Abstract
A rapid, simple and sensitive biosensor system for tetracycline detection is very important in food safety. In this paper we developed a label-free aptasensor for electrochemical detection of tetracycline. According to the electrochemical impendence spectroscopy (EIS) analysis, there was a linear relationship between the concentration of tetracycline and the electron transfer resistance from 10 to 3000 ng ml−1 of the tetracycline concentration. The detection limit was 10 ng ml−1 in 15 min detection duration. The prepared aptasensor showed a good reproducibility with an acceptable stability in tetracycline detection. The recoveries of tetracycline in spiked milk samples were in the range of 88.1%–94.2%. The aptasensor has sensitivity 98% and specificity of 100%.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Tetracycline (TCN) is one of the most common antibiotics that are widely used for treatment of infectious diseases in fodder animals. The extensive use of tetracycline, including tetracycline in veterinary medicine, as an antibiotic and growth promoter has led to its accumulation in diary food products, such as meat [1], milk [2], honey [3], eggs/chicken [4] and has been found to cause serious threats to human health. Therefore, it is very important to develop a rapid, simple, sensitive and specific detection method to detect tetracyclines in food products. To safeguard human health, the European Union (EU) has set safe maximum residue limits (MRL) for residues of veterinary drugs in animal tissues entering the human food chain. The MRL of tetracycline in milk, for example, is 100 μg kg−1.
The common techniques used to detect tetracyclines include microbiological assay [5], high-performance thin-layer chromatography (HPTLC) [6], capillary electrophoresis (CE), enzyme-linked immunoassay (ELISA) [7], electrochemical [8], liquid chromatography-mass spectrometry (LC/MS) [9], high performance liquid chromatography (HPLC) [10]. Microbiological assay is a simple method with low cost, but time-consuming, and lack of sensitivity and specificity. ELISA has high specificity, but is time-consuming (multiple incubations and washing steps) with high background absorption and susceptible to being influenced by sample matrix. CE has the advantages of rapid separation, analysis, and low cost, but not suitable for small molecular detection. LC/MS, HPTLC and HPLC need expensive and sophisticated instruments. The electrochemical technologies have received a great deal of attention, mainly because of their ease in operation, high specificity and sensitivity, ease to miniaturization and amenability to automation.
Wang et al [11] developed a tetracycline sensor using a molecularly imprinted polymer modified carbon nanotube-gold nanoparticles electrode which has high specificity, but the linear range is narrow and the sensitivity cannot meet the requirements. The linear range varied from 0.1 to 40 μg ml−1, and the detection limit was 40 ng ml−1. Que et al [12] developed a sensitive electrochemical immunoassay of tetracycline residues by using platinum-catalyzed hydrogen evolution reaction on an anti-tetracycline antibody modified immunosensor, which has high sensitivity and specificity. However, this immunosensor is complex and has some difficulties for real sample analysis in complex systems, and the catalytic property is easily affected by external conditions [12].
The aptamers are short, single-stranded oligonucleotides of DNA or RNA origin which can be selected in vitro to bind nearly any target molecules, from small molecules to proteins [13]. In most diagnostics and biomolecular sensing studies, the antibodies were used for their high affinity and specific molecular recognition, but with some limitations, including animals or cell lines requirement with complicated purification steps for antibody production. Unlike antibodies, aptamers have many advantages over using antibodies because of the following: (a) advantages of aptamers to rival antibodies, (b) they can be selected by in-vitro methods for their target molecules, (c) aptamers can be chemically synthesized and modified using well-established nucleic acid, (d) they are highly stable under elevated temperatures, unlike antibodies, and (e) they can be stably used as biorecognition elements in conjunction with a detection systems, such as surface plasmon resonance (SPR) and electrochemical methods [14]. Applications of DNA/RNA aptamers as analytical tools have now emerged because of high affinity, specificity, and selectivity for their target molecules [15].
Kim et al [8] developed a label-free electrochemical aptasensor on a screen printed gold electrode for the detection of tetracycline using square-wave voltammetry (SWV) technique. The detection limit is 10 nM, but the stability and reproducibility of the method were not discussed. In this work we developed a simple label-free electrochemical aptasensor for the specific detection of tetracycline using tetracycline-binding aptamer. The interaction between tetracycline and aptamer was investigated by the electrochemical probe of ferricyanide.
2. Materials and methods
2.1. Materials
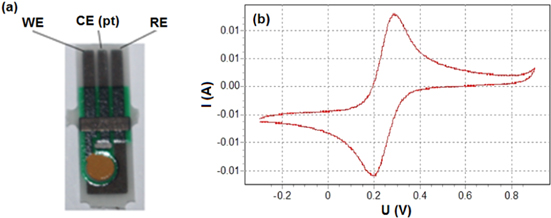
Tetracycline (purity 98%), oxytetracycline, streptomycin, and neomycin was purchased from Sigma Company (St. Louis, MO, USA). Electrochemical analysis was performed at room temperature using an electrochemical analyzer IM6 (USA) for cyclic voltammetry (CV) and electrochemical impendence spectroscopy (EIS) analysis. All experiments were carried out with a conventional three-electrode system which consisted of an aptamer modified gold electrode or gold electrode as the working electrode (WE), a platinum wire as the counter electrode (CE), and a Ag/AgCl reference electrode (RE), electrodes were integrated on a single chip, and the diameter of working electrode was 2.2 mm. The impedance measurements were performed in the presence of a K3[Fe(CN)6]/K4[Fe(CN)6] (5 mM : 5 mM) mixture, in a solution that contained KCl (100 mM). They were recorded by applying a potential equivalent to that of the open circuit, 0.22 V (versus Ag/AgCl) over the frequency range of 100 kHz–10 MHz.
2.2. Methods
2.2.1. Fabrication of aptamer-based electrochemical sensor
The electrode chip immersed in 0.5 M H2SO4 for 10 min, then the scan of cyclic voltammetry (CV) characterization was performed in dissociation solution (K4Fe(CN)6 5 mM + K3Fe(CN)6 5 mM and 100 mM KCl to determine reversible redox system with following modes: U1 = −0.3 V, U2 = 0.9 V, scanning speed v = 100 mV s−1. Later, the active gold electrode was rinsed with ultrapure water and then immersed into 2 μM aptamer solution to self-assemble for 2 h at 4 °C. After incubating the gold electrode in aptamer solution, the incubated gold electrode was rinsed again with ultrapure water to remove the non-fixed aptamer. After each step of the fabrication procedure, the corresponding EIS response was recorded. Thus the aptasensor was fabricated and its performance was controlled.
2.2.2. Electrochemical measurements
The fabricated aptasensor was incubated in phosphate buffer saline (PBS) containing TCN with the concentrations of 1 ng ml−1, 10 ng ml−1, 100 ng ml−1, 500 ng ml−1, 1000 ng ml−1 and 3000 ng ml−1 at room temperature for 15 min. After washing aptasensor with ultrapure water to remove the non-specific components, EIS responses of the aptasensor were recorded. The relationship between TCN concentration and electron-transfer resistance (Ret) was analyzed to evaluate the sensitivity of aptasensor.
2.2.3. Detection of tetracycline in milk
Collected milk samples for detecting TCN were pretreated as follows: add 2% trichloroacetic acid solution to the milk contained in the tube of a centrifuge, mix well under the action of the ultrasound for 30 min, then perform the centrifugation with the speed of 10 000 rpm for 8 min. The 0.2 micron centrifugal filter from polyvinylidene fluoride (PVDF) was used in the centrifuge to remove lipids. Adjust the pH to neutral (pH = 7.0) with NaOH. Store at −40 °C until the beginning of electrochemical analysis. The concentrations of TCN in the pretreated milk samples were then determined by using the preparated aptasensor.
3. Results and discussion
3.1. Sensing mechanisms of the aptasensor
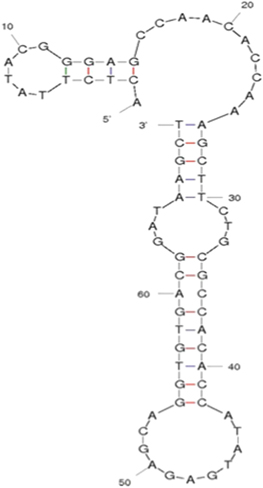
The aptamer is selected through an in-vitro selection process known as SELEX by the Laboratory of Animal Cell Technology, Institute of Biotechnology. Aptamer specifically binds to tetracycline with high affinity with Kd = (52.5 ± 3.6) nM, primary structure of stem-loop shape with thermal power is low (ΔG = −10.06 kcal mol−1). The secondary structure of tetracycline binding ssDNA aptamer was predicted by m-fold program according to the free energy minimization algorithm (figure 1).
Figure 1. Secondary structure of the ssDNA aptamer. The structure was predicted by m-fold program according to the free minimization algorithm.
Download figure:
Standard image High-resolution imageThe gold electrode chip was polished, then the CV curve was scanned in dissociation solution ferro/ferri cyanide, with the mode as described in subsection 2.2.1.
CV is an effective and convenient technique for probing the feature of the modified electrode surface. Here CV was used to investigate the electrochemical behaviors of  The redox-label
The redox-label  revealed a reversible CV at the bare Au with a peak-to-peak separation ΔEp of 59 mV and redox system is reversible, ensuring the accuracy of the electrochemical reaction later.
revealed a reversible CV at the bare Au with a peak-to-peak separation ΔEp of 59 mV and redox system is reversible, ensuring the accuracy of the electrochemical reaction later.
Figure 2. (a) System chip electrode; (b) CV curve obtained in buffer containing 5 mM  and 100 mM KCl at a scan rate of 100 mV s−1 at a bare Au.
and 100 mM KCl at a scan rate of 100 mV s−1 at a bare Au.
Download figure:
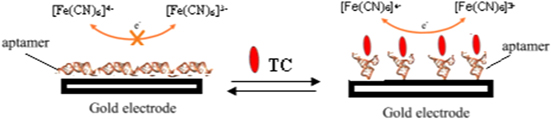
Standard image High-resolution imageThe aptasensor was constructed by covalently attaching an amino-modified aptamer to an activated gold electrode. The ferricyanide solution was used as an electrochemical indicator to generate electron flow between bulk solution and work electrode. Aptamer was at a high-level structure of double-stranded form on the surface of electrode. In the absence of tetracycline, the nitrogen moieties within the nucleotide bases prompted aptamer nonspecifically bound to the gold electrode surface, and formed a disordered orientation [16]. The negative charge of aptamer rejected the anionic redox probe [Fe(CN)6]3−/4− in the bulk solution and hindered the electron transfer of [Fe(CN)6]3−/4−, which led to increased electron-transfer resistance (Ret). The tetracycline molecules were inserted into the double-stranded area of aptamer and resulted in high level spatial structure with further extension which forced the aptamer to change from lying flat or reclining into upright (figure 3), inducing the electron transfer of [Fe(CN)6]3−/4− between bulk solution and work electrode with a detectable signal response.
Figure 3. The schematic diagram of the electrochemical aptasensor detecting tetracycline.
Download figure:
Standard image High-resolution image3.2. Electrochemical measurements and EIS analysis
Electrochemical impendence spectroscopy (EIS) is a powerful technique to characterize the interface properties of the modified electrode. EIS is often analyzed using an equivalent circuit. The equivalent circuit is applied to fit the experimental data and extract the necessary information about the electrical parameters responsible for the impedimetric change. The impedance spectra obtained in the presence of different concentrations of tetracycline from 1 to 3000 ng ml−1 were modeled by the equivalent electrical circuit shown in figure 4(a). The circuit is a combination of resistor solution (1), double layer capacitance (2), membrane electrical polarity point chip (3), power diffusion index (4), double layer capacitance pores (5), charge transfer resistance (6).
Figure 4. (a) The applied equivalent electrical circuit (resistor solution (1), double layer capacitance (2), membrane electrical polarity point chip (3), power diffusion index (4), double layer capacitance pores (5), charge transfer resistance (6)); and b) Nyquist plots obtained in reality and from simulation of modeled equivalent circuit.
Download figure:
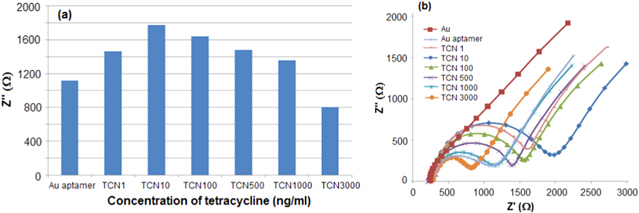
Standard image High-resolution imageAs shown in figure 5(a), increased concentrations corresponded to the increased responses at concentrations of 1–10 ng ml−1 and decreased responses at concentrations of 10–3000 ng ml−1, respectively. This might be due to that the orientation of aptamer on the surface of electrode was changed from lying flat or reclining to upright after bonding tetracycline with aptamer. The upright orientation of aptamer–tetracycline compound was able to reduce the electron-transfer resistance (Ret) and increase the conductivity when [Fe(CN)6]3−/4− was bound on the gold electrode.
Figure 5. (a) The variation of aptasensor response to the increased concentrations of tetracycline: TCN 1, TCN 10, TCN 100, TCN 500, TCN 1000 and TCN 3000 are 1 ng ml−1, 10 ng ml−1, 100 ng ml−1, 500 ng ml−1, 1000 ng ml−1 and 3000 ng ml−1, respectively; (b) Nyquist plots obtained in the presence of 5.0 mmol L−1 [Fe(CN)6]3−/4− as a redox probe, after incubating the aptasensor in tetracycline solutions of various concentrations.
Download figure:
Standard image High-resolution imageFigures 5(b) and 6(a) showed the Nyquist plots of aptasensor response to the concentration change of tetracycline. The semicircle diameter at higher frequencies correlated to the electron-transfer resistance (Ret). As the figure shows Nyquist plot of bare Au was a straight line. After aptamer casting on the surface of Au electrode, the Ret increased dramatically (1112 Ω). The Ret decreased after incubating this aptasensor with various concentrations of tetracycline over the range of 10–3000 ng ml−1.
Figure 6. (a) Nyquist plots was obtained in the presence of 5 mM [Fe(CN)6]3−/4−as a redox probe, after the aptasensor incubating in tetracycline solution with various concentrations TCN 10 = 10 ng ml−1; (b) dependence of Ret on the concentration of tetracycline 10, 100, 500, 1000 and 3000 ng ml−1.
Download figure:
Standard image High-resolution imageFigure 6(b) shows that the plot of the electron-transfer resistance (Ret) versus the concentrations of tetracycline was linear over the interval of 10–3000 ng ml−1, Ret (Ω) = −0.3 C ng ml−1 + 1680, R2 = 0.970), limit of detection (LOD) was 10 ng ml−1. It is significantly lower than the maximum residue limit in milk (100 ng ml−1).
3.3. The specificity of the aptasensor to tetracycline
In order to evaluate the selectivity of Au-aptamer to tetracycline in the presence of interferents, oxytetracycline, streptomycin, and neomycin were selected as interferents in the electrochemical analysis with tetracycline. Firstly, three interferents were mixed to a mixture shown in table 1. The test results indicated that no signal had changed when Au-aptamer aptasensor was incubated within the three-interferent mixtures. When tetracycline with final concentration of 10 ng ml−1 was added into the mixture, the signal changed significantly (figure 7). These tests indicated that the developed strategy could be used to identify tetracycline with high specificity.
Table 1. The concentration of elements in mixture.
| Interferents | Concentration in mixture (ng ml−1) | Maximum residue limit in milk (ng ml−1) |
|---|---|---|
| Tetracycline | 10 | 100 |
| Oxytetracycline | 10 | 100 |
| Streptomycin | 20 | 200 |
| Neomycin | 150 | 1500 |
Figure 7. Selective evaluation of aptasensor: (1) Au-aptamer, (2) mixture of three interferents: oxytetracycline (OTC), streptomycin (Str), neomycin (Neo) and (3) mixture of tetracycline and three interferents.
Download figure:
Standard image High-resolution image3.4. Sensitivity and specificity
A total of 60 specimens were used to determine the sensitivity and specificity of aptasensor. 30 specimens are negative, 30 specimens are positive; the sensitivity and specificity were calculated. 30 positive specimens were tested using the aptasensor, one specimen (specimen 24) tested negative in the aptasensor. Thus, the sensitivity is 98%. A total of 30 specimens negative and all of these specimens were identified as negative by aptasensor. Thus, the specificity was 100%.
3.5. Detection of tetracycline in milk samples
In order to examine the ability of this aptasensor for the determination of tetracycline in milk samples, the recoveries of three different concentrations of tetracycline in spiked milk samples were determined using aptasensor. As shown in table 2, the recoveries were in the range of 88.1%–94.2%, which indicated that the developed aptasensor is adequate for the determination of tetracycline in milk samples.
Table 2. Recoveries of tetracycline from spiked milk samples.
| Standard value of tetracycline (ng ml−1) | Found (ng ml−1) | Recovery (%) |
|---|---|---|
| 30 | 26.43 | 88.1 |
| 200 | 186.22 | 93.1 |
| 600 | 565.71 | 94.2 |
4. Conclusion
In this study we developed a label-free electrochemical aptasensor for specific detection of tetracycline. The interaction between tetracycline and aptamer was investigated by the electrochemical probe of ferricyanide and monitored by electrochemical impedance spectroscopy (EIS). The aptasensor in our study had a high sensitive and specificity with fast response. The aptasensor was sensitive to tetracycline and showed a detection limit of 10 ng ml−1, with linear range varied from 10 to 3000 ng ml−1 in 15 min detection duration. The recoveries of tetracycline in spiked milk samples were in the range of 88.1%–94.2%.
The electrochemical aptasensor described here offered several advantages:
Unlike antibody-based immunoassays, the aptasensor responded directly to the presence of tetracycline without the need for multiple labeling and washing steps.
The aptasensor in our study had high sensitivity and specificity with fast response. Comparing to the electrochemical aptasensor for tetracycline reported by Kim et al [8] and Zhou et al [10], this aptasensor had higher sensitive, shorter incubation time and simpler steps to develop (incubation time: 15 min in this work, 30 min in Kim's and Zhou's works).
Future work will focus on: (1) improving the stability of aptamer in real samples; (2) performing the quantitative detection of tetracycline in a variety of samples.
Acknowledgments
This work was supported by Ministry of Science and Technology in KC-04 program for project KC04.12/11-15.