mixed with 200 mL de-ionized water and boiled at 90 °C for 90 min and then cooled at room temperature. The mixture was then filtered, and the filtrate here termed as an aqueous leaf extract of Bryophyllum pinnatum which was used as a reducing and capping agent. The aqueous leaf extract was then added into 0.3 M KMnO4 solution (1:1; v/v) dropwise with constant stirring using a magnetic stirrer. The mixture was then heated at 90 °C on a hot plate until the color changed to blackish and a colloidal suspension was obtained. The suspension was then centrifuged and washed repeatedly with de-ionized water to remove the unreacted substances. The pellet was then dried at 60 °C. The schematic representation of the synthesis of α-MnO2 nanoparticles is shown in figure 1.
Figure 1. Schematic representation of the synthesis of α-MnO2 NPs.
Download figure:
Standard image High-resolution imageCharacterization of α-MnO2 nanoparticles
The crystalline phase of the product was confirmed using an x-ray powder diffractometer (XRD) (Philips PANalytical X'PERT PRO) equipped with CuKα radiation (1.5418 Å). The presence of functional groups in the Bryophyllum pinnatum aqueous leaf extract and their incorporation with the synthesized α-MnO2 nanoparticles was evaluated from their Fourier transform infrared (FT-IR) studies using a JASCO-FTIR-6300, Japan. Energy dispersive x-ray (EDX) was carried out by JEOL, JSM 7600 F, Japan, for the elemental analysis of the samples. The thermal analysis of the material was carried out by TA instrument (SDR Q-600) operating from 25 to 800 °C with a heating rate of 10 °C/min. The surface morphology was observed by FESEM (JEOL, model JSM 7600 F, Japan). TEM analysis was performed using a JEOL, JEM 2010, Japan in order to find out the surface modification of the samples and particle size measurement. The magnetic property was evaluated using a vibrating sample magnetometer (EV-9 MicroSense, German).
Results and discussion
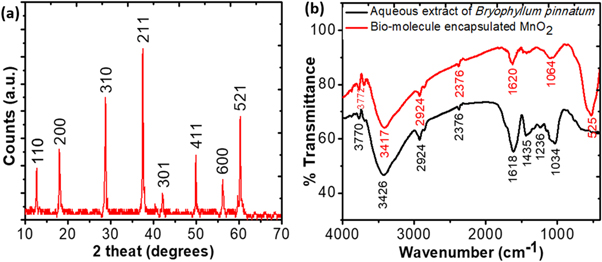
Figure 2(a) shows a typical XRD pattern of the synthesized samples. All the diffraction peaks of the product can be exclusively indexed to tetragonal phase (JCPDS Card, No. 44–0141, a = 9.784 Å; c = 2.863 Å) with space group 14 /m. No impurity peak was observed, indicating high purity and crystallinity of the product. The average crystallite size (D) was measured using the Debye–Scherrer equation [26] as follows:
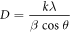
where, k is the shape factor of value 0.89, λ is the wavelength of x-ray (0.154 nm), β is the full width at half maxima, θ is the Bragg angle. The value of D was found to be about 10 nm. Our experimental results demonstrate that green synthesis of materials can enforces to crystalize on pure tetragonal phase.
Figure 2. (a) XRD pattern of α-MnO2 nanoparticles, (b) FT-IR spectra of aqueous leaf extract of Bryophyllum pinnatum (BP) and BP aqueous leaf extract mediated synthesized α-MnO2 nanoparticles.
Download figure:
Standard image High-resolution imageThe FT-IR spectra obtained for the aqueous leaf extract of Bryophyllum pinnatum and the synthesized samples are shown in figure 2(b). Three strong peaks appeared in the spectrum obtained for aqueous leaf extract at the wavenumber values of 1034, 1618, and 3426 cm−1 are representing the stretching vibration bands of C–O–C, –C=O, and –OH, respectively [27]. Some weak peaks were also found at the values of 1236, 1435, 2376, 2924, and 3770 cm−1. These peaks indicate the stretching vibration bands of C–OH, the C–N of the amine, C≡C, C–H, and −OH respectively [27–29]. These functional groups may be originated from a large number of bio-molecules, such as alkaloids, flavones, etc in the aqueous leaf extract which can act as reducing and capping agents [11]. It can also be seen from the figure 2(b) that almost similar functional groups existed in the samples like aqueous leaf extract indicating the encapsulation of MnO2 nanoparticles with bio-molecules originated from the aqueous leaf extract. Moreover, an extra prominent peak appeared in the FT-IR spectrum of MnO2 at 524 cm−1 which can be assigned to Mn–O bending vibrations [30].
The elemental composition of the prepared α-MnO2 nanoparticles was analyzed using EDX analysis as shown in figure 3(a). The lines observed at 5.90, 6.52, 0.64, and 0.52 keV are associated for K (α, β), L lines of the Mn element, and K line of the O elements, respectively [6]. The atomic percentage of Mn and O were 29 and 41 respectively indicating the formation of non-stoichiometric MnO2 with oxygen vacancy which indicates that the material might show better photocatalytic activity [31]. Another intense peak obtained at 0.28 is assigned as C. This impure element originated from the aqueous leaf extract and depict their conjunction with the synthesized α-MnO2 nanoparticles [32].
Figure 3. (a) EDX spectra and (b) TGA and DSC of α-MnO2 nanoparticles and bio-organic components of Bryophyllum pinnatum aqueous leaf extract.
Download figure:
Standard image High-resolution imageFigure 3(b) shows the thermogravimetric (TG) and differential scanning calorimetric (DSC) analyses of the materials. The slope of the TG curve shows that the weight loss of the sample occurred at different stages up to 800 °C. The first weight loss of about 1% was observed at 100 °C, which was due to the release of water molecules absorbed to the surface of the synthesized sample. The second weight loss up to 450 °C was found to be occurred due to the crystallinity and release of unreacted bio-molecules present in the sample originating from the aqueous leaf extract. Finally, changes in weight loss from the TG plot were observed from 450 °C to 750 °C which were attributed to the release of coating molecules from the surface of the synthesized α-MnO2 nanoparticles. DSC report indicates that the crystallization peak appeared at 175 °C. Further DSC peak appeared at 450 °C attributed to the nano-crystallinity of the material [33]. A broad peak was also obtained at 600 °C which was due to the transition from α-MnO2 to another polymorph of MnO2 [34].
Figure 4(a) shows the FESEM image of the samples indicating that the synthesized α-MnO2 nanoparticles are uniformly distributed over the surface and appeared to be less agglomerated. The minimal agglomeration of the samples might be due to the surface encapsulation which hinders the agglomeration of the particles. The synthesis procedure was repeated for three times and the surface morphology was found to be reproducible indicating the potentiality of Bryophyllum pinnatum aqueous leaf extract as a reducing and capping agent for the synthesis of bio-molecule capped α-MnO2 nanoparticles. The TEM image (figure 4(b)) demonstrates that the samples are spherical in shape with the size in the range of 4–18 nm calculated using ImageJ software. Moreover, an intense look on the nanoparticles clearly indicates that almost all the particles are encapsulated by a thick layer of bio-molecules which accentuated one of the objectives of the present study, i.e., encapsulation of α-MnO2 nanoparticles with bio-molecules.
Figure 4. (a) FESEM image showing the surface morphology of α-MnO2 nanoparticles and (b) TEM image showing the encapsulated α-MnO2 nanoparticles synthesized from the reduction of KMnO4 using BP aqueous leaf extract.
Download figure:
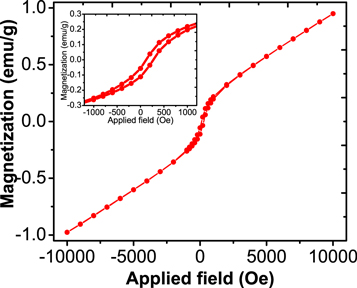
Standard image High-resolution imageRoom temperature VSM measurement of our studied α-MnO2 nanoparticles has been represented in figure 5. This figure demonstrates that studied α-MnO2 nanoparticles show very weak ferromagnetic behaviour at room temperature. Figure 5 (inset) shows the enlargement view of the hysteresis loop obtained from VSM measurement at room temperature. Generally, symmetric nature of Mn-O-Mn bonds are responsible for the antiferromagnetic nature of α-MnO2 at ground state. Several reports of low temperature (5 K) ferromagnetic α-MnO2 nanorods are available in literature which were synthesized by hydrothermal method using KMnO4 as the precursor [35, 36]. In this work, we found the hysteresis loop with 1000 Oe coercivity appears at room temperature, indicating weak ferromagnetic ordering. From figure 5, it could be demonstrated that at higher applied field, the antiferromagnetic phase is predominant. The week hysteresis loop at room temperature implies the presence of mixed ferromagnetic and antiferromagnetic interaction. The ferromagnetic interaction of the synthesized α-MnO2 nanoparticles is due the Mn4+-O2--Mn4+ coupling [7]. These types of materials have reported as diluted magnetic semiconductors with room temperature ferromagnetism nature, are interesting materials for spintronic applications [37].
Figure 5. Room temperature magnetization curve of α-MnO2 NPs synthesized from the reduction of KMnO4 using Bryophyllum pinnatum aqueous leaf extract (inset shows the enlargement view of the hysteresis loop).
Download figure:
Standard image High-resolution imageConclusion
In the present study, we have successfully developed a facile green synthesis route for the synthesis of crystalline α-MnO2 nanoparticles with sizes ranging from 4 to 18 nm. The method for synthesis of MnO2 nanocrystals is simple, eco-friendly, cost-effective, and efficient, which excludes external reducing agents and stabilizers. Magnetic property of α-MnO2 nanoparticles prepared by this method was investigated using a vibrating sample magnetometer (VSM) at room temperature, and a small hysteresis loop was obtained in the VSM analysis indicating weak ferromagnetic property of the bio-molecule capped α-MnO2 nanoparticles. The room temperature week ferromagnetic property of the presently synthesized α-MnO2 nanoparticles might have a notable impact on spintronic applications.
Acknowledgments
The authors gratefully acknowledge the lab personnels of Nanoscience and Technology Research Laboratory and Materials Science Laboratory of Atomic Energy Centre, Dhaka for their generous support during the research work.
Competing interest
The authors declare no competing interest.