Abstract
In this work the surface modification and functionalization of carbon nanotubes (CNTs) were investigated. CNTs were firstly treated by acid mixture H2SO4/HNO3 to introduce the carboxylic group onto the surface of CNTs. This carboxylic group was used as reaction precursor in the functionalization. Two functional groups, dodecylamine (DDA) and 3-aminopropyl triethoxysilane (3-APTES), were successfully covalently attached to CNTs. The functionalized CNTs were characterized by Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, differential scanning calorimetry and thermal gravimetric analysis (DSC/TGA) and transmission electron microscopy (TEM) methods. The CNTs attached to the organofunctional moieties have greater versatility for further utilization in different application fields such as biology, nanocomposites, solar energy, etc.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since the discovery of the carbon nanotube (CNT) by Iijima [1], it has been become a potential candidate for a wide range of applications such as optoelectronics, advanced nanocomposites and gas storage. CNTs possess unique properties including extraordinary strength and stiffness. Previous researches have shown that mechanically CNTs are 100 times stronger than steel but their density is six times lower [2]. Extensive research has been done by incorporating different types of CNTs into polymeric materials to form new composites that possess high mechanical strength, electrical and thermal conductivity. Good dispersion and good interfacial adhesion between CNTs and the polymer matrix is essential to enhance the mechanical properties of CNT polymer nanocomposites [3]. Because of their nature, CNTs are inherently insoluble in most organic and aqueous solvents as well as poorly chemically compatible with the polymer matrix. Thus, surface modification of CNTs in order to improve the compatibility and solubility is one of the main research points in the last years. Noncovalent or covalent functionalization of CNTs can enhance their compatibility and solubility [4]. The noncovalent functionalization of CNTs includes noncovalent coating with surfactants [5], surface wrapping with long polymer chains such as polystyrene sulfonate [6] and noncovalent adsorption of noncharged polymer chains such as polyvinylpyrrolidone [7], etc. The advantage of noncovalent functionalization is that the structure and original properties of CNTs are not changed after modification. However, the surfactants, polymer chains and electric acceptor which can be used for this method are very limited, their dispersion is not very stable and most importantly, it is difficult to further modify CNTs with different functionalities. Direct covalent functionalization can greatly improve the solubility and compatibility of CNTs. Generally, long alkyl chains, polymer chains and biomolecules can be grafted onto CNTs by esterification or amidation reactions [8, 9]. The disadvantages of covalent functionalization of CNTs are that the structure and the original properties of CNTs are changed after modification. Generally, the more modification on the surface, the more the outstanding properties of CNTs will be altered [4]. How to modify CNTs with altering very little the perfect structure and the original special properties has become a major challenge at the present time.
In this work the modification and functionalization of CNTs with some organic groups such as carboxylic group (COOH), amine group and silane group was investigated. The characterization of the modification process was characterized by FT-IR spectroscopy, Raman spectroscopy, DSC/TGA and TEM methods.
2. Experimental
2.1. Materials
Multi-walled carbon nanotube (MWCNTs) with the purity >90% was purchased by Showa Denko K K (Japan). Dodecylamine (DDA), 3-aminopropyl triethoxysilane (3-APTES), dimethylformide (DMF), thionyl dichloride (SOCl2), nitric acid (HNO3 65%), sulfuric acid (H2SO4 98%) were supplied by Merck Company (Germany). All chemical reagents are analytical grade and were used as received.
2.2. Modification procedures and characterization methods
2.2.1. Attachment of COOH group onto the surface of MWCNTs.
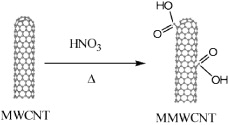
The procedures for modification of MWCNTs with COOH group are done according to [10]. Figure 1 shows the scheme for modification of MWCNTs with COOH group.
Figure 1. Scheme for modification of MWCNTs with COOH group.
Download figure:
Standard image High-resolution image0.5 g of MWCNTs was immersed in 80 ml mixture of H2SO4/HNO3 (3:1, v/v) at 70 °C for different times under continuous sonication. The black solid obtained after filtration was washed several times with distilled water, and dried at 80 °C in vacuum for 8 h. The resulting COOH modified MWCNTs (MWCNTs–COOH) were used for further modification.
2.2.2. Attachment of amine group onto the surface of MWCNTs.
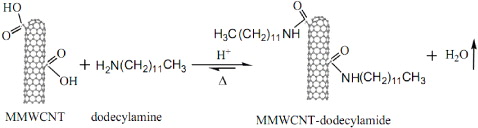
Figure 2 shows the scheme of MWCNTs modification with DDA [11, 12].
Figure 2. Modification scheme of MWCNTs with DDA.
Download figure:
Standard image High-resolution imageFirstly, 0.5 g MWCNTs–COOH and 3 g DDA were mixed at 70 °C for 0.5 h under continuous sonication. Then, many drops of H2SO4 as catalyst were poured into the mixture and the reaction took place for a further 5 h. After that, MWCNTs modified with DDA (MWCNTs–DDA) were filtered and washed several times with distilled water. Finally, MWCNTs–DDA was dried at 80 °C in vacuum for 8 h.
2.2.3. Attachment of silane group onto the surface of MWCNTs.
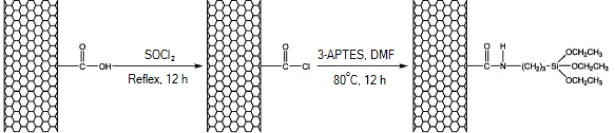
Figure 3 shows the scheme of MWCNTs modification with 3-APTES [13].
Figure 3. Modification scheme of MWCNTs with 3-APTES.
Download figure:
Standard image High-resolution imageMWCNTs–COOH is reacted with excess SOCl2 for 12 h under reflux to convert the carboxylic acids into acyl chlorides. Then, the residual SOCl2 is removed through reduced pressure distillation using a rotary evaporator. The as-prepared MWCNTs–COCl is dispersed in DMF and then sonicated for 0.5 h to create a homogeneous suspension. Subsequently, an excess portion of 3-APTES is added into the mixture. Then, the mixture is placed in a constant temperature oil bath at 80 °C for 12 h with vigorous stirring under the protection of argon. After the return of the reaction mixture to the ambient temperature, the resulting reaction medium is filtrated through a 0.22 μm PTFE membrane and washed with enough DMF. Finally, MWCNTs–3-APTES was dried at 80 °C in vacuum for 8 h.
The FTIR and Raman spectra of the samples were recorded on the FTIR Impact − 410 and Renishaw Invia instruments, respectively. The TEM images were taken by a TEM-1010, JEOL. The DSC/TGA thermograms were recorded on the DSC/TGA, Labsys-instrument. The heating rate was 5 °C min−1 in nitrogen atmosphere.
3. Results and discussion
3.1. Modification of MWCNTs with COOH group
Figure 4 shows the FTIR spectra of pristine MWCNTs and MWCNTs–COOH by different reaction times. Because of its nature, MWCNTs show very weak peaks of  aromatic ring stretching at the wavenumber of around 1600 cm−1. After treatment with acid mixture H2SO4/HNO3, a strong peak appears at wavenumber of 3400–3500 cm−1. This peak is assigned to the vibration of
aromatic ring stretching at the wavenumber of around 1600 cm−1. After treatment with acid mixture H2SO4/HNO3, a strong peak appears at wavenumber of 3400–3500 cm−1. This peak is assigned to the vibration of  group. Besides, a new other peak at 1000–1200 cm−1 also appears by the MWCNTs–COOH. This peak is assigned to
group. Besides, a new other peak at 1000–1200 cm−1 also appears by the MWCNTs–COOH. This peak is assigned to  bonding. This result clearly indicates that the COOH group is successfully attached onto the surface of MWCNTs.
bonding. This result clearly indicates that the COOH group is successfully attached onto the surface of MWCNTs.
Figure 4. FTIR spectra of pristine MWCNTs (a); MWCNTs–COOH (b, c, d) with reaction time of 2, 4 and 6 h, respectively.
Download figure:
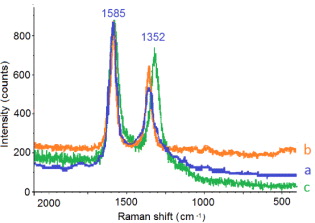
Standard image High-resolution imageFigure 5 show the Raman spectra of pristine MWCNTs and MWCNTs–COOH with different reaction times. The Raman spectra of pristine MWCNTs show two characteristic peaks. One at the Raman shift of 1352 cm−1 named D-band is assigned to the disordered graphitic structure of MWCNTs. The other peak at the Raman shift of 1585 cm−1 named G-band is assigned to the  bond in the graphitic plane. Thus, the intensity ratio of D-band to G-band (ID/IG) can be used for evaluation of graphitic structure of CNTs. From the Raman spectra, ID/IG ratio of pristine MWCNTs and MWCNTs–COOH by the reaction time of 2 and 6 h is 0.58, 0.66 and 0.87, respectively. The explanation for the increasing ID/IG ratio with increasing reaction time is that by the attachment of COOH group onto the surface of MWCNTs it is expected that the graphitic structure of MWCNTs is destroyed. That means that the intensity of D-band increases while the intensity of G-band decreases. The longer the reaction time is, the more the graphitic structure is destroyed. In other words, the intensity ratio of D-band to G-band (ID/IG) increases with increasing reaction time.
bond in the graphitic plane. Thus, the intensity ratio of D-band to G-band (ID/IG) can be used for evaluation of graphitic structure of CNTs. From the Raman spectra, ID/IG ratio of pristine MWCNTs and MWCNTs–COOH by the reaction time of 2 and 6 h is 0.58, 0.66 and 0.87, respectively. The explanation for the increasing ID/IG ratio with increasing reaction time is that by the attachment of COOH group onto the surface of MWCNTs it is expected that the graphitic structure of MWCNTs is destroyed. That means that the intensity of D-band increases while the intensity of G-band decreases. The longer the reaction time is, the more the graphitic structure is destroyed. In other words, the intensity ratio of D-band to G-band (ID/IG) increases with increasing reaction time.
Figure 5. Raman spectra of pristine MWCNTs (a), MWCNTs–COOH (b, c) with reaction time of 2 and 6 h, respectively.
Download figure:
Standard image High-resolution imageFigure 6 shows the TEM images of pristine MWCNTs and MWCNTs–COOH samples. It is clear that the pristine MWCNTs have a diameter from 8 to 18.2 nm. After attachment of COOH group onto the surface of MWCNTs, the diameter of MWCNTs–COOH slightly increases up to 22.2 nm. This result reconfirms the successful attachment of COOH group onto the surface of MWCNTs.
Figure 6. TEM images of pristine MWCNTs (a); MWCNTs–COOH (b) by reaction time of 6 h.
Download figure:
Standard image High-resolution imageFor the quantitative determination of the COOH group at the surface of MWCNTs, DSC/TGA measurements were performed. Figure 7 shows the DSC/TGA thermograms of pristine MWCNTs and MWCNTs–COOH at different reaction times.
Figure 7. DSC/TGA thermograms of pristine MWCNTs (a); MWCNTs–COOH (b, c) with reaction times of 2 and 6 h, respectively.
Download figure:
Standard image High-resolution imageBy the TGA thermogram of pristine MWCNTs, a slight weight loss at 100 °C could be observed. This weight loss is assigned to weight loss of moisture absorbed in MWCNTs. By DSC thermogram of pristine MWCNTs, there is evidence for a reaction taken during the measurements. In contrast, several weight loss steps are found by TGA thermogram of MWCNTs–COOH samples. The weight loss around 100 °C is also from the moisture absorbed in the sample. The weight loss from 250 to 600 °C is assigned to the weight loss of COOH group attached onto the surface of MWCNTs. By the reaction time of 2 h, the weight loss of COOH group is about 3.18% and by the reaction time of 6 h it increases to 8.39%. According to [12], the weight loss in the temperature range from 600 to 800 °C by MWCNTs–COOH sample corresponds to the oxidation of CNTs due to the decomposition of COOH group releasing oxygen into the chamber of DSC/TGA system. In addition, there are exothermic peaks at 353.9 °C by DSC thermograms for MWCNTs–COOH samples.
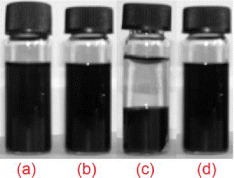
The attachment of COOH group onto the surface of MWCNTs has a strong effect on the solubility of MWCNTs in aqueous medium. Figure 8 shows photographs of MWCNTs before and after attachment with COOH in aqueous solution and the same solution after storage in 72 h.
Figure 8. Photographs of pristine aqueous solution of MWCNTs (a), aqueous solution of MWCNT attached with COOH (b) and after storage in 72 h of pristine aqueous solution of MWCNT (c), aqueous solution of MWCNT attached with COOH (d).
Download figure:
Standard image High-resolution imageThe dispersion of MWCNTs in aqueous solution is conducted by sonication. In the case of pristine MWCNTs, shortly after finishing the sonication, the accumulation of MWCNTs happens. In contrast, the aqueous solution with MWCNTs–COOH is stable during the store in 72 h.
3.2. Functionalization of MWCNTs with amine and silane groups
Figure 9 shows the FT-IR spectra of MWCNTs with amine and silane groups on the surface.
Figure 9. FT-IR spectra of MWCNTs–DDA (a) and MWCNTs–3APTES (b).
Download figure:
Standard image High-resolution imageBy the FTIR spectrum of MWCNTs–DDA, the sharp peak at wavenumber of 3356 cm−1 is assigned to the characteristic  bonding in the CONH group. Especially, the very strong peaks at 2950 and 2879 cm−1 from the C–H bonding indicate that the long carbon hydro chain (CH2)11 of dodecylamine has been attached onto the surface of MWCNTs. This result indicates that DDA was successfully attached onto the surface of MWCNTs.
bonding in the CONH group. Especially, the very strong peaks at 2950 and 2879 cm−1 from the C–H bonding indicate that the long carbon hydro chain (CH2)11 of dodecylamine has been attached onto the surface of MWCNTs. This result indicates that DDA was successfully attached onto the surface of MWCNTs.
By the FTIR spectrum of MWCNTs–3APTES, the peaks at wavenumber of 1073 and 805 cm−1 are assigned to the characteristic  and
and  bonding, respectively. The peak at 3450 cm−1 is from OH group, 1165 cm−1 from the out-of-plane vibration of
bonding, respectively. The peak at 3450 cm−1 is from OH group, 1165 cm−1 from the out-of-plane vibration of  bonding and the peaks at 2955, 2882 cm−1 from C–H bonding. This result confirms the attachment of 3APTES onto the surface of MWCNTs.
bonding and the peaks at 2955, 2882 cm−1 from C–H bonding. This result confirms the attachment of 3APTES onto the surface of MWCNTs.
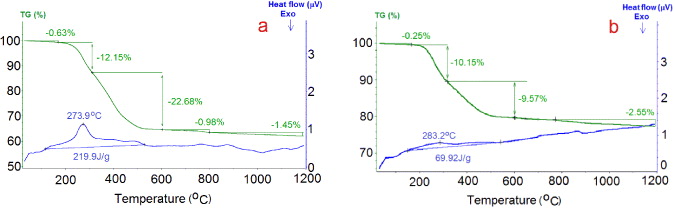
For the quantitative determination of the attachments of DDA and 3APTES onto the surface of MWCNTs, figure 10 shows the DSC/TGA thermograms of MWCNTs–DDA and MWCNTs–3APTES.
Figure 10. TGA/DSC thermograms of MWCNTs–DDA (a) and MWCNTs–3APTES (b).
Download figure:
Standard image High-resolution imageFor MWCNTs–DDA, a strong weight loss of about 34.8% in the temperature range from 200 to 600 °C is observed. This weight loss is due to the decomposition of DDA. Similarly, a strong weight loss of about 19.7% in the temperature range from 200 to 600 °C is also observed by TGA thermograms of MWCNTs–3APTES. This weight loss is from the decomposition of 3APTES. The decomposition of DDA as well as of 3APTES is accompanied by an endothermic peak in this temperature range observed by DSC thermograms.
4. Conclusion
The surface modification and functionalization of MWCNTs with carboxylic group, DDA and 3APTES were investigated. The attachment of COOH group, DDA and 3APTES onto the surface of MWCNTs could be proved by FT-IR spectra and Raman spectra. By the modification with COOH group, the content of COOH group on the surface of MWCNTs increases with increasing reaction time from 3.18% for 2 h to 9.39% for 6 h. The content of DDA and 3APTES found by MWCNTs–DDA and MWCNTs–3APTES are 34.8 and 19.7%, respectively. The successful attachments of these organic compounds onto the surface of MWCNTs open diversified application of CNTs in biology, nanocomposite, solar cell, etc.
Acknowledgments
We acknowledge the National Foundation for Science and Technology Development (NAFOSTED) for financial support (project no. 104.04.28.09).