Abstract
Long-wavelength luminescent probes and sensors become increasingly popular. They offer the advantage of lower levels of autofluorescence in most biological probes. Due to high penetration depth and low scattering of red and NIR light such probes potentially enable in vivo measurements in tissues and some of them have already reached a high level of reliability required for such applications. This review focuses on the recent progress in development and application of long-wavelength analyte-sensitive probes which can operate both reversibly and irreversibly. Photophysical properties, sensing mechanisms, advantages and limitations of individual probes are discussed.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
| BP | benzoporphyrin |
| BS | brightness |
| Cys | cysteine |
| ESIPT | excited state intramolecular proton transfer |
| FRET | Förster resonance energy transfer |
| GSH | glutathione |
| Hcy | homocysteine |
| ICT | internal charge transfer |
| LOD | limit of detection |
| NIR | near infrared |
| 2-P | two photon |
| PET | photoinduced electron transfer |
| ROS | reactive oxygen species |
| QY | quantum yield |
| UC | photon upconversion |
1. Introduction
Recent decades have seen an enormous progress in development of luminescent probes and sensors. Compared to more conventional analytical tools, optical probes offer the advantage of being only minimally invasive and can be prepared in a variety of formats including small molecule probes, analyte-sensitive nanoparticles, fiber-optic or planar optical sensors. Long-wavelength probes offer significant advantages over more conventional UV, blue and green light excitable probes due to low scattering of excitation and emission light, low levels of autofluorescence in most biological systems (photosynthetic systems represent a notable exception) and less disturbance and photodamage caused to cells and living organisms. Due to good penetration depth of red and near-infrared light, long-wavelength probes offer a unique possibility of analyte quantification in vivo e.g. in normal and diseased tissues of living organisms or in biofilms. Particularly in recent years many new luminescent long-wavelength probes for various important analytes were developed. Whereas most of the published probes still have many limitations and require further improvement, some of them proved to be robust enough to enable a variety of important applications. For example, near-infrared oxygen probes are applied for reliable quantification of the analyte in living organisms e.g. for tumor imaging.
This review will focus on recent progress in development and application of long-wavelength analyte-sensitive probes. Some of these probes have been covered in recent reviews describing UV–vis and NIR probes [1–3]. For older probes published prior to 2012 one can refer to some excellent reviews [4–7]. However, the probes not covered in these sources and those necessary for better understanding of the recent work will also be mentioned here. Analyte-insensitive probes (fluorescent labels) which are only aimed at visualization of an object (organelles, cells, tissues) will not be included and are covered elsewhere [8–13].
2. Important properties of the probes
Obviously, it becomes increasingly difficult to orient in the large amount of published probes and select the one which is most suitable for a particular application. Unfortunately, there is no 'ideal' probe, and any probe has advantages and disadvantages. To make the selection of potential candidates easier, several most important properties should be considered.
2.1. Photophysical properties
Absorption and emission spectra will be the first to consider. The choice of spectral properties will be guided by the desired application. For example, for measurements in animal tissues and live animals, the absorption and emission bands should be located in the so called 'optical window' [14] which covers the 650–950 nm wavelength range. At shorter wavelengths the light is strongly absorbed by haemoglobin and other pigments, and the autofluorescence and scattering by tissue are greatly increased, whereas at longer wavelengths the absorption by water becomes rather strong. A second window for in vivo imaging (1000–1350 nm) [15] exists, but it is challenging to design adequate probes for this part of electromagnetic spectrum. Since the probability of non-radiative deactivation of the low energetic excited state is significantly higher, most chromophores absorbing above 900 nm do not possess detectable emission.
Another possibility to measure at depths exceeding several hundred micrometers is to use NIR light for excitation of either 2-P probes or UC based-systems [16]. In order to explore the full potential of these probes for in vivo measurements, the emission should be located in the red part of the spectrum. Unfortunately, conventional indicators are poorly suited for this method due to low 2-P absorption cross-sections whereas most lanthanide upconverting (UC) systems either emit in the blue–green part of the spectrum or are not analyte-sensitive. Multi-photon laser scanning microscopy is also suitable for imaging in phototrophic biofilms, where excitation of down-converting probes with red light can result in strong autofluorescence originating from chlorophyll and other pigments. For many other important applications (e.g. intracellular imaging) the probes excitable in the orange–red part of the spectrum are already highly advantageous compared to conventional UV- and blue light-excitable dyes due to less photodamage caused to the cells. Thus, in this review we will include luminescent probes excitable above 560 nm (yellow, orange, red and NIR light) but also some two photon and UP probes.
Spectral compatibility of the probes with available excitation sources and photodetectors is also important [16]. Whereas various efficient light sources (LEDs, laser diodes, lasers etc) are available for the whole visible spectrum the compatibility of the probes with photodetectors may still remain an issue particularly, in case of older systems. For example, standard photomultipliers are virtually 'blind' above 750 nm. Despite affordability of newer PMTs as well as CCD and CMOS cameras having good spectral sensitivity in NIR, recently developed long-wavelength probes may not be compatible with available instrumental set-ups and the upgrade is often not possible for financial reasons. On the other hand, very cheap and compact photodiodes are an excellent choice for use in read-out devices for optical sensors due to maximal sensitivity in NIR. Avalanche photodiodes represent a good compromise between photomultipliers and photodiodes in terms of sensitivity and spectral response and may be the best choice for measurement in NIR [17].
Luminescence BS is another important parameter. It is defined as a product of molar absorption coefficient (ε) and luminescence quantum yield (QY). High BS is of utmost importance for in vivo measurements in tissues since most excitation and emission light is lost due to light scattering and absorption, even in case of NIR probes. To reach acceptable BS, ε should exceed 20 000–30 000 M−1 cm−1 and be preferably above 80 000 M−1 cm−1. Due to more efficient radiativeless deactivation of the excited state in case of NIR dyes QYs exceeding 30% can be considered good, and only a few dyes feature QYs above 50%. The probes with QYs below 1% ('on' state) are less likely to be suitable for precise quantification of analytes in tissues.
2.2. Encapsulation of the indicator and probe solubility
Many reported long-wavelength probes are small molecules which are likely to interact with biological matter present in the analyzed media. Such interaction significantly affects the photophysical and sensing properties of the probe, particularly absorption and emission spectra, luminescence quantum yield, luminescence decay time, pKa value of the pH indicators, etc. Thus, small molecule probes are most reliable in a defined environment after the calibration is performed in identical conditions, which is challenging for many in vivo measurements. Protection of the probe from undesired interactions is typically performed either by assembling a dendimeric or PEG-based shell around the core indicator [18] or by its encapsulation into polymeric nanoparticles. The first approach results in small probes of several nanometers in diameter, however it also requires significant synthetic effort. Most of the reported dendrimers are designed to remain in vasculature and do not penetrate the cell membranes. On the other hand, encapsulation of indicators inside polymeric nanoparticles can be performed using simple techniques such as swelling [19] or nanoprecipitation [20]. The polymer used in the nanoparticles, the charge and the nature of the groups on their surfaces determine the cell-penetrating properties. This can be useful for analyte imaging in cells and even organels. The size of the nanobeads (typically > 10–20 nm) is larger than that of dendrimers. Leaching of physically entrapped dyes into surrounding medium may be critical, therefore covalent immobilization is preferable. However, the synthetic effort needed for preparation of indicators and polymerization is much higher than for the nanobeads prepared via physical entrapment of the dyes. For the latter, immobilization of lipophilic indicators inside a hydrophobic shell often completely eliminates leaching.
One should distinguish between luminescent probes and optical sensors (optodes) [21]. Optical sensors are usually prepared immobilization of the indicators inside a bulk polymer which acts as a solvent for the dye and as a permeation-selective matrix to ensure the necessary protection from interfering species. This material is typically coated onto a planar support or onto a tip of an optical fiber, to produce planar optodes and fiber-optic sensors, respectively. The sensors are used in combination with a dedicated read-out device or a measurement set-up including a light source, photodetector and necessary electronics. In case of fiber-optic sensors spectral properties of the indicator are of less importance since undesired effects (e.g. background fluorescence from the probe) can be eliminated by using an additional layer of optical isolation and the conventional UV–vis indicators may be as suitable as NIR indicators.
2.3. Reversibility of the response
The chemical mechanisms responsible for the response of the probe are rather diverse and vary from reversible physical quenching (oxygen probes), formation of complexes (most metal probes), protonation/deprotonation of the indicator (pH probes) to irreversible oxidation of the dye (probes for reactive oxygen species) and cleavage of a certain chemical bond (e.g. hydrogen sulfide, thiol and enzymatic activity probes). In contrast to the reversible probes, the irreversible probes are only suitable for quantification of the increasing concentration of the analyte. Despite this inconvenience, irreversible probes are also of great practical importance since design of fully reversible probes for many analytes is challenging. Only few of these probes can be regenerated (such as some redox probes) [22, 23] and used for next cycle.
2.4. Signal referencing for quantitative measurements
Luminescence intensity is an ambiguous parameter which is influenced by many factors including intensity of the light source, sensitivity of the photodetector, scattering, coloration of the media, concentration of the probe, etc. Therefore, reliable quantification of the analyte by measuring luminescence intensity at a single wavelength is only possible if all this conditions are kept constant which is difficult or impossible to achieve in many if not most applications. Notably, many of the probes described below do not use any referencing and thus need further optimization to achieve reliable quantification of the analyte.
Several referencing schemes are widely employed [24]. The ratiometric (two wavelength, excitation or emission) scheme is popular, particularly in microscopy and imaging applications [25]. However, it is difficult to design a molecule which will possess two spectrally different emissions (in the absence and in presence of the analyte) having overlapping excitation spectra for both forms and similar BS. In fact, only a few such long-wavelength probes have been reported [26]. On the other hand, many irreversible probes are suitable for ratiometric read-out, since both forms (before and after the reaction) are fluorescent. Alternatively, an analyte-insensitive luminescent dye or phosphor can be added into the sensing material along with an indicator to ensure ratiometric response. It is often challenging to find a pair of dyes which possess similar excitation but different emission spectra, good BS and high photostability. Evidently, leaching or photobleaching of one of the components will result in a shift of the calibration. It should be also noted that all ratiometric probes are still liable to wavelength-dependent effects such as light scattering.
Measurement of the luminescence decay time represents a self-referenced technique which is widely used in optical sensing [24]. The phosphorescence lifetime (oxygen probes) can be measured in frequency domain using relatively cheap and compact instrumentation [27]. However, such read-out devices are limited to single point measurements (fiber-optic sensors, sensor spots, nanoaparticle suspension in vials). Imaging of phosphorescence or fluorescence lifetime (PLIM and FLIM, respectively) becomes increasingly popular [28] but requires significantly more expensive instrumentation. Whereas PLIM enables reliable read-out of oxygen probes even in vivo, FLIM may not be useful for many analyte-sensitive probes based on fluorescent dyes. In fact, in case of 'on–off' probes (many PET pH probes, irreversible thiol probes etc.), only the fluorescence decay time of the 'on' form will be detected. In this case, FRET based concepts are more promising since it becomes possible to modulate the fluorescence decay times.
2.5. Photostability
Photostability of a probe can be critical for many applications in which light intensities are comparably high such as in high resolution imaging. Photobleaching results in a drift of the calibration and is particularly critical for unreferenced measurements of luminescence intensity. Photostability varies greatly for different chromophore classes. Unfortunately, this property is difficult to compare, since the conditions in which the photobleaching experiments are performed are not uniform. The light intensities can vary several orders of magnitude and so can the photobleaching rates. Often, higher light intensities have to be used for less bright samples (either due to low BS of the probe or to low relative concentration in a biological sample) inducing quicker photodamage. In general, some classes of long-wavelength probes, particularly those with extended conjugation (cyanine dyes) are more vulnerable to photobleaching than conjugated aromatic systems (porphyrins, aza-BODIPYs).
2.6. Applications
Three important application areas of long-wavelength probes can be distinguished. Monitoring in vivo is the most challenging application and only a few probes can currently fulfill the necessary requirements [29]. Apart from adequate spectral properties and high luminescence BS determined by efficient absorption and scattering of light in tissues, suitability for referenced measurements is of utmost importance. The probes based on luminescence intensity can provide only semi-quantitative information since the light losses are difficult to quantify. On the other hand, the lifetime-based probes (e.g. for oxygen) offer the advantage of being self-referenced and retain reliable calibration in tissue.
Photophysical properties of the probes are less important in case of in vitro and ex vivo intracellular measurements since light absorption and scattering are not as pronounced as in in vivo experiments (but still depend on the type of tissue). Here, major selection criteria are cell/tissue staining efficiency, cell specificity, intracellular distribution, toxicity, compatibility with the available instrumentation and capabilities of multiplexing with conventional fluorophores [30]. Also here, long-wavelength probes are advantageous since most conventional fluorophores emit at shorter wavelengths.
Finally, many NIR probes represent a key component in sensing materials which are applied in vitro. Such conventional formats as fiber optic sensors, planar sensors and spots are common. Similarly to UV–vis indicators, the candidates are chosen according to some property most relevant for desired application, such high BS, good photostability, simplicity of synthesis, etc.
3. Oxygen probes and sensors
Optical oxygen probes rely on dynamic quenching of luminescence of an indicator dye and this process is fully reversible. Typically, the indicator is embedded into a polymeric matrix which acts as a solvent for the dye and as a permeation-selective membrane which protects the probes from interferences [31]. As will be shown below, dendrimeric probes represent an excellent alternative. In contrast to the amperometric Clark electrode optical oxygen probes do not consume the analyte unless highly reactive singlet oxygen reacts with the indicators, polymeric matrix or the components of the media [32]. However, the biggest advantage of optical oxygen sensors compared to more conventional analytical methods is the possibility of remote read-out.
NIR oxygen probes and sensors become increasingly attractive particularly for biological and medical applications. Nevertheless, most state-of-the-art oxygen sensors still rely on UV–vis indicators such as Ru(II) polypyridyl complexes, Pd(II) and Pt(II) porphyrins and some cyclometallated Pt(II) and Ir(III) dyes [33]. The absorption of Ru(II) polypyridyls is located below 500 nm, whereas the Pt(II) and Pd(II) porphyrins (e.g. the most prominent representatives—the complexes of octaethylporphyrin and meso-pentafluorophenylporphyrin) can be excited up to 500–545 nm (Q bands). Unfortunately, excitation in the Q-bands of these porphyrins is not very efficient (ε ~ 10 000–20 000 M−1 cm−1). Apart from coumarin complexes [34] most cyclometallated Pt(II) and Ir(III) dyes as well as Cu(I) chelates absorb poorly in the visible part of the spectrum (ε < 10 000 M−1 cm−1) and do not represent viable oxygen probes due to this reason.
3.1. Classes of NIR oxygen probes
Several classes of NIR oxygen probes have been reported but only some of them seem to be promising for practical applications. Oxidation of octaethylporphyrin and meso-pentafluorophenylporphyrin gives porphyrin-ketones (figure 1, (1)) [35] and porphyrin-lactones 2 [36], respectively, and results in pronounced bathochromic shift of the absorption spectra (table S1, ESI) (stacks.iop.org/MAF/4/042005/mmedia). The absorption of the Pd(II) complexes is red-shifted by ~20 nm compared to that of the Pt(II) complexes. The complexes of porphyrin ketones and porphyrin lactones show phosphorescence in NIR part of the spectrum (759 nm and 733 nm for Pt(II) porphyrin ketone and lactone, respectively) with moderate QYs (~12% for Pt(II) porphyrin-ketone). These dyes were used in numerous oxygen-sensing materials for application in pressure-sensitive paints [37, 38], for non-destructive monitoring of oxygen ingress and residual oxygen in food packages [39, 40] and in pre- and post-pasteurized bottled beer [41], in lab on a chip microfluidic devices [42–44], for sensing of intracellular oxygen with help of nanosensors [45, 46], and in fiber-optic sensors [47].
Figure 1. Chemical structures of the long-wavelength oxygen probes. M = Pd(II) and Pt(II) in all cases.
Download figure:
Standard image High-resolution imageIn contrast to porphyrins, chlorins consist of only three pyrrols and one pyrroline. Since the chlorins possess efficient absorption in the red part of the spectrum and show fairly strong fluorescence (such as chlorophyls) the metal complexes of the dyes could be potentially suitable as NIR oxygen probes. Indeed, the Pt(II) and Pd(II) complexes of chlorins (3) and (4) were found to be excitable with red light and were phosphorescent in NIR [48] but with fairly low QYs (1–2%).
Back in 1995 Vinogradov and Wilson reported synthesis and properties of phosphorescent tetraphenyltetrabenzoporphyrin complexes [49] and their oxygen-sensing properties [50]. These chromophores attract increasing attention in recent years. The π-extension of the porphyrin macrocycle resulted in a pronounced bathochromic shift of the absorption and emission spectra compared to other porphyrins. The Pd(II) complex with tetraphenyltetrabenzoporphyrin 5 was found to have the best photophysical properties and its sulfonated derivative 6 was used to monitor oxygen distribution in tissue [50]. The authors demonstrated for the first time that NIR oxygen probes enable time-resolved mapping of oxygen concentration in tissues through substantially thick layers (~1 cm) which was previously impossible to achieve using conventional UV–vis indicators. Unfortunately, synthesis and isolation of pure BPs by the template condensation was not possible which hindered their practical application [51]. Later, the BP complexes became accessible via Lindsey method which yielded analytically pure BPs bearing various functional groups [52], for example in meso-position [48, 53] such as phenyl (5), 4-fluorophenyl (7), fluorenyl (8), biphenyl (9) and others. Meso-substitution only slightly affects the position of the Q-band (located at 613–617 nm for the Pt(II) complexes, table S1, ESI) (stacks.iop.org/MAF/4/042005/mmedia) and the emission spectrum. It was demonstrated that the BPs possess very high photostability which is only slightly lower than that of highly photostable meso-pentafluorophenylporphyrin [54]. Due to much better BS compared to conventional indicators the new dyes proved to be promising for application in planar optodes and fiber-optic sensors (mainly based on polystyrene and its derivatives). These sensors were used for measurement of oxygen solubility in organic solvents [55], online measurement of antioxidant activity using a capillary-based sensor coupled to an HPLC system [56], imaging of oxygen dynamics of symbiotic cyanobacterium Prochloron [57], luminescence imaging of physiological wound oxygenation [58], ultrafast measurement of oxygen flux with fiber-optic microsensors [59], preparation of structured materials for food packaging applications [60]. In vitro experiments using simulated subcutaneous read-out demonstrated superiority of the new sensors over those based on UV–vis indicators which were found to be virtually useless due to the dramatic signal loss [54].
New planar optodes reported by Ehgartner et al [61] made use of Pt(II) complex with 7 embedded into polystyrene along with a reference fluorescent dye. They allowed imaging of oxygen distribution under excitation with red light using a commercially available compact dual chip camera (RGB/NIR). The oxygen-sensitive NIR emission of the BP (NIR chip) was referenced against the analyte-insensitive emission of the fluorescent dye (red channel of the RGB chip). This low cost set-up was also used to read out a ratiometric pH/oxygen dual sensor albeit under excitation with blue light.
Sensors based on indicators physically entrapped into the polymeric matrix can suffer from aggregation, leaching or migration of the dye (into the sample or a polymeric support). Such effects are greatly accelerated at high temperatures, which are typical for sterilization via autoclavation. In order to overcome these limitations Hutter et al developed a simple strategy for covalent immobilization of the BPs [62]. Readily available in large amounts in a simple three-step synthesis, monobromo- or tetrabromo-substituted BPs 10 were either directly grafted onto the copolymer of styrene and styrene boronic acid or converted into styrene derivatives 11. The modified dyes could be copolymerized with a variety of monomers such as acrylates or styrene to yield several functional materials. The new sensing materials based on covalently coupled indicators did not show any migration or leaching of the dye even in organic solvents and at high temperature. In the later work [63], tetra-styrene derivatives were covalently embedded into silicon rubbers to produce mechanically and chemically robust trace oxygen sensing materials. The new sensors were applied for quantification of oxygen ingress into wine bottles. Due to attractive photophysical properties, the BPs were found to be particularly suitable for measurement through highly absorbing glass of the bottles.
As was mentioned above, Pt(II) and Pd(II) complexes of BPs efficiently absorb at 600–630 nm and already fulfill the requirements for many important applications due to this reason. However, the indicators with longer excitation wavelength are of high interest due to even better penetration of the excitation light into the tissue. Further π-extension of BPs results in naphthoporphyrins (12) [64, 65] which feature about 80 nm bathochromically shifted absorption and emission spectra. The synthetic strategy used for the benzorporphyrin dyes can be adapted to produce naphthoporphyrins with a variety of substituents [66, 67]. Niedermair et al [68] showed that it is also possible to prepare molecular hybrids of benzo- and naphthoporphyrins (13–15) and thus systematically tune the photophysical properties. Such an approach makes it possible to use a variety of excitation sources including red LEDs and laser diodes. The luminescence decay time decrease with increasing amount of naptho-moieties. Unfortunately, luminescence QYs and photostability of the dyes decreases as well. Thus, inferior photophysical properties of napthoporphyrins compared to BPs may make them less attractive for in vivo applications despite more favorable spectral properties.
Phthalocyanines possess intense narrow absorption in the NIR part of the spectrum and unmatched photostability. However, the Pd(II) and Pt(II) complexes of phthalocyanines show very weak NIR phosphorescence (QY < 1%) [69] and for this reason are poorly suitable for oxygen-sensing applications. Metal complexes of aza-tetrabenzoporphyrins combine the advantages of phthalocyanines and BPs. Mono-aza-triphenyltetrabenzoporphyrins (16) feature narrow absorption of the Q-band which is bathochromically shifted (~20 nm) compared to the respective BPs [70]. The phosphorescence maxima are red-shifted by ~75 nm compared to the benzoporpyhrins (table S1, ESI) (stacks.iop.org/MAF/4/042005/mmedia). Good photostability and compatibility with red laser diodes and He–Ne laser are particularly attractive features. Introduction of bulky tert-butyl groups (17) [71] drastically enhanced solubility of the dyes in organic solvents and polymeric matrices.
As was shown above, porphyrin dyes and their analogues represent by far the most promising class of NIR oxygen indicators. However, alternative indicators are highly welcomed to enable greater flexibility in choice of spectral properties and realization of some emerging applications such as e.g. oxygen monitoring in food packages where cheap indicators are required. Readily available Pd(II) and Pt(II) complexes with donor–acceptor Schiff bases (18, 19) [72] feature efficient absorption in the orange part of the spectrum (ε > 100 000 M−1 cm−1) and NIR phosphorescence (λmax 760–790 nm) which is fairly strong in case of the Pt(II) complexes (QY ~ 10%). The spectral properties are tuneable depending on the nature of the respective ligand. The Pt(II) complexes were shown to be promising indicators in oxygen-sensing materials and can be a good alternative to the porphyrin-based systems, particularly for low cost applications. However, lower BS of the indicators and lower photostability should be kept in mind. Papkovsky and co-workers investigated alternative sensing materials based on a metal-free diiodo-BODIPY dye 20 [73]. The iodination promoted inter-system crossing and broad phosphorescence peaking at 790 nm was observed additionally to the fluorescence of the BODIPY dye. Immobilization in polysulfone and polypropylene resulted in materials with rather high oxygen sensitivity due long phosphorescence decay times of about 300 µs.
3.2. NIR emitting dendrimers for oxygen sensing
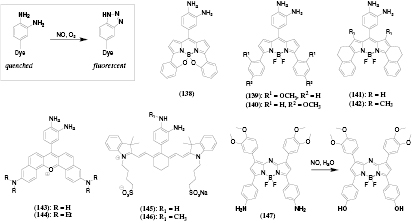
The NIR oxygen indicators described above were mostly applied for manufacturing of planar optodes (for imaging of oxygen distribution on flat surfaces) and fiber-optic sensors which proved to be excellent tools for application in research and industry. However, their application in vivo is limited due to high invasiveness and inability to measure in small objects such as cells. Dendrimeric oxygen probes (referred to as Oxyphors) developed by Vinogradov, Wilson and co-workers represent promising candidates for in vivo applications such as vascular imaging [74]. The first iteration of Oxyphors relied on generation 2 [75] and generation 4 [76] dendrimers (21) bearing polyglutamic chains in the meso-position of the porphyrin. The polymeric chains render the hydrophobic BP water-soluble, prevent aggregation of the dye, provide necessary protection from interfering species and control oxygen permeability in order to adjust the sensitivity to physiologically relevant range. For example, the dendrimers G2 and G4 absorbed at 632 nm and emitted phosphorescence at about 790 nm with rather high QY of about 10%, were highly soluble in blood plasma and showed quenching optimal for physiological measurements when pre-bound to albumin [75]. Although application of G2 for in vivo monitoring of oxygen distribution in growing tumours of rats was demonstrated [75] the dendrimers G2 and G4 showed some degree of core to core aggregation accompanied by diminished lifetime in water, and required pre-binding with albumin to achieve sensitivity matching physiological conditions. To overcome these the same group prepared several dendimers where the polyarylglycine dendrons were attached to both the three and five positions of the meso-phenyl ring rather than to the single position 4 of the phenyl ring (22) [77, 78]. Additionally, polyethylene glycol chains were placed on the periphery of polyarylglycine dendrons in order to minimize non-specific binding. Indeed, such modification ensured good protection and the probes did not interact with biological solutes. They can operate both in albumin-free (interstitial space) and albumin-rich (blood plasma) environments and were used for in vivo imaging of intravascular and interstitial oxygenation in murine tumors (figure 2(a)) [78]. Pt(II)-dendrimers prepared analogously to the Pd(II)-based probes demonstrated significantly better luminescence BS due to higher QYs approaching 30% for some dendrimers [77]. In the same work protected π-extended dendimers based on tetranaphthoporphyrin were prepared. The absorption of the Pt(II) complexes peaked at 699 nm whereas moderate phosphorescence (QY = 7%, τ = 12 µs) was observed at 923 nm.
Figure 2. (A) Imaging of intravascular oxygenation in a mouse using Oxyphor G4. The probe was injected via the tail vein to achieve a final concentration in blood plasma of ~4 µM. (a) Photograph of the anesthetized animal; (b) the zoomed-in region, as imaged by the ICCD camera with the tumor indicated by yellow arrow); (c) and (d) calculated false-color images of phosphorescence lifetime and oxygen partial pressure, respectively. Reproduced with permission from [78], copyright American Chemical Society. (B) Measurement of pO2 in cortical microvasculature. (a) Measured pO2 values in microvasculature at various depths, overlaid on the MIP image of the vasculature structure (gray). (b) Composite image showing a projection of the imaged vasculature stack. Reproduced with permission from [92], copyright Nature publishing group.
Download figure:
Standard image High-resolution imageNichols et al [79] presented an alternative route to the BP dendrimers which was assembled using the click reaction of azide with alkyne. Although the phosphorescence was relatively weak, the authors demonstrated the ability of the dendrimers to spontaneously penetrate spheroids (in contrast to Oxyphors which do not penetrate cells and multicellular structures) and therefore their high potential for oxygen imaging in 3D cancer models.
3.3. NIR dyes in oxygen-sensitive nanoparticles
In contrast to dendimers, the parent BP dyes such as 5 are highly hydrophobic and insoluble in water. To enable (intracellular) imaging these dyes can be embedded into water-dispersible polymeric nanoparticles. Similarly to the dendimeric shell, the polymer protects the dye from undesired interferences (e.g. ionic species) and determines oxygen sensitivity. This protection may be more efficient than in case of dendrimers, particularly in case of much larger microparticles, which is useful for measurements in the challenging environment of gastrointestinal tract [80]. Additional functional groups can be introduced on the surface of the beads, e.g. quaternary ammonium groups for cell penetration capabilities. Rather simple techniques such as nanoprecipitation [20] or staining by swelling [19] were used to prepare oxygen sensitive nanoparticles by immobilizing the Pt(II) complexes with 16 and 7 into cationic Rl-100 [70] and neutral poly(styrene-block-vinylpyrrolidone) beads [54], respectively. Pt(II) complex with 7 embedded into Rl-100 nanoparticles ('NanO2-IR' [81]) was used as cell-penetrating probe for monitoring of oxygen metabolism in the brain of mice following peripherial sensory stimulation of the whiskers. The work demonstrates high potential of the new probe for visualization and real-time analysis of sensory-evoked neural activity in vivo.
Napp and co-workers [82] prepared oxygen nanosensors by immobilizing the Pd(II) complex with 5 into amino-functionalized polystyrene nanoparticles (diamerer 100 nm) along with a fluorescent dye DY-635. The nanoparticles were suitable for both ratiometric and lifetime read-out modalities and could be further functionalized with polyethylene glycol and the monoclonal antibody herceptin. The binding of the nanobeads to HER2/neu-overexpressing tumor cells was confirmed in vitro. The authors demonstrated a distinct ratiometric in vivo response in tumors of a mouse upon inducing hypoxic condition by animal sacrifice.
Cao et al [83] prepared polystryrene nanoparticles via emulsion polymerization and subsequently embedded the complex of Pt(II) with 5 into the nanobeads by swelling in an organic solvent. The new nanosensors (diameter 350 nm) were applied for monitoring of bacterial growth and characterization of dose-response functions in microfluidics. Measurements of oxygen concentration and cellular autofluorescence were realized by a two channel micro flow-through fluorimeter, whereas the cell density was measured with a flow-through photometer.
Mistlberger et al [84] presented multi-functional nanoparticles (size ~185 nm) based on a copolymer of styrene and maleic acid which were doped with Pt(II) and Pd(II) BPs 7 and lipophilic magnetite nanoparticles. These oxygen-sensitive nanobeads could be collected and manipulated with a magnet. Covalent attachment of glucose oxidase to the surface of the beads enabled reproducible response to glucose (0.5 to 5 mM) monitored via oxygen consumption during the enzymatic reaction.
Fabricius-Dyg and co-workers demonstrated high potential of magnetic microparticles (size ~80 µm) based on Pt(II) complex with 7 for in vivo imaging of oxygen dynamics on uneven surface of a coral [85]. These microparticles were dispersed over the coral and fixed on its surface with help of a magnet. Measurements under increasing irradiance showed typical saturation curves of O2 concentration during the photosynthesis.
Kumar et al [86] embedded Pt(II) complex with 12 into phospholipid micelles (irregular shape, size ~100 nm) and used them for in vivo visualization of tumors in mice. The micelles were accumulated in tumors within 1–4 d after the injection which was clearly visible via imaging. Unfortunately, the BS of the dye in the beads was much lower (~20-fold) and the decay time much shorter compared to the dissolved indicator which can be attributed to aggregation of the indicator. This indicates the necessity of further optimization if the probe is intended to be used for oxygen quantification in vivo, and not solely for visualization of tumors.
Wang et al [87, 88] reported a new type of nanosensor based on a soft core rigidized with a silane reagent and an outer poly(ethylene glycol) shell. Oxygen indicators (Pd(II) tetraphenyltetrabenzoporphyrin 5 and conventional UV–vis dyes) were embedded into the core of the nanobeads, whereas the lipophilic shell enabled dispersibility and high stability of the small particles (diameter 12 nm) in various kinds of aqueous media. Importantly, no significant aggregation of the indicators could be noticed and the sensitivity was optimal for measurement at physiologically relevant concentrations. The particles were not internalized via endocytosis which made them promising for extracellular monitoring of oxygen concentration e.g. in blood, brain fluid or in microfluidic devices.
3.4. Two-photon oxygen probes
Two photon (2-P) spectroscopy became a popular technique due to high resolution, lower photobleaching of the probes, ability to use NIR excitation with most conventional fluorophores [89] and, particularly, because of higher penetration depth of the excitation light in tissues. Unfortunately, most of the conventional analyte-sensitive dyes are poorly suited for this method due to low 2-P absorption cross-sections. Although many efficient multi-photon emitters have been prepared in the last decade, the research was mostly focused on the development of inert fluorescent labels but not analyte-sensitive probes. In order to minimize scattering of the luminescence in vivo such probes should emit in the red or NIR part of the spectrum analogously to the down-converting probes described above (red excitation/NIR emission). Brinas et al designed several dendrimeric probes bearing a metalloporphyrin core and a shell containing 4–8 coumarin chromophores (24) [90]. Large amplification (~10-fold) of the phosphorescent signal (peaking at about 650 nm) upon 2-P excitation was observed due to the antenna effect of the coumarin and its higher 2-P absorption cross-sections compared to the porpyhrin. However, the phosphorescence QY decreased significantly (<7%) which was attributed to phosphorescence quenching via electron transfer. To improve the performance of the 2-P-probes the same group designed another polyarylglycine dendrimer modified with the coumarin 343 antenna and oligoethyleneglycol to prevent non-specific binding [91]. Importantly, the coumarin was placed at the maximal distance from the porphyrin core in order to reduce the interaction between the porphyrin and the coumarin. Indeed, the phosphorescence QY of the dendrimer was similar to that of the porphyrin bearing no coumarins. Efficient oxygen-sensitive phosphorescence (λmax = 680 nm) from the Pt(II) porphyrin was observed under 1P and 2-P excitation which was tested for intracellular imaging of oxygen distribution in the lifetime detection mode. The probe proved to be useful for high resolution in vivo oxygen imaging under 2-P excitation and a number of studies have been published recently. For example, it was applied for 3D imaging of oxygen tension in microvasculature and tissue at various depth (figure 2(b)) [92] and in deep cerebral vessels [93], for imaging of tissue oxygenation in the rat primary sensory cortex in response to sensory stimulation [94], for monitoring of oxygen in cortical microvessels before and after occlusion [95], for measurement of local oxygen concentration in bone marrow of live animals [96], etc.
In an alternative approach, a 2-P antenna (polyfluorene) was used as an energy donor for highly photostable oxygen-sensitive Pt(II) porphyrin [97]. Both dyes were embedded into polymeric Rl-100 nanobeads (diameter ~70 nm). Upon 2-P excitation (λmax 760 nm) the red emission from the porpyhrin was enhanced by 25–30 times compared to the probe containing no antenna. The positive charge of the beads enabled efficient staining of neurospheres. In a later work, Dmitriev et al [98] prepared conjugated polymer nanoparticles based on alternating fluorene and benzothiadiazole units with integrated Pt(II) BP and positively and/or negatively charged groups. Oxygen-sensitive NIR emission and the nanosensors could be read-out either in the ratiometric 2 wavelength mode (using residual emission of the conjugated polymer) or via measurement of the luminescence decay time. High BS of the probes under 2-photon excitation was due to the high 2-photon absorption cross-section of the conjugated polymer antenna and efficient energy transfer from the antenna to the oxygen indicator. It was demonstrated that the new nanosensors represent efficient tools for multi-modal (ratiometric intensity/lifetime, 1P or 2-P) O2 imaging with a broad range of cell and 3D tissue models such as tumor spheroids.
Asymmetrical dyes are expected to possess much higher 2-P absorption cross-sections than the symmetrical analogous so that the antenna may not be needed. Thus, Esipova and Vinogradov [99] prepared Pt(II) and Pd(II) complexes with dibenzo- and dinaphthoporphyrins (23). The dibenzo- and dinaphthoporphyrins featured strong red and NIR phosphorescence, respectively, and ~25-fold higher 2-P absorption cross-sections compared to symmetrical porphyrins which indicated their high future potential for imaging applications.
4. Monosaccharide probes
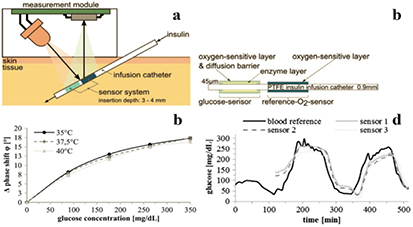
Optical glucose sensors exploit several concepts and are mainly based on glucose-binding proteins (conconavalin A), boronic acid receptors or make use of the enzyme glucose oxidase (GOx) [100]. The enzymatic systems are very selective; they rely on different transducers such as an amperometric hydrogen peroxide sensor or an electrochemical/optical oxygen sensor. New NIR oxygen indicators enable realization of a 'smart tattoo' [101] concept, i.e. subcutaneous glucose sensing which is the first step to creation of an artificial pancreas. Thus, Nacht et al [71] reported a novel glucose sensor positioned on the outer wall of an insulin catheter (figure 3). The sensor includes a layer of GOx cross-linked with bovine serum albumin which is covered by a polymeric layer containing poly(tert-butyl)styrene microparticles doped with Pt(II) BP ((7), figure 1). In presence of glucose oxygen is consumed which results in an increase of the phosphorescence decay time. The dynamic range of the sensor was 0–360 mg dl−1 glucose which covers the physiologically relevant ranges of hypo-, normo- and hyperglycemia. In order to compensate the glucose sensor for variation in pO2, another part of the catheter was coated with an oxygen sensor based on Pt(II) aza-benzoporphyrin 17 dissolved in polystyrene. The spectral properties of the indicators are significantly different, so that both information (glucose concentration and pO2) can be separated. Long-wavelength absorption and emission of both probes enables subcutaneous sensing. The in vivo measurements in pigs were performed with a specially constructed compact dual-channel phase fluorimeter and the obtained glucose levels were similar to the reference method (figure 3) thus demonstrating high potential of the sensor for continuous glucose monitoring in vivo.
Figure 3. Optical sensor system for subcutaneous glucose monitoring: (a) cross-sections of the sensor system for subcutaneous glucose monitoring; (b) cross-section of the catheter with integrated optical sensors; (c) response of the sensor to glucose in air-saturated aqueous solution; (c) comparison of reference blood glucose levels with system-generated data (120 min run-in time) for in vivo measurement in pigs. Reproduced with permission from [71]. Copyright Elsevier Science.
Download figure:
Standard image High-resolution imageIn an alternative approach the glucose binding properties of the apo-GOx were made use of (figure 4, left) [102]. An amino-dextran (which competes with the analyte for the binding sites of the apo-enzyme) was labeled with NIR AF-647 dye whereas the apo-GOx was stained with the QSY-21 quencher. Both components were embedded into alginate microspheres. A polyelectrolyte labeled with a NIR fluorophore AF-750 enabled ratiometic referencing. In presence of glucose a reproducible enhancement of the fluorescence intensity of Alexa Fluor-647 was observed. The dynamic range of the sensor was 0–540 mg dl−1.
Figure 4. Left: glucose sensing mechanism for the long-wavelength probe reported by Chaudhary et al [102]; right: NIR fluorescent saccharide probes based on boronic acids and possible sensing mechanism.
Download figure:
Standard image High-resolution imageThe boronic acid receptors profit from high chemical stability but most systems reported so far operate only in solution and are based on UV–vis receptors which cannot enable subcutaneous sensing. Saito et al [103] prepared a long-wavelength saccharide probe based on a squarylium cyanine dye bearing a boronic acid (figure 4, (25)). In aqueous solution, the dye forms soluble but non-emissive aggregates. In presence of a monosaccharide, the dye aggregates dissociate to give fluorescent monomers (λexc = 630 nm, λem = 660 nm). An 18-fold enhancement of fluorescence was observed in presence of fructose; similarly to other mono-boronic acid saccharide probes, the sensitivity to other saccharides was lower. The sensitivity was the highest at pH 10, which may be inconvenient for practical applications.
Lopez et al [104] described a new boronic acid-modified cyanine dye 26. In the absence of a saccharide this probe showed poor fluorescence in aqueous solution which was attributed to PET involving the piperazine-group, but is more likely to be due to quenching induced by aggregation. In presence of saccharide, but also some glycoproteins (particularly mucine) strong enhancement of NIR fluorescence was observed (λexc = 720 nm, λem = 820 nm). At pH 7.4, the highest sensitivity was observed for fructose and sorbitol, and the dynamic range of the probe was 0–300 mM for these analytes.
The group of Strano reported an assay for glucose based on NIR luminescence of carbon nanotubes [105]. Carbon nanotubes benefit from unmatched photostability and compatibility of the spectral properties with NIR 'optical window' but have only moderate luminescence QYs which are significantly lower than that of conventional fluorophores, particularly in aqueous solutions. They demonstrated that aromatic boronic acids adsorb on the nanotube side walls and induce fluorescence quenching (broad excitation 400–1000 nm, λmax em = 985 nm) due to PET (27). Fluorescence was recovered upon addition of glucose since quenching was disrupted by boronate ion formation. 4-chlorophenylboronic acid and 4-cyanophenylboronic acids were identified as the most promising ones due to fluorescence response in the physiologically relevant range. In fact, the fluorescence intensity was enhanced by a factor of 3.5 from 0 to 30 mM of glucose. The fluorescence quenching and recovery were reversible.
5. pH probes and sensors
Monitoring of pH is of great importance in many fields of science and technology. Measuring of intracellular pH is of particular interest due to complex regulation mechanisms in cells [106] and optical probes proved to be useful for this purpose [107]. Although up to date most of biological studies has been done with UV–vis probes, much progress in development of long-wavelength probes has been achieved in the last couple of years including genetically encodable fluorescent probes (chapter 13). The intracellular pH values in mammalian cells can vary greatly from slightly basic in mitochondria to acidic (pH 4.5–5.0) in lysosomes. pH 6.5–7.5 is optimal for growth of most bacteria, however, optimal pH for alkaliphiles is higher than 9 [108]. Since the dynamic range of optical pH probes rarely exceeds 3 pH units and the highest sensitivity is observed within 1 pH unit, it is evident that numerous probes are necessary to cover all potential applications.
5.1. Porphyrin-based probes
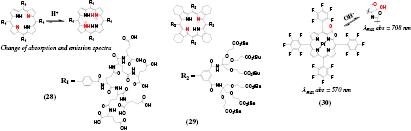
It is well known that the pyrrol nitrogen atoms of the metal-free porphyrins and their analogues can be protonated in acidic media to result in formation of a dication. Due to absorption in the red part of the spectrum (Q band) and emission in the red/ NIR, the metal-free porphyrins represent interesting candidates for designing NIR pH probes. Vinogradov and co-workers demonstrated that electrostatic stabilization of the core porphyrin charge by peripheral negative charges (carboxylate groups) is necessary to shift the intrinsically low pKa into the physiologically relevant range [109]. Polyglutamic dendrimers (figure 5 28) absorb in the red part of the spectrum (Q-band) and show fairly strong fluorescence (QY 18%) which shifts bathochromically from 647 to 685 nm upon protonation. Dendrimers based on tetrabenzoporphyrins have bathochromically shifted absorption and emission due to π-extension but lower QYs (~5%) [110]. Unfortunately, binding of metal cations by the carboxylate groups greatly affected the apparent pKa which shifted from 6.7 at very small ionic strength (~0.2 mM) to ~5.3 at 200 mM of K+ or already at 8 mM of Mg2+ [111].
Figure 5. Chemical structures of porphyrin-based pH probes.
Download figure:
Standard image High-resolution imageTo overcome the ion-sensitive behavior, the same group prepared a pH sensitive dendrimer based on a highly non-planar porphyrin [112] showing weak NIR fluorescence (~1%, table S2, ESI) (stacks.iop.org/MAF/4/042005/mmedia) (29). Strongly saddled conformation was responsible for the rather high pKa values of 7.8 and 6.0 attributed to the formation of a mono and a di-cation, respectively. The probe was useful for quantification of proton transport across phospholipid bilayers under excitation with blue light but excitation with red light was not very efficient. Non-symmetrical porphyrinoids may be more promising candidates for designing bright red light-excitable probes. For example, metal-free porphyrin ketones and particularly porpyhrin-diketones show rather bright pH-sensitive fluorescence (QYs about 30% [113] and 50% [114] respectively). Rather low pKa values can be expected from highly electron-deficient character of the macrocycle, which nevertheless may be fully adequate for many biotechnological applications.
An interesting approach was realized by Khalil et al [115] who observed a reversible nucleophilic attack of the OH− ions on an electron-deficient Pt(II) porpholactone 30. The characteristic band at 570 nm disappeared and the product absorbing in the NIR region (λmax = 708 nm) was formed. The inflection point was very high (12.6), making the system suitable only for some specific applications. The optical sensors showed slow response (~30 min) probably due to poor proton permeability of the polymer matrix used for the pH indicator.
Finally, some NIR-to-red UC pH sensing materials were reported by Esipova and co-workers [116]. The probe combined UC lanthanide nanoparticles (Yb3+ and Er3+-doped β-NaYF4) and red light-emitting polyglutamic porphyrin dendrimers which are highly soluble in aqueous solutions, have good biocompatibility and are not toxic. Excitation of ~40 nm large nanoparticles with NIR light (980 nm) resulted in red emission which was modulated in the emission–reabsorption mechanism, whereas the intensity of the green emission (540 nm) from Er3+ remained constant.
5.2. Probes based on cyanine dyes
Cyanines are popular NIR dyes with efficient absorption which makes them promising candidates for designing pH probes. Recently reported hemicyanine pH probes (31–34, figure 6) [26] are highly promising for ratiometric imaging due to high fluorescence BS for both protonated and deprotonated forms (table S2, ESI) (stacks.iop.org/MAF/4/042005/mmedia). The probe bearing benzothiazole (34) is particularly promising due to the largest shifts in the emission upon deprotonation (76 nm) which enables simple spectral separation of the fluorescence in ratiometric read-out. The pKa value is tuneable from 6.2 to 7.4 so that the dyes can cover wide range of medical and biotechnological applications. The authors demonstrated high potential of the probe for intracellular imaging and for imaging in vivo in small animals. Wan et al [117] synthesized a very similar fluorescent probe with dual NIR absorption and emission and lysosome-targeting capabilities due to morpholine introduced in the dye skeleton (35). The pKa value of 5.0 and high photostability make the new probe promising for monitoring of intracellular pH in acidic environment of lysosomes, but also for other applications.
Figure 6. Chemical structures of pH probes based on cyanine dyes.
Download figure:
Standard image High-resolution imageWiktorowski et al [118] designed a series of water-soluble pyrrolopyrrole cyanines for pH monitoring in highly acidic environment (36, 37). The chromophores are excitable at ~800 nm and possess very high ε (~200 000 M−1 cm−1) [119]. Only the form obtained via protonation of the methyloctylamino-group (pKa values 2.4–3.4) is fluorescent (λmax 790–825 nm). Despite presence of two PEG chains (MW 1000) in the molecule the probes showed high tendency to aggregate in aqueous solutions and the QYs were reduced ~10-fold to 1–3%. Such high dependency of the QY on the polarity of the environment is likely to make precise calibration in biological environment challenging.
A new type of cyanine-based probes was presented by He et al [120]. The pyrrol core rendered the dyes (38) pH sensitive in two different pH ranges. The pH increase from acidic to neutral resulted in an enhancement in fluorescence (λmax 639 nm) whereas further increase of the pH (7.5–11) resulted in the bathochromic shift of both absorption and fluorescence spectra which enabled ratiometric imaging of pH under basic conditions. The probes were demonstrated to be suitable for pH imaging in living cells and for real-time sensing of pH changes induced by alkaline phosphatase.
5.3. Probes based on BODIPYs and aza-BODIPYs
Because of their good fluorescence BS, BODIPYs are popular dyes for designing analyte-sensitive probes [121]. However, structural modification is necessary to shift the absorption and emission to longer wavelengths [122]. Zhang et al [123] presented a family of π-extended BODIPY dyes (λmax abs 670–710 nm) bearing piperazine groups (figure 7, 39–41). These groups are responsible for almost complete quenching of fluorescence at physiological pH, which is attributed to PET. The probes emit in NIR (λmax 716–760 nm) in acidic media when the piperazino group is protonated. Six oligo ethyleneglycole chains are necessary to provide sufficient hydrophilicity and avoid aggregation in aqueous media (QY 16% for 41), whereas QYs are significantly lower for the derivatives with 4 and 2 chains. The estimated pKa values (2.9–3.57) are surprisingly low for the piperazino group. Intracellular measurements demonstrated that the new probes can target lysosomes and are therefore potentially useful for monitoring of lysosomal pH. Unfortunately, the cellular uptake of the larger probe 41 was about 10-fold less efficient than for the probe 39.
Figure 7. Chemical structures of long-wavelength pH probes based on BODIPYs and aza-BODIPYs.
Download figure:
Standard image High-resolution imageCompared to BODIPYs, the BF2-chelated tetraarylazadipyrromethenes (aza-BODIPYs) possess bathochromically shifted absorption and emission spectra, which makes them attractive for designing NIR pH probes. The probe reported by Murtagh et al (42) [124] features efficient absorption and emission in NIR. The fluorescence was quenched upon deprotonation of the phenolate due to PET (pKa ~ 6.9). Simultaneously, the absorption shifted from 700 to 775 nm upon deprotonation. The parent dye also contained the alkyne rest which enabled further functionalization with a saccharide or carboxyl-group via alkyne-azide cycloaddition reaction. Jokic et al [125] demonstrate that the pKa value of aza-BODIPYs (43) can be systematically tuned (7.0–8.9) by introducing electron-donating (alkyl, alkoxy-groups) or electron-withdrawing (chlorine) substituents into the dye. All the indicators showed outstanding photostability greatly exceeding that of the state-of-the-art NIR pH indicators such as SNARF. The authors immobilized the dyes into polyurethane hydrogel D4 to prepare fiber-optic sensors. In vivo measurement in the gastric cavity of a coral showed the decrease of the pH value with increasing distance from the mouth opening. In a more recent work by another group [126], symmetrical dihydroxy-aza-BODIPYs were synthesized and applied as intracellular pH probes. After short incubation time, the compounds were internalized across the cellular membrane and accumulated in cytoplasm. Clearly, the excellent photostability of the probes is advantageous for microscopic applications.
Schutting et al [127]. demonstrated that aza-BODIPYs are also valuable indicators for monitoring of carbon dioxide. Symmetrical dyes (44) bearing two phenolates showed two deprotonation equilibria in presence of tetraoctylammonium hydrogencarbonate when dissolved in ethyl cellulose. The doubly deprotonated form (characteristic for low pCO2) and mono-deprotonated form (high pCO2) absorbed at 780–830 nm and 720–760 nm, respectively. An inner-filter effect read-out system was used in order to convert the absorption changes into referenced luminescence information. All the sensors profited from high photostability of the aza-BODIPYs and tuneability of the pKa. Ultra-sensitive sensors based on the CH3-substituted phenol resolve far below the atmospheric carbon dioxide levels (LOD ~ 0.007 kPa pCO2) whereas the sensors operating from 1 to 100% CO2 make use of the Cl-substituted phenol.
5.4. Probes based on other chromophores
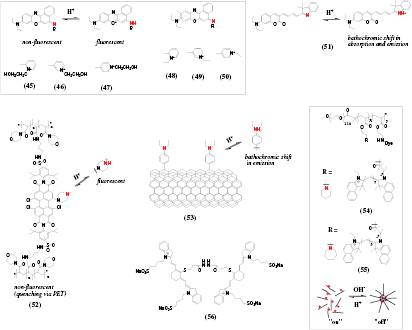
A family of water-soluble NIR pH probes based on benzo[a]phenoxazine was introduced by Liu et al [128].The dyes (figure 8, 45–47) possess electron-withdrawing aromatic group attached to the nitrogen of the protonable imino group. Only the protonated form is fluorescent albeit with moderate QYs of 1–2%. The pKa of the probes can be tuned over a broad range by varying the position of the electron-withdrawing positively charged substituent: pKa of 2.7, 5.8 and 7.1 for the dyes 45–47, respectively. He et al [129] designed similar dyes which upon protonation featured either hypsochromic shift of the absorption in case of ortho- and para-substituted dyes (48 and 50, respectively), or a bathochromic shift in case of 49, which lacks conjugation. Interestingly, the protonated forms possess rather strong fluorescence (QY 12–29%), which is in contrast to the results of Liu et al [128] obtained for very similar dyes.
Figure 8. Chemical structures of long-wavelength pH probes based on different chromophores: phenoxazines (45–50), coumarins (51), perylenes (52), carbon nanotubes (53) as well as the probes based on pH-responsive polymers (54, 55) and an irreversible probe for monitoring of acidosis (56).
Download figure:
Standard image High-resolution imageAnother Nile blue derivative was used for pH-dependent modulation of the UC emission (λexc 980 nm) of NaYF4 : Er, Yb nanorods where both components were embedded into plasticized polyvinylchloride membrane [130]. Measurement in blood showed that the green emission (542 nm) was not analytically useful due to reabsorption of the light by hemoglobin but the red emission (656 nm) was suitable for measurement of pH from 6 to 9 (pKa 8.5). The background fluorescence was completely eliminated and the sensor showed reproducible response. Similarly to other UC systems, calibration of the sensor is likely to be challenging due to non-linear dependency of the emission intensity on the intensity of excitation light.
A π-extended coumarin dye (51) [131] showed long-wavelength absorption and emission for the protonated form (572–700 nm and 722 nm, respectively) which shifted hypsochromically upon deprotonation. Relatively low pKa (3.9) enables measurement of pH in acidic microenvironment. The indicator showed no interferences from common metal ions and amino acids.
Aigner et al [132] prepared a perylene bearing an N-methylpiperazine group in the bay position (52) which was responsible for almost complete quenching via PET. The absorption spectrum (λmax ~ 660 nm) changed only slightly upon protonation of piperazine, but the NIR fluorescence (λmax 740 nm) was greatly enhanced (QY ~ 25%). A functionalized dye was photopolymerized with acryloylmorpholine and a cross-linker to result in a pH-sensitive hydrogel. The sensor showed high photostability typical for perylenes and highly reproducible response due to complete elimination of migration and aggregation of the dye. The pKa value of ~6.0 made the new sensors promising for biotechnological applications.
Kwon and co-workers [133] presented a pH probe whose emission matches the second optical window (1100–1400 nm). Carbon nanotubes were covalently functionalized with diethylaniline (53) which resulted in a new defect photoluminescence. The long-wavelength band shifted from 1117 to 1136 nm at acidic pH which enabled sensing pH with moderate resolution of 0.2 units (pKa 6.28). A dedicated set-up including emission monochromator and photodetector sensitive in this part of NIR spectrum may be a limiting factor for practical applications.
A very interesting approach was presented by Zhou et al [134]. They designed a library of pH-sensitive nanoparticles based on common pH-insensitive dyes covalently attached to a block copolymer capable of pH-induced micellization. The conjugates were highly fluorescent in acidic media, and micellization at higher pH greatly enhanced quenching of the dyes via homoFRET mechanism. The size of the unimers was about 3 nm, whereas the formed micelles were 24 nm in diameter. Two NIR cyanine dyes were used along with UV–vis fluorophores. In contrast to common small molecule pH indicators, the 'on–off' behavior was achieved within 0.25 pH units, which potentially enables ~10-fold enhancement in the resolution. Importantly, the pH transition can be controlled within the range of 5.0–7.4 by using different amines in the block copolymer.
5.5. Irreversible probes for imaging of acidosis
The probes described above respond fully reversibly to pH changes. However, in some applications it may be sufficient to monitor the pH rise. Thus, Wang and co-worker developed NIR probes for visualization of acidosis in tumors which can be useful for non-invasive prediction of the tumor metastasis potential [135]. A non-fluorescent cyanine dimer 56 forms fluorescent monomeric species (λmax 810 nm) upon hydrolysis of acid liable hydrazone bond. The same principle was used to develop the acidosis probe by conjugating multiple fluorophore molecules to a dextran [136].
6. Probes for metal ions
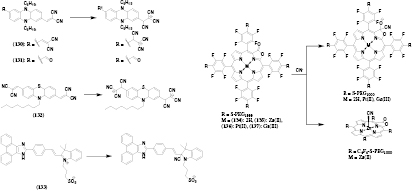
Optical measurement of various biologically relevant metal ions is of great interest in many applications. Especially for imaging applications the deeper light penetration, lower background fluorescence and reduced scattering are beneficial. Despite vital role of alkali and alkaline metal ions in physiological processes in living organisms virtually all probes for these analytes rely on UV–vis dyes [1]. Some of NIR probes reported prior to 2012 including those for potassium [137] and calcium [138] represent a notable exception. Only a few long-wavelength probes for calcium have been reported recently, both based on fluorophores [139, 140] and genetically encoded fluorescent proteins (chapter 13). On the other hand, numerous probes for heavy metal ions have been published. However, the solubility of many reported probes in water is limited and they are utilized in mixtures of organic solvents with water; these probes are only suitable for in vitro assays but not intracellular and in vivo measurements. Incorporation of the indicators in water dispersible beads [141] is an elegant way to overcome poor aqueous solubility. Main detection mechanisms rely on complexation [142–146], spirocyclate opening [147–151] and cleavage of quenching groups [152–154].
6.1. Complexation-based probes
Complexation of the analyzed cation by a fluoroionophore was a principle of choice in many metal cations probes. Various different ligands for zinc [142, 155], copper [144], silver [143], mercury [145], calcium [135–137], and aluminum [146] were developed (figure 9).
Figure 9. Chemical structures of complexation-based probes for metals.
Download figure:
Standard image High-resolution imageA nanoprobe for quantification of Ca2+ in cells (57) designed by Si et al [141] used a commercially available calcium sensitive dye (λem 580 nm) and a referenced fluorphore (λem 670 nm) incorporated in polyacrylamide beads which protected the dyes from undesired interactions with cellular components. Collot and co-workers [139] developed three calcium probes (58–60) based on a rhodamine dye and differently modified 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) based ligands. All three probes have the absorption and emission maxima at 586 nm and 604 nm, respectively. Notably, their Ca2+ affinity is tuned by the different substituents on the BAPTA-ligand. All three probes show a drastic increase in QY upon binding of calcium (~0.005 to ~0.55, table S3, ESI) (stacks.iop.org/MAF/4/042005/mmedia) and an approximately ten-fold increase in fluorescence lifetime (~0.2 to 3.6 ns). Furthermore the probes are 2-P excitable (λmax 912 nm) which was enabled in vivo imaging of calcium in the cortex of rats. Additionally, an azido group enables covalent coupling of the probes to various biomolecules which may be useful for design of subcellulary targeted probes.
Similar probes were designed by modification of Si containing fluorescein analogues with BAPTA (61–63) [140]. The probes showed absorption maxima at 585 and 597 nm and emission maxima at 603 nm and 607 nm for 61 and 62, respectively. The QYs of these probes increased from 0.066 to 0.37 and 0.024 to 0.39, respectively, after addition of 39 µM calcium. Both probes showed pH dependency but the pKa of the chlorine substituted probe 62 was 5.1 which reduced the interference at physiological conditions. This probe was further modified with acetoxymethylester groups (63) which rendered it cell permeable.
Zhang et al [155] developed a Zn2+ turn-on probe 64 based on a phenoxazinium dye coupled to a 2-aminoethyl-bis(2-pyridylmethyl)amine acting as a PET quencher. Red fluorescence is recovered upon binding of Zn2+. Cd2+, Ni2+ and Cu2+ were found to interfere significantly but other cations showed no effect. Another Zn2+ probe was based on a squaraine dye bearing two 2,2'-bipyridine groups as ligands (65) [142]. Complexation of Zn2+ leads to a small bathochromic shift of the emission (from 698 to 720 nm) and an enhancement of fluorescence intensity. Unfortunately, many other metal cations also cause spectral shifts or quenching of the fluorescence.
Li and co-workers [143] developed a selective Ag+ probe (66) based on a cyanine dye and piperazine as recognition unit. Upon binding of Ag+ the absorption and fluorescence spectra showed a blue-shift of ~200 nm which was attributed to a reduction of a dye carbon after coordination of Ag+. A cyanine dye containing 2-aminoethylpyridine (67) was used to detect Cu2+ in sub-micromolar range (LOD 93 nM) [144]. Complexation resulted in reduction of absorbance at 643 nm and quenching of fluorescence at 715 nm. A similar cyanine dye bearing a N-(3-aminopropyl)-imidazole (68) [146] was not fluorescent due to PET from imidazole. Coordination of Al3+ to imidazole resulted in a strong fluorescence increase at 730 nm with a low LOD of 19.5 nM. This reaction was selective for Al3+ and no response for various other metal cations was found. Imaging of Al3+ in cells was demonstrated. Furthermore the probe was useful for measurements of deoxyribonuclease activity.
An extended BODIPY dye modified with a mercury specific ligand (69) [145] was used for imaging in HeLa cells. The dye showed a strong fluorescence enhancement at 637 nm and a shift of the absorption maximum from 548 nm to 591 nm. No cross-sensitivities to other metal cations were found but the dye showed strong pH-dependent emission and absorption properties which limits its practical applicability.
6.2. Probes exploring a cleavage of a trigger group
Palladium is widely used in industry and can tightly bind biomolecules potentially disturbing many biological processes. Several probes for palladium used specific cleavage of a trigger group (unsaturated ether or ester) bound to a NIR fluorophore (figure 10). After the catalytic cleavage a strong fluorescence enhancement and a spectral shift can be observed. This reaction proved to be very selective for all the presented probes and no sensitivity to other metal cations was observed. Particularly, the probes based on a novel NIR chromophore (70 [152],) and cyanine dyes (71 [153], 72 [154]) were prepared and used for detection of the analyte in HeLa cells and in vivo [153]. In case of 72 a huge hypsochromic shift of absorbance from 740 to 540 nm and fluorescence from 825 to 660 nm enabled ratiometric colorimetric and fluorimetric measurements, as well as naked eye detection.
Figure 10. Chemical structures of the palladium probes based on the cleavage of a trigger group.
Download figure:
Standard image High-resolution image6.3. Metal probes based on spirocyclization of chromophores
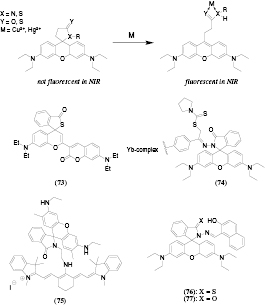
Another mechanism for metal ion detection is the opening of spiro-rings (which disrupt the π-conjugation in a dye) resulting in recovery of the chromophore, most commonly a rhodamide or a rhosamine (figure 11). This leads to a strong fluorescence enhancement accompanied by a red shift in the emission and absorption spectra. The method was mostly used for mercury [148, 149, 151] and copper sensing [147, 150]. The LODs are typically in nanomolar range and selectivity against other metal ions is good. Thus, opening of the thiolactone spiro ring (73, [148]) in presence of Hg2+ causes a red-shift of the absorption (from 425 to 655 nm) and fluorescence (from 480 nm to 695 nm) which can be used for ratiometric read-out. Suitability of the probe for intracellular imaging of Hg2+ (HeLa cells) was demonstrated. In another Hg2+ probe a rhodamine was coupled to a NIR emitting Yb(III) complex (74, [149]). Ring opening causes a 8-fold absorption increase at 570 nm and a strong fluorescence enhancement at 590 nm and 1030 nm. A similar strategy was used by Xu et al [150] who coupled a rhodamine spyrolactam to a cyanine dye (75). In the closed form, fluorescence from cyanine (λmax 732 nm) is observed. The fluorescence is quenched after opening of the ring in presence of Cu2+ due to PET from the cyanine to rhodamine. Simultaneously, the rhodamine emission increases at 549 nm, enabling ratiometric read-out of the probe.
Figure 11. Chemical structures of the metals probes exploring spirocyclization of chromophores.
Download figure:
Standard image High-resolution imageUC lanthanide nanoparticles were added to the solution of Hg2+ probes based on rhodamine (76, 77) [151] to enable NIR excitation (980 nm). The emission of the nanoparticles (λmax 539 nm) decreased upon opening of the spiro-ring due to increase of absorption at ~530 nm. Simultaneously, fluorescence from the rhodamine (λmax ~ 580 nm) was enhanced which enabled ratiometric read-out. Among the tested metal ions, Cu2+, Ag+, Fe2+ and Fe3+ were found to interfere, and the cross-sensitivity was found to be more pronounced for 77 than for 76. Similar spectral changes were observed for a Cu2+ probe (78) obtained by loading a copper sensitive rhodamine B hydrazide onto silica-coated NaYF4 UC nanoparticles [147].
7. Probes for hydrogen sulfide and (bio)thiols
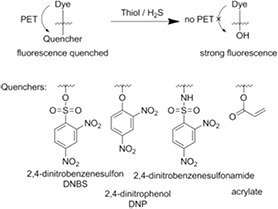
Measurement of various (bio)thiols with optical probes has gained increasing attention recently. Optical probes offer many advantages like low limits of detection, remote read-out and the possibility of imaging. In contrast to oxygen or pH probes the detection reactions for hydrogen sulfide or thiols are irreversible and therefore the probes can only act as dosimeters.
Currently, the most commonly employed measurement principle utilizes thiolysis of quenching groups connected to a dye molecule (figure 12). The quenching is very efficient, so that virtually no fluorescence is observed in the absence of the analytes. Upon cleavage of these groups by thiols or hydrogen sulfide a highly fluorescent dye is generated. Due to the turn-on behavior the LOD for these systems is often in the low µM range and can be as low as 5–10 nM [156] for certain systems (table S4, ESI) (stacks.iop.org/MAF/4/042005/mmedia). 2,4-dinitrobenzene derivatives are common quenchers; they are often attached to various dyes as ethers [157–159], sulfonyl-esters [160, 161] or sulfonyl-amides [162, 163]. Besides 2,4-dinitrobenzene also other cleavable quenching groups were utilized including acrylate [164–166], 2-carboxybenzaldehyde [156], nitroazo aryl ether [167] and 4-methoxythiophenol [168]. Furthermore probes with different detection mechanisms like reaction with an aldehyde [164, 169] or spirocyclization after an initial substitution reaction [170] were developed. In general the selectivity of this method is often not high and many thiols are detected simultaneously [160, 161]. However, many selective probes for hydrogen sulfide [156–159, 171–181], GSH [167, 168], the combination of Cys and Hcy [165, 168, 182, 183], Cys [164, 166], Hcy [169], aromatic thiols [162, 163] and polysulfides [184, 185] were also developed.
Figure 12. General mechanism for optical probes for hydrogen sulfide and thiols based on cleavage of a quencher group.
Download figure:
Standard image High-resolution image7.1. Probes based on cleavage of a quencher group
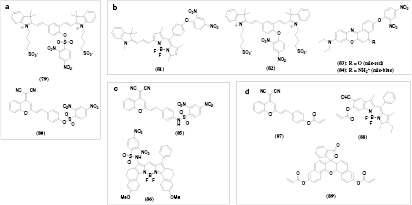
The cleavage of a 2,4-dinitrobenzensulfonyl-group (DNBS) was used for detection of biothiols like GSH, Cys or Hcy. Generally, it was not possible to distinguish between the thiols, but no cross-sensitivities to many metal cations [160] and non-thiol amino acids [160, 161] were observed. For example, a cyanine dye with an attached DNBS-group (figure 13, 79) [160] exhibits strong fluorescence (λmax 695 nm) in presence of GSH, Cys or Hcy. The probe was useful in pH range of 6.5–8 and its applicability was demonstrated by measurements in fetal bovine serum. Another probe for the same thiols utilized dicyanomethylene-benzopyran chromophore modified with a DNBS-group (80, [161]). Low LODs (18 nM) were obtained and applicability of the probe for intracellular measurements was demonstrated. However, the released fluorescent dye (λmax 690 nm) shows pH-dependent emission between pH 5.7 and 8 which can be a serious drawback.
Figure 13. Chemical structures of optical probes for hydrogen sulfide and thiols based on cleavage of a quencher group: (a) 2,4-dinitrobenezenesulfonyl-ester; (b) 2,4-dinitrophenyl-ether; (c) 2,4-dinitrophenylsulfonamide and (d) acrylate ester.
Download figure:
Standard image High-resolution imageCompared to DNBS, 2,4-dinitrophenyl-ether (DNP-ether) forms a more stable ether bond which is efficiently cleavable by more reactive hydrogen sulfide, although GSH, Cys, Hcy can also interfere. A π-extended BODIPY dye modified with DNP-ether (81) [157] showed a 18-fold enhancement of NIR fluorescence (table S4 ESI) (stacks.iop.org/MAF/4/042005/mmedia) after cleavage of the quencher by NaHS and reached a LOD of 50 nM. Various cations, anions, reactive oxygen species and reducing agents were tested and caused very little response but GSH and Cys caused a significant fluorescence increase. Cross-sensitivity to these thiols was significantly lower for a probe based on a cyanine dye 82 [158]. The cleavage oft DNP-ether resulted in a reduction of fluorescence intensity at 555 nm and a strong enhancement of the emission at 695 nm which enabled ratiometric measurements. The probe based on Nile red 83 [159] showed an enhancement of fluorescence (666 nm) after reaction with hydrogen sulfide, whereas the fluorescence was quenched for a similar probe based on Nile blue 84. The probes were applied for imaging of hydrogen sulfide in HeLa cells.
Because of high stability of amide bonds, the selectivity toward more reactive aromatic thiols can be achieved by using 2,4-dinitrobenzensulfonamide. For instance, only aromatic thiols (thiophenol as model analyte) caused large fluorescence enhancement (670 nm, LOD 0.15 µM) of the intracellular probe based on a dicyanomethylene-benzopyran dye 85 [163] whereas Cys, Hcy, GSH, other amino acids and anions did not interfere. A BODIPY-dye probe 86 [162] possessed similar characteristics (high selectivity to aromatic thiols, fluorescence enhancement at 633 nm) with much better LOD of 37 nM but the addition of a detergent was needed for better solubility of the probe.
Several thiol probes utilized the quenching ability of acrylate. The acrylate moiety attached to a chromophore is cleaved in a two step reaction. First, the thiol reacts with the double bond and in a second reaction the nearby amine group of Cys or Hcy cleaves the ester bond and forms a 7 or 8 membered ring for Cys or Hcy, respectively. Such probes do not respond to GSH since it has no amino group near its thiol group and cannot complete the cleavage reaction. For example, strong enhancement of NIR fluorescence was observed upon reaction of Cys or Hcy with a probe based on a cyanomethylene-benzopyran dye (87) [165]. Cross-sensitivities to biothiols, other amino-acids, anions, hydrogen sulfide and reactive oxygen species were found to be negligible.
Combination of an aldehyde and an acrylate groups in a π-extended BODIPY-dye 88 [164] allowed to achieve selectivity for Cys over Hcy and GSH. First, Cys forms a thiazolidine ring with the aldehyde group, then the thiol group of another molecule of Cys reacts with the acrylate double bond and finally the acrylate is cleaved under cyclization of the Cys with the acrylate. All these reaction steps are required to obtain the active dye with strong ESIPT-type (excited state intramolecular proton transfer) fluorescence. Other advantages of this probe are the large Stokes shift of 112 nm and cell permeability. The probe based on a naphthofluorescein dye 89 [166] is selective to Cys over Hcy due to faster reaction kinetics with the former. The probe was applied for selective detection of Cys in HeLa cells.
Other quenching groups were also found to be useful for designing probes for hydrogen sulfide and thiols. Although reduction of the m-nitrophenol was proposed as mechanism for an intracellular cyanine-based probe (figure 14, 90) [176], it is more likely that fluorescence enhancement (755 nm) is due to cleavage of the phenylether similarly to the dinitrophenol-based probes. The probe developed by Wang et al (91) [156] relies on a two-step reaction of 2-carboxybenzaldehyde ester with hydrogen sulfide and biothiols. In a first reaction step the analyte reacts with the aldehyde group which results in quenching of the NIR fluorescence. In the second step the bound hydrogen sulfide attacks the linking ester bond and cleaves the 2-carboxybenzaldehyde from the dye. Thereby a new weaker fluorescence peak at 625 nm appears which enables ratiometric measurements. The LOD was 5–10 nM. 7-nitro-1,2,3-benzoxadiazole groups (92) can also be selectively cleaved by hydrogen sulfide [177] thus generating fluorescent naphthofluorescein (λmax 662 nm).
Figure 14. Chemical structures of optical probes for hydrogen sulfide and thiols based on cleavage of the quencher groups other than those shown in figure 13.
Download figure:
Standard image High-resolution imageGao et al [184, 185] reported two probes selective for polysulfide and showing no response to sulfur-containing biomolecules. A quencher (p-nitrofluorobenzoate) was attached to an aza-BODIPY dye (93, 94). In the first step, the fluorine is substituted by a polysulfide and then a second sulfur atom of the polysulfide attacks the ester bond and cleaves the quencher group. This causes a bathochromic shift of the absorbance and enhancement of NIR fluorescence which was used for imaging was in cells and in living animals.
7.2. Probes based on a substitution of a trigger group
These probes utilize a substitution of a trigger group by the thiol group of the analyte (figure 15) resulting in formation of a thioether in the first stage. In an optional second reaction step the thioether is replaced by the nearby amino group which is only possible for Cys and Hcy but not for GSH. These analytes can be distinguished since the spectral properties of the thioether and amine are significantly different. Additionally, the faster reaction kinetics of Cys can enable discrimination of Cys over Hcy. For example, strong fluorescence enhancement (λmax 830 nm) was observed for the cyanine dye modified with nitrophenol (95) [186] after reaction with Cys, Hcy or GSH. However, only Cys underwent a fast replacement reaction resulting in the formation of fluorophore emitting at 750 nm. Similarly, the probe (96) [168] based on a pyronin dye and 4-methoxyphenol formed green light-emitting amino-derivative after reaction with (homo)cycteine, whereas the emission of the thio-derivative obtained via reaction with GSH was located in the red and NIR parts of the spectrum. Nitroazoaryl ether as PET quencher was also found to be useful [167]. Only GSH adduct formed from (97) showed strong fluorescence (λmax 810 nm) which allowed its detection without interference from Cys and Hcy.
Figure 15. Chemical structures and sensing mechanism of optical probes for thiols based on substitution of a trigger group.
Download figure:
Standard image High-resolution image7.3. Probes exploring reactions with an aldehyde
Another method of selective Cys and Hcy detection relies on their reaction with an aldehyde resulting in formation of a 5- or 6-membered ring (figure 16). GSH and hydrogen sulfide do not undergo this reaction. For example, relatively weak NIR fluorescence (QY 3.6% at 778 nm) of (98) [182] is significantly enhanced only after reaction with Cys and Hcy. The LOD was determined to be 7.9 nM and imaging in living cells was demonstrated. Similarly, an 9-fold and 30-fold enhancement of fluorescence (λmax ~ 680 nm) was observed for a BODIPY probe (99) [169] after reaction with Cys and Hcy, respectively, whereas the effect of other amino acids was very weak.
Figure 16. Chemical structures and sensing mechanism of aldehyde-modified optical probes for thiols.
Download figure:
Standard image High-resolution image7.4. Reduction of azide
The reduction of azide groups to amines is another frequently utilized principle of hydrogen sulfide detection (figure 17). The reaction can lead to turn-on [173, 174] and turn-off [172, 175] signals as well as the subsequent cleavage of other quenching groups [179]. A probe based on a dicyanomethylene-benzopyran dye 100 simultaneously reported by Sun et al [173] and Zheng et al [174] showed strong enhancement of red fluorescence after reaction with hydrogen sulfide but no effect of other biologically relevant sulfur species. It was useful for imaging in cells and in living animals particularly due to suitability for two photon excitation. In case of for an intracellular probe based on a cyanine dye 101 [179] hydrogen sulfide caused a decrease of fluorescence at 744 nm and an enhancement at 810 nm thus making the probe suitable for ratiometric imaging. On the contrary, fluorescence quenching was observed for a BODIPY dye (102) modified with two azide groups after their reduction to amine [175]. The LOD was 0.34 µM and imaging in MCF-7 cells was demonstrated. An aza-BODIPY dye modified with two azide groups (103) showed similar behavior [172]. Apart from fluorescence quenching (λmax 750 nm) the absorption spectrum changed significantly, which enabled colorimetric read-out. Interestingly, the reaction product is in itself a probe for nitric oxide (see chapter 11).
Figure 17. Chemical structures and sensing mechanism of optical probes for hydrogen sulfide based on reduction of azide group.
Download figure:
Standard image High-resolution image7.5. Probes exploring other detection mechanisms
More specific mechanisms were also found to be useful for detection of hydrogen sulfide and thiols (figure 18). Zhang et al [187] used lanthanide-doped UC phosphors (λexc 980 nm) modified with dopamine-quinone (104) acting as fluorescence quencher. In presence of GSH the quinone was reduced to dopamine which resulted in enhancement of the UC fluorescence at ~550 and 670 nm. Selective addition of hydrogen sulfide to the indolinium unit of a π-extended coumarin 105 [181] disrupted the conjugation and leaded to a strong decrease of absorbance at 588 nm and fluorescence at 652 nm. The probe preferably targeted mitochondria and showed quick response for intracellular hydrogen sulfide. Similarly, nucleophilic addition of (homo)Cys to the enolate double bond of squaraine 106 [183] resulted in a decrease of NIR absorption and emission and no interference from GSH or other amino acids was observed. This rather sensitive probe (LOD ~ 60 nM) was utilized for intracellular imaging of biothiols.
Figure 18. Reactions of the probes for hydrogen sulfide and thiols based on the mechanisms not covered in 7.1–7.4.
Download figure:
Standard image High-resolution imageThe NIR emission (λmax 694 nm) of a benzopyrylium dye 107 [170] shifts to 474 nm after selective reaction with (homo)cysteine. Cleavage of the 3,5-bis(trifluoromethyl)-benzenethiol group is followed by intramolecular spirocyclization which disrupts the conjugated system. Wu et al [180] developed two hydrogen sulfide probes based on the removal of Cu(II) from the cyclen complex attached to a BODIPY dye. This promotes NIR fluorescence of the probes (λmax = 765 and 680 nm for 108 and 109, respectively) which are virtually non-emissive in the absence of the analyte. Due to long-wavelength absorption and emission, the probes were promising for imaging in cells and particularly in living animals.
Finally, Wang et al [188] and Yu et al [178] developed probes for reactive oxygen species based on the oxidation of selenium and tellurium (chapter 8). These oxidation reactions are reversible by hydrogen sulfide [188] and GSH or Cys [178], which can be employed for their sensing. Photophysical properties of the probes for hydrogen sulfide and thiols are summarized in table S4 ESI.
8. Probes for reactive oxygen species (ROS)
ROS play an important role in cell metabolism but also cause cell damage during oxidative stress. Overproduction of ROS is an important indicator of various pathological processes. Since 2012 several long-wavelength probes based on cyanine [22, 178, 189, 190] and BODIPY [188] dyes were reported (table S5 ESI) (stacks.iop.org/MAF/4/042005/mmedia). Although these probes generally react irreversibly with the dyes and function as dosimeters, they can be regenerated by reaction with thiols or hydrogen sulfide (figure 19). In fact, this property can be utilized to design probes for these analytes (chapter 7). Oxidation of selenium or tellurium atoms by ROX resulting in enhancement of fluorescence is the most common detection principle. For instance, oxidation of tellurium in a cyanine dye 110 [178] by peroxynitrile results in strong fluorescence enhancement (λmax 820 nm). The reaction is rather selective against other ROX and can be completely reversed by GSH or Cys. NIR absorption and emission make it possible to visualize the redox state of cells and living animals. Similarly, oxidation of selenium in a BODIPY-based probe 111 [188] by hypobromous acid results in appearance of strong red fluorescence and can be reversed by hydrogen sulfide which can be utilized for detection of the latter. The probe is selective against other ROX and only hypochlorous acid interferes. A cyanine dye modified with a selenomorpholine 112 [189] shows strong fluorescence enhancement in aqueous solution after oxidation with hydrochlorous acid due to disruption of non-fluorescent aggregates. The probe is selective against hypobromous acid and other ROX and was suitable for in vivo imaging. A probe for investigation of the redox status of cells, determined by GSH and hydrogen peroxide, utilizes ebselen as a reporter 113 [22]; the 5-membered ring is reductively opened in presence of GSH. This highly selective process results in quenching of NIR fluorescence (λmax 794 nm) of the probe and can be reversed by hydrogen peroxide whereas other ROX do not interfere. Another remarkable feature is localization of the probe in mitochondria. Thus, imaging of hydrogen peroxide generation in induced apoptotic cells and in wounds of young zebrafish larvae was demonstrated.
Figure 19. Chemical structures and reactions of long-wavelength probes for reactive oxygen species.
Download figure:
Standard image High-resolution imageSun et al [190] reported a probe for hypochlorous acid which does not contain selenium or tellurium (114). Oxidation of a double bond promoted by the electron-donating amine leads to disruption of the conjugation in the cyanine dye. This results in quenching of NIR fluorescence and ~200 nm hypsochromic shift of the absorption. The probe was selective against other ROS which enabled measurement of myeloperoxidase activity catalyzing the conversion of hydrogen peroxide and chloride into hypochlorous acid.
9. Probes for monitoring of enzyme activity
Measurements of enzyme activity are of particular interest since increased activity of certain enzymes can be a good indicator for malfunctioning cells. Long-wavelength probes enable in vivo imaging, e.g. in tumors. Cyanines are most commonly utilized [190–200], but other emitters including hemicyanines [201, 202], squaraines [203] or semiconductor quantum dots [204] were also found to be useful. Enzyme catalyzed cleavage of a peptide linker between a fluorescent dye and a quencher [191, 192, 198–200] is one of the most popular strategies. Other methods are based on reduction of nitro groups [194, 195], detection of enzyme generated reactive oxygen species [190] and cleavage of an ICT group [192]. The detected enzymes were nitroreductase [194, 195, 201], cathepsin B [192, 199], cathepsin S [197], metalloproteases [193, 200], FAPα [198], β-lactamase [202], legumain [191], myeloperoxidase [190], phosphatase [203], N-acetyltransferase 2 [196], DNase [146] and alkaline phosphatase [204].
9.1. Probes based on cleavage of a (peptide) linker
The probes utilizing this strategy are designed by linking a NIR fluorescent dye and a quencher with a short peptide linker (figure 20, table S6, ESI) (stacks.iop.org/MAF/4/042005/mmedia). The fluorescence of the dye is almost completely quenched due to FRET but is recovered upon cleavage of the peptide by the target enzyme. A probe for cathepsin B, which elevated level is associated with a wide variety of diseases, was prepared by linking the fluorescent dye Cy5.5 and the quencher BHQ-3 (115) with a short peptide chain which was itself connected to tumor-targeting glycol chitosan nanoparticles [199]. It was used for intracellular imaging and in vivo imaging in living animals. Another probe for cathepsin B utilized the Cy5 dye and a cyanine dye as a quencher (118) [192]. Several probes for measurement of cathepsin S activity, a biomarker in cancer and many inflammatory diseases, make use of the cyanine dye Cy5 and quenchers QSY21 and sulfo-QSY21 connected with a electrophile-cleavable non-peptide linkers such as 2,6-dimethyl-terephthalic acid and tetrafluoro-4-hydroxybenzoic acid (119) [197]. The probes were highly selective against other representative of the cysteine cathepsin family.
Figure 20. Sensing mechanism and chemical structures of the probes for enzymes.
Download figure:
Standard image High-resolution imageA probe for fibroblast activation protein-alpha (FAPα), a biomarker for human carcinomas, utilized the Cy5.5 dye and the quencher QSY21 connected by a short peptide chain (116) [198]. It showed fast tumor uptake and was used for imaging in living animals. The same general methodology was used by Lee et al [200] to prepare a probe for matrix metalloproteinase 3 (MMP-3), which is a biomarker for rheumatoid arthritis. Cy5.5 and BHQ-3 (117) were connected with a short peptide sequence which was in turn linked to glycol chitosan. This probe was selective against other metalloproteinases (except for MMP-7) and was applied for visualization of collagen-induced arthritis in mice.
Direct linkage of a dye to a cleavable group acting as an internal charge transfer (ICT) quencher can also be utilized. For example, another probe for cathepsin B explored cleavage of an ICT quencher bound to a cyanine dye 120 [192]. The quenching group is removed by two subsequent reaction steps after the enzymatic cleavage of an amide bond. Compared to the FRET-based probe design by the same authors, the probe exploring ICT had significantly reduced background fluorescence, and therefore higher sensitivity.
Li et al [202] developed a β-lactamase probe 121 based on a hemicyanine dye and a group containing β-lactam. A strong fluorescence enhancement (λmax 707 nm) is observed after opening of the β-lactam ring and subsequent release of the dye. A LOD of 0.02 nM of β-lactamase was determined in human urine samples and no cross sensitivities to various salts, amino acids and enzymes were found.
9.2. Probes exploring self-quenching of dyes
A probe for legumain expression and activity, which is linked to a number of pathological conditions including cancer, atherosclerosis and inflammation, explored the self-quenching of two almost identical cyanine dyes 122 [191] connected to a dimer with the legumain specific peptide. The authors utilized a similar methodology to design a cleavable magnetic resonance imaging (MRI) contrast agent which showed imaging enhancement after cleavage of the peptide. The utility of both probes was demonstrated for in vivo imaging in tumors.
A probe for metalloproteinase (MMP-2 and MMP-9) explored self-quenching of a cyanine dye 123 coupled to peptide strands [193]. The strands assembled to a helical structure resulting into high local concentration of the dye. The fluorescent monomers (λmax ~ 810 nm) were released in presence of the enzyme which was used for in vivo imaging of its activity in tumors. Analogously, a squaraine dye 124 formed non-fluorescent aggregates around sodium hexametaphosphate [203]. Upon hydrolysation of the hexametaphosphate by phosphatase the aggregates were destroyed leading to the recovery of fluorescence at 644 nm.
9.3. Probes based on reduction of nitro-group
A straightforward strategy is used in the probes for quantification of nitroreductase activity: a dye is modified with a quencher containing nitro-group. Reduction of the latter leads to strong fluorescence enhancement. For instance, a 50-fold fluorescence enhancement (λmax 708 nm) was observed for the probe 125 [194] after reduction of the p-nitrobenzyl group and subsequent detachment of the p-aminobenzyl group. The probe showed no response to various biological reduction agents and only NADH together with nitroreductase caused the strong turn-on response. The nitroimidazole group was also found to be a useful quencher for the cyanine dye 126 [195]. This highly selective and sensitive (LOD 77 pg l−1) probe was applied for visualization of hypoxic status of tumor cells via imaging of nitroreductase activity. Another probe based on p-nitrobenzyl quenching group and hemicyanine (127) [201] was conveniently prepared from IR 780 dye and used for imaging of the enzyme activity in zebrafish.
9.4. Probes for enzymes exploring other mechanisms
Several interesting concepts were reported to design long-wavelength probes for other enzymes. For example, Wang et al [196] developed a probe for the activity of N-acetyltransferase 2, one of the predicting factors for several human cancers. The probe was based on a cyanine dye and 4-aminothiophenol acting as PET-quencher (128). Acetylation of the amino group eliminated the PET-effect resulting in a strong fluorescence enhancement at 812 nm. The NIR fluorescence enabled monitoring of the enzymatic activity in vivo. A probe for Al3+ reported by Datta et al [146] can be employed for sensing of DNase activity. The probe is fluorescent when coordinated to Al3+ but upon creation of DNA fragments by DNase the Al3+ is bound resulting in a decrease of fluorescence at 730 nm. As was mentioned above, a probe for hypochlorous acid [190] is also able to measure myeloperoxidase activity by reacting with hypochlorous acid generated by the enzyme.
A probe for alkaline phosphatase 129 [204] makes use of W-CuInS2 quantum dots, which fluorescence is quenched by Cu2+ (stacks.iop.org/MAF/4/042005/mmedia). The quenching is reduced by pyrophosphate (PPi) and this effect is not observed for other anions. PPi is selectively destroyed by alkaline phosphatase resulting in quenching.
10. Probes for cyanide
Detection of cyanide is of great interest due to its high toxicity and widespread applications. Optical probes utilize high nucleophilicity of cyanide which addition results in shortening of the π-conjugated system of the dye (figure 21). For instance, phenazine dyes (130, 131) [205] possess two and one dicyano-vinyl groups, respectively. The long-wavelength absorption of both probes disappears after selective reaction with cyanide, which is accompanied either by reduction of NIR fluorescence in case of 130 or a shift of fluorescence from NIR to red in case of 131 (table S7 ESI) (stacks.iop.org/MAF/4/042005/mmedia). The LODs were 57.7 nM and 23.1 nM, for 130 and 131, respectively. A related probe based on phenothiazine 132 [206] showed a similar LOD of 67 nM and was used for imaging in HeLa cells. The π-conjugated system of a phenanthroimidazole-indolium dye 133 [207] is interrupted by nucleophilic addition of cyanide resulting in a decrease of fluorescence intensity at 743 nm but sulfide and Cys showed significant interference.
Figure 21. Chemical structures and reactivity of the probes for cyanide.
Download figure:
Standard image High-resolution imageA different approach was demonstrated by Worlinsky et al [208] who reported four water soluble probes 134–137 including free base PEGylated porpholactone and its Zn(II), Pt(II) and Ga(III) complexes. Coordination of the cyanide to the central atom of the Zn(II) complex resulted in a small spectral change, whereas other dyes reacted with the cyanide on the lactone ring and a new band peaking at about 700 nm appeared. The LODs were estimated to be 1.5 mM, 2 mM, 4 mM, 0.24 mM for the free base, the Zn(II), Pt(II) and Ga(III) complex, respectively. Red emission of the Ga(III) complex decreased upon reaction with cyanide and a new emission band (λmax 745 nm) appeared. Unfortunately, the QYs of both emissions were low (0.8% and 0.01%, respectively). An optical sensor prepared by immobilization of the Ga(III) complex into a Nafion® membrane showed LOD of 5 mM and almost reversible behavior which is remarkable considering that the most probes for cyanide are irreversible.
11. Probes for nitric oxide
Nitric oxide is an important regulator in numerous physiological processes in mammals and a key signalling molecule in plants. Therefore, long-wavelength probes for nitric oxide are particularly advantageous due to highly scattering character of these matrices and their autofluorescence. The most common detection strategy relies on reaction of nitric oxide and oxygen with an o-diaminophenyl-group, which is a PET quencher for the dye, leading to formation of a 1,2,3-triazole ring resulting in strong fluorescence enhancement (figure 22, table S8 ESI) [209] (stacks.iop.org/MAF/4/042005/mmedia). This reaction proved to be specific for nitric oxide and is not affected by reactive oxygen species, metal cations, amino acids and other nitrogen containing substances. BODIPYs were chromophores of choice for designing nitric oxide probes.
Figure 22. Sensing mechanism and chemical structures of the probes for nitric oxide.
Download figure:
Standard image High-resolution imageIn order to increase the absorption and emission wavelength, π-conjugation was extended by introducing phenyl rings (139, 140) [210], the effect of this modification being particularly pronounced in case of rigid dyes (138, 141, 142) [211–213]. Indeed, the emission of all the dyes was located above 590 nm. In all cases, 3,4-diaminophenyl groups caused almost complete fluorescence quenching. Fluorescence QY were rather high after reaction with nitrogen oxide: 21%, 7%, 25%, 16% and even 55% for 138, 139, 140, 141 and 142, respectively. Low fluorescent background in the 'off' state enables good sensitivity (e.g. LOD of 6.7 nM and 2 nM for 139 and 140, respectively). The BODIPY-based probes showed excellent intracellular retention [212] and were applied for imaging of nitric oxide in mammalian cells and animal and plant tissues [212, 213]. Apart from BODIPYs, naphthorhodamine dyes were also found to be useful (143, 144) [214]. Interestingly, o-diaminophenyl-group could not fully quench the fluorescence of this chromophore (QYs in the 'off' state 2.7% and 1.5%, respectively). After the reaction the QYs increased to 16% and 13%, respectively. Nevertheless, the probe was useful for intracellular imaging.
The probes described above rely solely on luminescence intensity measurements. Zhegalova et al [215] reported probes 145 and 146 based on a cyanine dye which might be useful for decay time measurements. Upon reaction with nitrogen oxide the QYs of NIR fluorescence increased by ~10-fold, but only about 25% increase of the decay time was observed. Such a huge difference between the BS in the 'on' and 'off' states is likely to complicate the lifetime measurements since mostly the molecules in the 'on' state contribute to overall signal. Undoubtedly, the probe is useful in the intensity mode.
A completely different reaction mechanism was explored by Adarsh et al [172]. The amino-groups of the aza-BODIPY-based probe 147 were replaced by hydroxyls after reaction with nitric oxide. A bathochromic shift and significant reduction of the absorbance of the dye was observed so that colorimetric and naked-eye detection of nitric oxide became possible.
12. Probes for other analytes
12.1. Probes for sulfur dioxide
Sulfur dioxide is one of the major pollutants which at high concentrations causes severe toxicological effects associating with respiratory problems, lung cancer, cardiovascular diseases and neuron damage in cells. Optical detection of sulfur dioxide relies on addition of the analyte to a double bound, which interrupts the π-conjugated system and results in a hypsochromic shift of absorption and emission spectra (table S9 ESI) (stacks.iop.org/MAF/4/042005/mmedia). For example, a coumarin-based probe (figure 23, 148) [216] showed a hypsochromic shift of the absorption maximum from 560 to 330 nm and the fluorescence maximum from 663 (QY = 0.07%) to 523 nm (QY = 0.45%). The water-soluble probe was selective toward SO2, 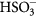 and
and  over other anions and reactive oxygen species but showed a cross-talk to pH due to deprotonation of the hydroxyl group. Despite very low fluorescence QYs (0.07% and 0.45% before and after the reaction, respectively) the probe could be applied for imaging of sulfur dioxide in cells. A compareable coumarin probe 149 [217] shows similar spectral changes upon reaction with bisulfite but benefits from much better BS (ε ~ 90 000 M−1 cm−1 at 585 nm and QY ~ 5.7% at 667 nm). The probe is highly selective against various anions, amino acids, amines etc., shows good LOD of 27 nM, and due to cell-permeability is suitable for intracellular measurements in the ratiometric mode. A benzopyrylium probe 150 [218] possessed similar spectral properties and BS, with ε ~ 80 000 M−1 cm−1 (λmax 650 nm) and QY ~ 4% (λmax 690 nm). The fluorescence maximum shifted to 489 nm after the reaction, which enabled read-out in the ratiometric mode. No interference from sulfhydryl-containing species, reactive oxygen species and various anions was found and the obtained detection limit was 10.4 nM. The cell-permeable probe was used for imaging of sulfur dioxide in HeLa cells.
over other anions and reactive oxygen species but showed a cross-talk to pH due to deprotonation of the hydroxyl group. Despite very low fluorescence QYs (0.07% and 0.45% before and after the reaction, respectively) the probe could be applied for imaging of sulfur dioxide in cells. A compareable coumarin probe 149 [217] shows similar spectral changes upon reaction with bisulfite but benefits from much better BS (ε ~ 90 000 M−1 cm−1 at 585 nm and QY ~ 5.7% at 667 nm). The probe is highly selective against various anions, amino acids, amines etc., shows good LOD of 27 nM, and due to cell-permeability is suitable for intracellular measurements in the ratiometric mode. A benzopyrylium probe 150 [218] possessed similar spectral properties and BS, with ε ~ 80 000 M−1 cm−1 (λmax 650 nm) and QY ~ 4% (λmax 690 nm). The fluorescence maximum shifted to 489 nm after the reaction, which enabled read-out in the ratiometric mode. No interference from sulfhydryl-containing species, reactive oxygen species and various anions was found and the obtained detection limit was 10.4 nM. The cell-permeable probe was used for imaging of sulfur dioxide in HeLa cells.
Figure 23. Chemical structures of the probes for other analytes: (a) sulfur dioxide; (b) hydrazine; (c) nitroxyl; (d) fluoride; (e) dopamine and (f) ascorbic acid.
Download figure:
Standard image High-resolution image12.2. Probes for hydrazine
Hydrazine finds widespread application in industry but is highly poisonous and causes serious damage to organs and central nervous system. Therefore, reliable detection of hydrazine in cells is of importance. The detection scheme utilizes the strong nucleophilic properties of the compound which is capable of cleaving ester bonds. For instance, a hydrazine probe based on a cyanine dye 151 [219] shows a strong hypsochromic shift of absorption (from 770 to 530 nm) and emission maximum (from 800 to 624 nm) after cleavage of the acetate ester. This reaction is specific to hydrazine and among many tested amines only hydroxylamine caused a weak signal. This sensitive probe (LOD 25 nM) was used for ratiometric imaging in cells and in living animals. Another highly selective probe for hydrazine relied on a hemicyanine dye modified with a levulinic acid ester (152) [220]. Cleavage of the ester resulted in a strong bathochromic shift of absorption (from 560 to 690 nm) and a 23-fold enhancement on NIR emission (λmax 725 nm).
12.3. Probes for nitroxyl
Understanding the effects of nitroxyl in biological systems is of much importance which stimulates development of methods for selective detection of this analyte in cells and tissues. Jing et al [221] developed a nitroxyl probe based on an aza-BODIPY dye bearing a diphenylphosphino-benzoyl moiety (153). This group is eliminated after reaction with nitroxyl leading to a strong fluorescence enhancement at 700 nm (QY increasing from 1 to 37%). The sensitive probe (LOD 60 nM) showed only weak cross-reactivity to S-nitrosoGSH, peroxinitrite, hydrogen peroxide and hypochlorite, but was not sensitive to other reactive oxygen species and nucleophiles. The probe localized in lysosomes and was highly promising for intracellular imaging and in vivo experiments in living animals.
A completely different strategy was proposed by Wrobel et al [222]. A push–pull chromophore was modified with a cyclam moiety with coordinated Cu2+ (154). Bound Cu(II) acts as a fluorescence quencher. It is selectively reduced by HNO to Cu(I) which results in a 5-fold fluorescence enhancement (λmax 715 nm). The selectivity of the probe was tested with various metal cations, reactive oxygen species, sulfhydryl-containing species, hydrogen sulfide and nitric oxide and only hydrogen sulfide caused a significant fluorescence enhancement. Intracellular imaging conducted with the probe helped to establish a relationship between HNO mobile zinc in a biological environment.
12.4. Probes for fluoride
Saravanan et al [223] reported a fluoride probe based on a benzoselenadiazole dye 155. The detection is achieved by blocking of an ESIPT effect by fluoride due to a deprotonation of the dye. This causes a bathochromic shift in absorption from 520 to 700 nm and hypsochromic shift in emission from 660 to 510 nm. Similarly, deprotonation of a diketopyrrolopyrrole dye 156 [224] by fluoride was also caused a bathochromic shift of absorption from 504 to 771 nm, and a quenching of fluorescence at 598 nm. Unfortunately, DMSO had to be used as a co-solvent for both probes, so that they may not be suitable for measurements in aqueous solutions.
12.5. Probes for dopamine
Liu et al [225] reported a CuInS2 quantum dot-based probe 157 for dopamine or other substances bearing vicinal diols (e.g. catechol, pyrogallol, and gallate). The nanoparticles were modified with a boronic acid and formed cyclic esters causing a fluorescence quenching at 736 nm. A LOD of 200 nM for dopamine was obtained. Although investigated biological substances did not cause fluorescence quenching (sugars, which are known to bind to boronic acids were not tested) they may cause decrease in the fluorescence quenching by dopamine by blocking the receptor.
12.6. Probes for ascorbic acid
A probe for ascorbic acid based on a cyanine dye 158 was proposed by Zhao et al [226]. The dye contains a hydrazone moiety which is hydrolyzed by Cu2+, resulting in a quenching of fluorescence at 660 nm. Ascorbic acid prevents the hydrolysis by reducing Cu2+ to Cu+ and, therefore, the fluorescence reduction. Evidently, the probe is only suitable for measurement of ascorbic acid in a defined copper containing environment which can only be created in vitro. Necessity of using DMSO further limits its practical application.
13. Genetically encodable fluorescent protein probes
Genetically encoded fluorescent proteins (GEFPs) are essential tools for labeling of proteins, cells, tissues and even whole organisms. Some of them also enable visualization of different analytes, mostly protons, calcium, zinc, halides, ROS, nitrogen oxide and the activity of some enzymes [227]. Thus, GEFP represent an interesting alternative to fluorophore-based probes. Similarly to fluorophore-based probes, intrinsic BS, photostability, environmental sensitivity and spectral properties of the GEFPs are the major factors to be considered [227]. Red (emission at 570–620 nm) and particularly far-red (emission > 620 nm) fluorescent proteins are advantageous for in vivo applications and great progress in their design and optimization was recently achieved [228]. Although only few analyte-sensitive probes based on such FPs have been reported, the situation is likely to significantly improve in future. Starting from the expanded palette of genetically encoded calicum probes [229] Campbell and co-workers systematically improved the spectral properties of these probes. They managed to shift the excitation maximum of the FP to 560 and the emission maximum to 610 nm [230]. At pH 7.4 the chromophores are in their non-fluorescent protonated form. In presence of Ca2+ the emission was enhanced 27-fold due deprotonation of the chromophore achieving appreciable QYs of 21%. The probe was most sensitive in the range of 100–1000 nM of Ca2+. Due to photoactivation of the probes, a special measurement protocol should be followed to achieve reliable performance. Low affinity genetically encoded Ca2+ probes (dynamic range 1–100 µM) designed by the same group [231] enabled imaging of the analyte in mitochondria and endoplasmic reticulum where the Ca2+ concentration is significantly higher than in cytosol. The absorption and emission of the probes were at ~570 nm and 595 nm, respectively, and ~10-fold enhancement in BS was observed upon interaction with the analyte. The spectral properties of the probe enabled simultaneous two-color imaging of Ca2+ dynamics in cells in endoplasmic reticulum (with the new probe) and in cytosol (with help of a green GEFP). Finally, the same group developed a Ca2+ GEFP probe, which was fluorescent at 585 nm and was suitable for 2-photon excitation at λ < 1000 nm due to large Stokes shift between the one photon absorption and fluorescence [232]. It should be noted that red FPs will typically show 2-P absorption at λ > 1000 nm, i.e. outside of NIR optical window. The authors demonstrated high potential of the new probe for 2-P imaging of Ca2+ in vivo.
pH represents another important parameter which can be reliably sensed with GRFPs. Particularly, green FP SEP is an established tool for imaging of exocytosis and endocytosis. Tantama and co-workers [233] designed a ratiometric probe for intracellular pH imaging 'pHRed' featuring fluorescence at 610 nm and two excitation peaks at 440 nm and 585 nm for deprotonated and protonated forms, respectively. Although the individual peaks respond with a pKa of 7.8, the apparent pKa for the fluorescence ratio is 6.6. Importantly, the ratio was not affected by differences in buffer composition (K+, Na+, Mg2+, Ca2+, Cl− etc), oxidative stress (H2O2) and temperature. The probe was suitable for 2-P excitation at 860 nm, and showed a change in the decay time of the red emission (from ~1.7 ns to 2.3 ns at pH 5 to 8, respectively), thus being potentially useful for self-referenced measurements in thick samples or even in vivo.
Another red fluorescent protein, 'pHuji', reported by Shen et al [234], combined attractive spectral properties (λexc 566 nm, λem 598 nm), high fluorescence change from pH 5.5 to 7.5 (22x enhancement of fluorescence) and sigmoidal calibration curve. The pKa of 7.7 was less optimal than for the established GEFP probe SEP (pKa 7.2). Pared with SEP, the new probe was promising for studying endocytic vesicle formation via simultaneous two color imaging.
Redox probes based on GEFPs are popular [235] and expanding of their spectral properties to the red part of the spectrum is of much interest. Ermakova et al [23] reported a first GEFP hydrogen peroxide probe, 'HyPerRed', featuring long-wavelength excitation and emission (λmax 575 nm and 605 nm, respectively). The probe showed response from 10 to 300 µM of H2O2 which was reversible: in cells, the oxidized form was reduced back within 10 min, whereas in vitro 2-mercaptoethanol could be used for regeneration. It was targeted to mitochondrial matrix and used for real-time simultaneous monitoring of H2O2 and GSH/GSSG or H2O2 and pH. The authors were able to detect H2O2 production in the mitochondrial matrix upon inhibition of the endoplasmic reticulum uptake of Ca2+.
Ai and co-workers [236] engineered another red-light emitting redox probe by introducing a reversible disulfide bridge to the N- and C-termini of a circularly permuted FP. The probe possessed excitation maximum at 576 nm and emission maximum at 600 nm and showed 4-fold fluorescence increase in the oxidized state at pH 7.4. It was used to image redox changes in living mammalian cells. Since the fluorescence of the new probe is pH-dependent, the authors also prepared a similar control pH probe (pKa 8.6) and demonstrated that the fluorescence changes of the new probe are caused by changes in the redox potential, but not pH fluctuations.
It can be concluded that the new GEFP probes for calcium, pH and H2O2 are not only highly promising for imaging of these parameters in cells and particularly in thick samples and potentially in vivo but also enable parallel imaging with a variety of other parameters using green light-emitting FPs or small molecular probes.
14. Temperature probes and inert reference materials for sensing applications
Temperature belongs to one of the most important parameters and its measurement is of particular interest in many fields of science and technology. Virtually all optical sensors show cross-sensitivity to temperature, therefore optical temperature compensation is of high interest. Moreover, optical methods potentially allow temperature monitoring in very small volumes, e.g. inside the cells (nanothermometry). Most of these probes rely on UV–vis dyes and are therefore beyond the scope of this review. Several thermographic phosphors represent a notable exception. Chromium(III)-activated yttrium aluminum borate [237] is excitable in both blue and red parts (550–650 nm band) of electromagnetic spectrum and emits in NIR (650–850 nm broad band). The temperature sensitivity of the decay time at ambient conditions is rather high (~1% K−1), whereas the luminescence intensity is only minor affected by temperature which ensures constant signal-to-noise ratio. The phosphor shows excellent chemical and photochemical stability which is favorably compared to molecular temperature probes. On the other hand, its ε is much lower than for (metal)organic dyes so that the phosphor can only be used in a relatively thick senor layers (microcrystalline powder dispersed in a polymer) applied onto a tip of an optical fiber or as a planar optode. As was demonstrated, the new phosphor can be successfully used for temperature compensation of the pH sensors based on NIR indicators [238].
Due to their high chemical and photochemical stability and spectral properties NIR phosphors represent promising luminescent emitters for application as reference materials. The established schemes include ratiometric 2-wavelength measurement via luminescence emission/excitation and dual lifetime referencing (DLR). Calcium copper silicate (Egyptian blue) and its strontium and barium analogous are particularly promising for this purpose [239]. These phosphors can be easily prepared from cheap materials, are chemically and photochemically robust, show rather strong absorption extending from the green part of the spectrum down to the NIR and emit bright luminescence (QYs about 10%) [240] in the NIR at about 920 nm. In order to prepare a ratiometric pH sensing material a NIR pH indicator (42) and Egyptian blue micropowder were dissolved/dispersed in a polyurethane hydrogel. The sensor is excitable with red light and features two emission bands in different parts of the NIR region thus enabling reliable 2-wavelength measurement. The same material can be used in the DLR scheme due to the long luminescence decay time of Egyptian Blue (>100 µs) and in a new scheme for conversion of absorption changes into ratiometric luminescence response. Additionally, the phosphors can be used as intrinsic temperature probes to enable compensation of optical sensors for temperature effects, albeit with lower sensitivity at ambient conditions.
15. Other applications of NIR probes
As was shown in the review, NIR probes represent viable tools for quantification of various analytes. However, these dyes are also promising candidates in completely different application areas. Particularly, efficient NIR OLEDs can be constructed on basis of platinum(II) complexes of tetrabenzo- and tetranaphthoporphyrins [64, 241, 242]. Phosphorescent metal complexes are also useful due to their ability to populate the triplet excited state with high probability. One potential application area includes photodynamic therapy in which photosensitized singlet oxygen destroys cancer cells [243, 244]. Even though the new dyes can be more promising for such applications than the state-of-the-art photosensitizers, their testing requires considerable resources and is not easy to realize in practice. Moreover, additional synthetic modification of the dyes is often necessary to make the candidates suitable. Phosphorescent NIR dyes also represent promising sensitizers in organic UC systems [245, 246]. Briefly, after the excitation of the dye and population of the triplet excited state, the energy is then transferred to the acceptor molecules which undergo triplet-triplet annihilation thus producing the highly fluorescent dye in the singlet excited state. The resulted emission is located at shorter wavelengths than the light used for the excitation of the sensitizer. This allows utilization of the otherwise not used red and NIR light which is expected to enhance the efficiency of some photovoltaic systems. Since high molar absorption coefficient is also an important property of the sensitizer, benzo- and naphthoporphyrins proved to be particularly valuable sensitizers for TTA UC [247–250]. Pt(II) BP was found to be useful as absorber in organic solar concentrators [251]. Unfortunately, oxygen quenching represents a serious problem in these systems and a simple and efficient strategy for its elimination has yet to be found.
16. Concluding remarks
As was demonstrated in this review, a significant progress in development of long-wavelength analyte-sensitive probes has been made in the recent years. The probes cover a wide variety of analytes including gaseous species, protons, metal ions, thiols etc. Some of them (such as oxygen probes) already enable reliable measurements in vivo in tissues of living animals. On the other hand, most of the probes are not yet advanced enough for such challenging applications, but nevertheless they represent promising analytical tools for monitoring of various parameters in cells and biological fluids. One of the challenges includes moderate or even poor BS of most reported long-wavelength probes, and the luminescence QY of a few per cent are rather common. New classes of bright long-wavelength chromophors continue to appear but new probes based on these systems are yet to be realized. The other challenge includes inadequate referencing of luminescence signals. Most reported probes do not make use of any referencing and therefore can be only applied in strictly defined conditions. Nevertheless, they are still useful for visualization of different analytes, and particularly the change of the concentration in time, also in vivo. Only some classes of the probes rely on self-referenced measurement of luminescence decay time (such as oxygen probes) whereas for majority of the probes ratiometric measurement remains the only possibility. However, few dyes have been reported for which both forms (without and with analyte) are highly fluorescent. More often, addition of an inert fluorophore would be necessary, which however requires more complex synthesis, and potentially results in high dependency of such systems on leaching and photobleaching of either probe or reference. Compatibility of the long-wavelength probes with photodetectors can be another critical issue and many available set-ups are still equipped with photodetectors which are virtually 'blind' in the near-infrared part of the spectrum. This, of course, hinders the application of the new materials, and therefore conventional UV–vis probes which are spectrally compatible with the available set-ups are still more common. The situation is likely to change in future since NIR-sensitive photodetectors are already available for competitive price. Despite these practical limitations, long-wavelength analyte-sensitive probes already represent viable analytical tools for numerous applications.
Acknowledgments
The authors thank Professor Ingo Klimant (Graz University of Technology) and Dr. Ruslan Dmitriev (University College Cork) for valuable comments on the manuscript.
Funding
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors acknowledge financial support from the European Commision (project SenseOcean, number 614141) and European Research Council (Project Oxygen, number 267233)
Competing interests
The authors have declared that no competing interests exist.