Abstract
A chemical reduction method for preparing colloidal copper nanoparticles in water and ethylene glycol (EG) is reported. The obtained copper nanoparticles were characterized by powder x-ray diffraction (XRD), transmission electron microscopy (TEM) and UV-visible spectrophotometry (UV-vis). Surface plasmon resonance peaks immediately after the synthesis appeared at 579 and 551 nm for the colloidal copper in water and EG, respectively. The stability of colloidal copper in EG was longer than that in water. The color of solution in water changed from light-red to black and the nanoparticles mostly precipitated after 22 days, which is attributed to the oxidation of copper nanoparticles in copper oxide (I), as was confirmed by optical absorption measurements. In EG, copper nanoparticles were red and stable even after 2 months. Ascorbic acid plays a role as antioxidant for colloidal copper, due to its ability to scavenge free radicals and reactive oxygen molecules. Polyvinyl pyrrolidone works both as size controller and polymeric capping agent because it hinders the nuclei from aggregation through the polar groups, which strongly absorb the copper particles on the surface with coordination bonds.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metallic nanoparticles have received significant attention by researchers due to their unique properties, such as depression of melting temperature, conductivity, magnetism and light absorption in comparison with bulk metals [1–4]. Among various metal particles, copper nanoparticles are attracting increased attention because of their optical and electrical conducting properties at a significantly lower cost than gold and silver [5, 6]. Copper nanoparticles are particularly attractive for applications in printed circuit boards (PCBs) and flexible electronics [3, 7–10], catalysts [11–14], light emitting diodes (LED) [10] and biocompatibility [15, 16]. However, the difficulty of copper reduction in mild reaction conditions or the easy oxidation of copper nanoparticles in air under ambient atmosphere conditions in comparison to noble metals like Au and Ag are the disadvantages of copper, which must be overcome.
Copper nanoparticles have been synthesized by different methods. To date, thermal reduction [17], thermal decomposition [5, 18], direct electrochemical reduction from CuO nanoparticles [6], mechano-chemical process [19], polyol process [7, 9, 20], chemical reduction [21–24], in-situ synthesis in polymers [15], electro-exploding wire (EEW) [25] and ion beam radiation [26] have all been developed to prepare nanostructured copper. Chemical reduction is one of the most convenient methods for the synthesis of metallic nanoparticles because the synthesis process is simple and the size and shape of nanostructured copper can easily be controlled. Compared with conventional methods, it is much faster, cleaner and more economical.
However, copper nanoparticles tend to be fairly unstable in solution and therefore special precautions have to be taken to avoid their aggregation or precipitation and oxidation during the preparation of such colloidal particles in solution. To protect copper nanoparticles against oxidation during preparation and storage, the reaction is in many cases performed in non-aqueous media, at low precursor concentration, and in an inert atmosphere [27], ascorbic acid is often used to play the role of reducing agent and antioxidant of colloidal copper [28]. Surfactants such as tetraethylenepentamine [22], cetyltrimethylammonium bromide [29], tetraoctylammonium bromide [30], polyelectrolytes such as poly(ethylene imine) [23], polyethylene glycols [24], polyvinylpyrrolidone [26] and poly(etherether ketone) [31] were applied to the synthetic system as dispersant or modifier. These materials were used not only to control the particle size, particle size distribution and shape but also to prevent aggregation and further oxidation. Sodium borohydride [3], sodium hypophosphite [9] and ascorbic acid [24] were usually utilized as reductant for the chemical reduction synthesis of nanostructured Cu.
We previously reported that polyethylene glycol (PEG) works both as a size controller and as a capping agent of copper nanoparticles [32]. In the present paper, we report a chemical reduction method to synthesize copper colloids in polar solvent without protective gas and we improve stability against aggregation or precipitation of copper nanoparticles. Ascorbic acid (natural vitamin C) was employed as a protective agent to prevent the nascent Cu nanoparticles from oxidation during the synthesis process and in storage. Two reaction media, water and ethylene glycol, were used as solvents. Their influence on the stability of the nanoparticle solutions is discussed. The protective role of polyvinyl pyrrolidone (PVP) was also investigated.
2. Experimental
2.1. Material
All of the chemicals were analytical grade and were used as purchased without further purification. Copper (II) sulfate pentahydrate salt CuSO 4·5H 2 O was 98.0% pure (Merck). Ethylene glycol (EG) was purchased from Guangdong Guanghua Chemical Factory (China). Polyvinyl pyrrolidone (PVP, average molecular weight of 40 000) from Merck was used as the capping agent. Sodium borohydride (NaBH 4, Reagent Plus 99%) from Sigma-Aldrich was used as the main reducing agent while ascorbic acid (99.7%, Prolabo) was used as the antioxidant of colloidal copper. Sodium hydroxide NaOH (>98%, Shantou Xilong Chemical Factory Guangdong, China) was also used to adjust the pH. Aqueous solutions were all made in deionized water.
2.2. Synthesis of copper nanoparticles
Ascorbic acid solution was prepared by dissolving 52.8 mg in 15 ml of distilled deionized water. A solution of copper (II) sulfate pentahydrate CuSO 4·5H 2 O (0.01 M) in deionized water was prepared separately and this was added to the solution of ascorbic acid under strong magnetic stirring. Various amounts of PVP were then added to explore its influence on nanoparticle size. Then the solution of NaOH (1 M) in deionized water was added dropwise to adjust the pH up to 12. After stirring at room temperature for about 1 h, a solution of NaBH 4 (0.1 M) in deionized water was added to the mixture under continuous strong stirring for about 10 min. The initial blue color of the reaction mixture turned yellow and eventually light-red, as shown in figure 1(a). The copper nanoparticles were separated and washed with deionized water by centrifugation, while using acetone as a non-solvent in order to remove excess PVP. The resulting precipitates were dried under vacuum for 3–4 h.
Figure 1 Colloid solutions of Cu nanoparticles in water (a) and Cu nanoparticles in EG (b).
A similar experiment was carried out in ethylene glycol instead of deionized water to see the effect of solvent on the stability of the copper nanoparticles. The obtained colloid solution is shown in figure 1(b) with a deeper red color.
2.3. Characterization
Synthesized samples were studied by UV-vis absorption spectroscopy from a double-beam spectrophotometer (Varian Cary 100) in the wavelength range from 190 to 1100 nm. Transmission electron microscopy (TEM) was used to study the particle size. Samples for TEM measurements were suspended in ethanol and ultrasonically dispersed. Drops of the suspensions were placed on a copper grid coated with carbon. The structure of the synthesized nanoparticles was analyzed with XRD (D8-Advance, Bruker AXS) with a Cu-Kα radiation source (λ=0.154 056 nm). XRD patterns were recorded from 20° to 80° with a scanning step of 0.01.
3. Results and discussion
3.1. The influences of solvent on solution stability
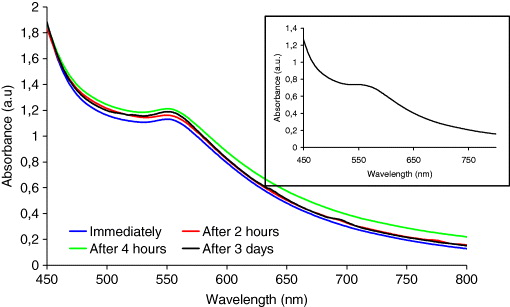
UV-vis absorption spectra of Cu nanoparticles are shown in figures 2 and 3. The absorption bands for Cu nanoparticles have been reported to be in the range of 500–600 nm [33]. In the present study, we have been able to prepare surface capped nanoparticles of copper and it is shown that the surface plasmon resonance can be maintained over time. The UV-vis absorbance spectra of Cu nanoparticles in water are shown in figure 2. The spectra were measured immediately after synthesis, 2 h, 4 h and 3 days from sample preparation. During the measurement period, a blue shift of the absorbance maximum for copper nanoparticles was observed from 579 to 576 and 575 nm, and finally to 548 nm for the surface plasmon resonance of copper nanoparticles in water. In our earlier work [32], we found that the surface plasmon resonance value was located around 560–570 nm in water when using PEG 6000. Many articles have reported surface plasmon absorbance blue shifts with decreasing size [34]. However, other effects could explain such a shift such as the oxidation of colloidal copper due to its reaction with dissolved oxygen in water. Partial oxidation also causes the blue shift because the absorbance of copper oxide occurs at shorter wavelengths [5, 28].
Figure 2 The UV-vis spectra of colloidal copper nanoparticles synthesized in water at different times. The insert is the UV-vis spectra of the colloidal copper nanoparticles after 22 days.
Figure 3 The UV-vis spectra of colloidal copper nanoparticles synthesized in EG at different times. The insert is the UV-vis spectra of the colloidal copper nanoparticles after 22 days.
According to [34, 35], when the particles are spherical, the surface plasmon resonance and the blue shift are affected by the size distribution. To study the stability in size, the surface plasmon resonance of colloid copper nanoparticles in water and EG was measured 22 days after preparation. Figure 3 shows the surface plasmon resonance of copper nanoparticles in EG. The result in table 1 shows that the surface plasmon resonance does not change during 3 days after preparation. A slight change is observed after 22 days at 547 nm, as this can be seen in the spectrum shown in the insert of figure 3. Furthermore, the solution was confirmed to be stable even after 2 months. However, the optical absorbance of copper nanoparticles in water 22 days after preparation showed no surface plasmon resonance, as shown in the inset of figure 2. This could be a consequence of oxidation to form copper oxide (I) nanoparticles since similar optical absorbance of copper oxide (I) nanoparticles have been reported in [36].
Table 1. Plasmon resonance of colloidal copper nanoparticles synthesized in water and EG at different times.
| Colloidal copper | 579 | 576 | 575 | 548 |
| nanoparticles | ||||
| synthesized | ||||
| in water | ||||
| Colloidal copper | 551 | 550 | 550 | 551 |
| nanoparticles | ||||
| synthesized | ||||
| in EG |
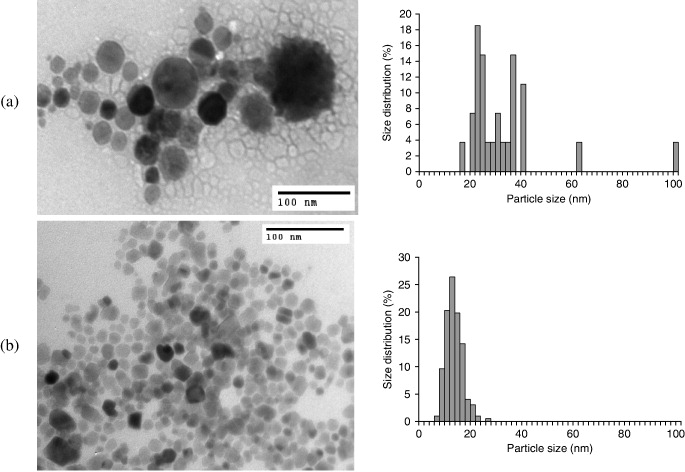
Figure 4 shows TEM images for colloidal Cu nanoparticles synthesized in water and EG together with histograms of particle size distribution. It is observed that the particles are nanosized and approximately spherical in both solvents. The mean diameter of the copper nanoparticles with water as the reaction solvent is 22 nm. We can see that those particles are large and widely dispersed. Figure 4(b) shows a TEM image of colloidal copper particles that were synthesized in EG. In comparison with the copper nanoparticles in water, the mean diameter of the nanoparticles is smaller (about 10 nm) as well as with a narrower distribution ranging from 4 to 26 nm. The reaction with dissolved oxygen in water leads to oxidation of colloidal copper in water, and this is shows by optical absorption measurements, as discussed above.
Figure 4 Transmission electron micrographs of Cu nanoparticles synthesized in water (a) and in EG (b).
3.2. The effect of PVP concentration on copper nanoparticles size
An important feature in the production of the metal nanoparticles is the ability to keep them physically isolated from each other to prevent agglomeration and oxidation processes. The stabilization is commonly achieved by using surfactants as protective molecules, which avoid the aggregation by binding to the nanoparticle surface [24, 26]. Among these molecules, PVP has been widely used as a steric stabilizer or capping agent to protect nanoparticles from agglomeration [18, 27].
The stabilization of metal colloids, and the size and shape of nanomaterials depend strongly on the solution concentration of PVP [25]. In this report, PVP works both as a size controller and as a polymeric capping agent because it hinders the nuclei from aggregation through the polar groups, which are strongly adsorbed at the surface of copper particles on the surface with coordination bonds.
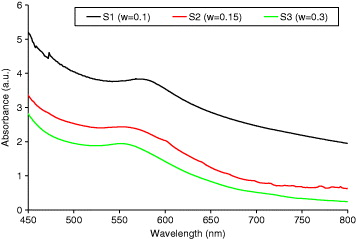
Figure 5 shows the UV-vis absorbance spectra of three colloidal solutions synthesized under the same conditions with different ratios of [PVP] to [Cu 2+] (as detailed in table 2). As is clearly seen, the maximum optical absorbance appears at a wavelength of around 550 nm, which is related to the surface plasmon resonance of copper nanoparticles. The peak position is blue shifted by about 1 nm with the increase in [PVP]/[Cu 2+] from 0.1 to 0.3. However, this small shift does not corroborate the particle size analysis by TEM since we should see a stronger shift as the particle size decreases, as predicted by Mie's theory [35].
Figure 5 The UV-vis spectra of colloidal copper nanoparticles with different ratios of [PVP] to [Cu 2+].
Table 2. Effects of the [PVP] to [Cu 2+] ratio on particle size.
| S1 | 0.1 | EG | 72 | 553 |
| S2 | 0.15 | EG | 22 | 552 |
| S3 | 0.3 | EG | 6 | 552 |
To obtain evidence that the nanoparticles formed in situ are pure copper metallic nanoparticles, XRD was performed on powder samples. Figure 6 shows the XRD patterns of the synthesized Cu samples. Samples S1–S3 were synthesized using different ratios [PVP]/[Cu 2+]. It can be found that diffraction peaks with strong intensities appear at angles corresponding to (111), (200) and (220) planes of fcc structure of copper with the space group of Fm3m (JCPDS No. 85–1326). Moreover, despite the fact that the measurements were done 1 week after powder preparation, there are no other peaks attributable to copper oxides as a secondary phase. This means that our copper nanoparticles are fairly stable against oxidation, at least at x-ray detection level. This stability against oxidation is likely attributable to the presence of the PVP capping layer at the surface of the particles. During particle synthesis, Cu ions can coordinately bond with N or O atoms present in PVP, so that the synthesized copper particles are covered by an absorbed layer of PVP [7].
Figure 6 X-ray diffraction (XRD) patterns of copper nanoparticles with different ratios of [PVP] to [Cu 2+] (w=0.1, 0.15 and 0.3).
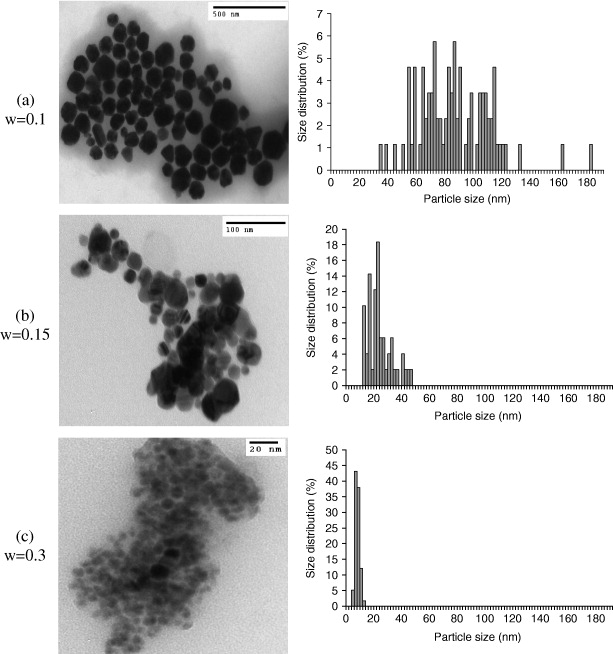
The surfactants can form an absorption layer at the surface of the copper nanoparticles to prevent the particles aggregating by increasing the repulsion force between them. Figure 7 shows the TEM images for the colloidal Cu nanoparticles synthesized and the histograms of particle size distribution at different ratios of [PVP] over [Cu 2+]. Figures 7(a) and (b) show a TEM image of copper nanoparticles and a histogram of particle size distribution at [PVP]/[Cu 2+]=0.1 and 0.15. We can see in the picture that the mean diameters of copper nanoparticles are about 72 nm and 22 nm (as reported in table 2), respectively. The diameters are widely distributed. Within this ratio, the particles are well separated and spherical. Figure 7(c) shows the TEM image of copper nanoparticles for w=0.3. We can see that the copper nanoparticles are spherical, their size is small (about 6 nm) and their size distribution is narrow. This clearly shows that the size of the Cu nanoparticles decreases with increasing [PVP]/[Cu 2+]. Consequently, at a higher molecular ratio of PVP over CuSO 4, more PVP molecules are adsorbed onto the Cu nanoparticles' surface, keeping them from excessive growth and leading to the formation of smaller nanoparticles.
Figure 7 Transmission electron micrographs of Cu nanoparticles with different ratios of [PVP] to [Cu 2+] (w=0.1, 0.15 and 0.3).
4. Conclusion
In this paper, colloidal copper nanoparticles were synthesized successfully by a chemical reduction method in water and EG. In both media, the particles are rather spherical, and it has been found that the average diameters of the copper nanoparticles are 22 nm and 10 nm in water and EG, respectively, for the most stable solutions. Stability of the colloidal particles and time evolution of the optical absorption were also investigated. Colloidal copper in EG is generally stable even after 2 months. In water, it was fairly precipitated after 22 days. Moreover, it was clearly shown that the ratio of [PVP] to [Cu 2+] has a remarkable effect on particle size and agglomeration of the produced nanoparticles. At higher [PVP]/[Cu 2+], the diameter of the copper nanoparticles was very small with narrow dispersion.
Acknowledgment
The authors appreciate the financial support of Vietnam National University in Ho Chi Minh City.