ABSTRACT
We present the results of an experimental study on the interaction of atomic deuterium with coronene films. The effects of D atom irradiation have been analyzed with infrared spectroscopy. The spectral changes provide evidence for deuteration of the outer edge coronene C sites via a D addition reaction. A cross section of 1.1 Å2 is estimated for the deuteration process of coronene. HD and D2 molecules form, through abstraction reactions, on deuterated coronene sites with a cross section of 0.06 Å2. The magnitude of both cross sections is in line with an Eley–Rideal type process. The results show that hydrogenated neutral polycyclic aromatic hydrocarbon molecules act as catalysts for the formation of molecular hydrogen.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
H2 is the most abundant molecule in the interstellar medium (ISM) and plays a key role in many astrophysical processes. No efficient gas-phase reactions exist for the conversion of atomic to molecular hydrogen under ISM conditions. There is a general consensus on the catalytic role of interstellar dust grains for the formation of H2. Laboratory experiments have indeed shown its formation via the recombination of atomic hydrogen on a variety of dust grain analog surfaces (e.g., Pirronello et al. 1997, 1999; Manicò et al. 2001; Hornekær et al. 2003). These measurements have revealed that this process is efficient at temperatures below 20 K. At higher temperatures, H atom recombination is not active as desorption of H atoms from weakly bound physisorbed sites is fast and recombination cannot take place (e.g., Vidali et al. 2005). More tightly bound states are necessary to form H2 at high grain temperatures. Strong binding sites on highly defected surfaces (Cuppen & Herbst 2005) and chemisorption states on graphite (Cazaux & Tielens 2004) have been proposed. Experiments have shown that molecular hydrogen formation on graphitic surfaces could be an efficient process under photodissociation region (PDR) and post-shock conditions (Hornekær et al. 2006a, 2006b). D atom irradiation experiments have revealed that the formation of H2 by abstraction of chemisorbed H atoms in the aliphatic CH2, 3 groups of hydrogenated carbon grains occurs with a negligible activation energy for a range of grain and H atom temperatures relevant to astrophysical environments such as diffuse interstellar clouds and PDRs (Mennella 2008).
H2 formation on polycyclic aromatic hydrocarbons (PAHs) has also been proposed. Theoretical studies have suggested that H2 can form through abstraction of edge H atoms of PAH cations by incoming H atoms (Cassam-Chenaï et al. 1994). Alternatively, it has been proposed that H2 can form on PAH cations in a two-step process: (1) an extra H atom is added to an edge site and (2) abstraction of the excess H atom by a second incoming H atom (Bauschlicher 1998). In the case of cations, the formation does not have significant energy barriers. Other processes involving PAH cations have been suggested. Le Page et al. (2009) have proposed that, after addition of an H atom on the edge of a PAH, H2 can be released through dissociative recombination of the hydrogenated ion with an electron. Recently, Szczepanski et al. (2011) have studied H2 ejection from a CH2 aliphatic group of excited protonated PAHs: large amplitude scissors vibrations lead to the formation and ejection of H2 from the C atom of this group. Low barrier routes to molecular hydrogen formation on neutral coronene have been computed with first-principle density functional theory (DFT): H2 should form by H abstraction on (super)hydrogenated coronene (Rauls & Hornekær 2008). Laboratory experiments have shown that atomic and molecular hydrogen react with benzene and small PAHs (Petrie et al. 1992; Scott et al. 1997; Le Page et al. 1997; Snow et al. 1998). More recently, the (super) hydrogenation of coronene has been observed with scanning tunneling microscopy and thermal desorption measurements following exposure of a coronene layer deposited on graphite to D atoms (Thrower et al. 2011). During D irradiation of aromatic carbon grains, addition and exchange reactions (with the formation of HD) have been observed with IR spectroscopy on hydrogenated graphene structures of the grains (Mennella 2011). However, experimental results supporting the hypothesis of PAHs as active catalysts for H2 formation under interstellar conditions have so far been lacking.
In this letter, we report on the IR spectrum evolution of coronene films exposed to D atoms. The spectral changes indicate deuteration of the outer edge sites of coronene and exchange reactions on these sites, with the formation of HD and D2 molecules. The results show experimentally, for the first time, the catalytic role played by neutral PAH molecules in the formation of H2 molecules.
2. EXPERIMENT AND RESULTS
The samples considered in the present study have been produced by coronene evaporation in an ultrahigh vacuum chamber with a base pressure of <1 × 10−9 mbar. Coronene (Sigma-Aldrich; 99% purity) was heated to 185°C in a thermal evaporation source. The source was degassed thoroughly prior to sample preparation in order to reduce the evaporation of any contaminants. Coronene molecules were deposited on CsI substrates located ca. 3 cm from the source resulting in a deposition rate of 0.6 Å s−1 as determined previously with a quartz crystal microbalance in the substrate position. The resulting samples are compact films of coronene with film thicknesses of 150 and 180 nm for the COR01 and COR02 samples, respectively. The infrared spectrum of the COR01 sample is shown in Figure 1. In addition to the vibrational modes of coronene, two very weak bands are present at 2925 and 2850 cm−1. They correspond to the sp3 asymmetric and symmetric C-H stretching modes of the CH2 group, respectively. These features are attributed to hydrocarbon contamination arising from air exposure during transfer of the samples. The contaminant features are also present in the spectrum of a blank CsI substrate used as a reference (see inset to Figure 1).
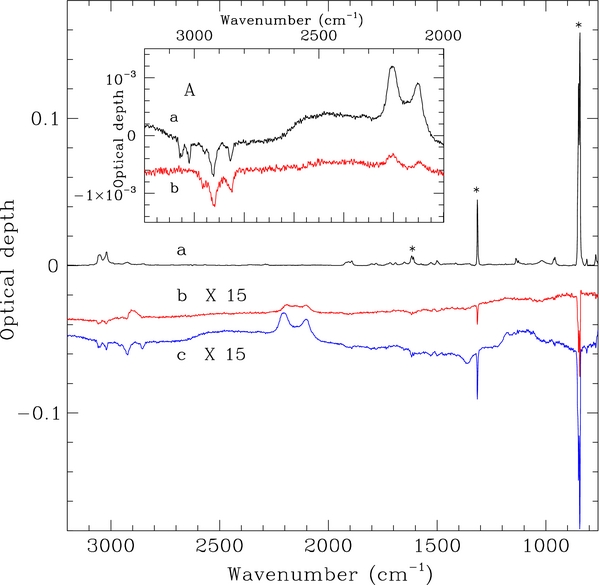
Figure 1. Evolution of the IR spectrum during D atom irradiation of the COR01 film. The initial spectrum (a) and those after irradiation (shown after subtraction of the initial spectrum) of 1.1 × 1016 (b) and 3.4 × 1018 D atoms cm−2 (c) are plotted. The difference spectra are offset for clarity. The bands with an asterisk correspond to the out-of-plane C-H bend doublet at 849 and 843 cm−1, the in-plane C-H bend at 1314 cm−1, and the C=C stretch doublet at 1616 and 1608 cm−1. Inset A: the C-H and C-D stretching bands of COR01 (a) and those of a blank CsI substrate (b) are compared after irradiation of 3.4 × 1018 D atoms cm−2.
Download figure:
Standard image High-resolution imageThe coronene films were subsequently irradiated with an atomic deuterium beam produced by microwave excited dissociation of molecular deuterium (99.96% purity). D atoms in the beam have a Maxwellian velocity distribution corresponding to 300 K (Mennella 2006). The experiments of the present work have been performed in a vacuum chamber with a base pressure of ∼2 × 10−8 mbar. The IR spectral evolution of the samples during irradiation has been studied with a resolution of 2 cm−1. Exposure of coronene to D atom at room temperature results in a general intensity decrease of the coronene IR bands. The intensity variations are small with respect to the initial band intensity (Figure 1), consistent with D atoms only being able to interact with the superficial layers of coronene films, due to the compact nature of the films. A decrease of the sp3 C-H stretching bands is also observed with increasing D atom fluence, FD. This is attributed to D atom interaction with contaminant hydrocarbon molecules. This conclusion is supported by experiments using blank CsI substrate where a similar intensity decrease of the aliphatic C-H stretching bands is observed during D atom irradiation (see Figure 1). In addition to the intensity decrease of the aromatic features of coronene, a simultaneous increase in the intensity of the sp3 C-D asymmetric and symmetric stretching modes of the CD2 group (at 2205 and 2100 cm−1, respectively) and the C-H and C-D stretching modes of the CHD group (at 2903 and 2152 cm−1, respectively) occurs during D irradiation.
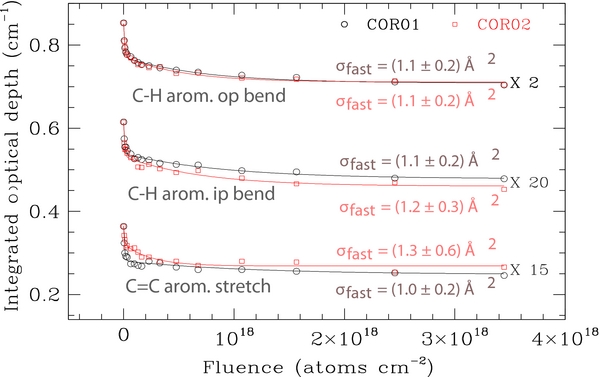
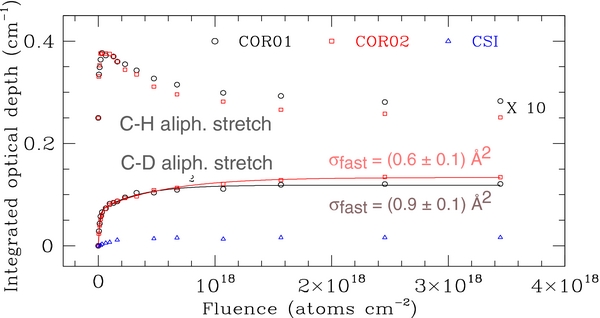
The trend of the integrated optical depth, τ, as a function of FD of the out-of-plane C-H bend, in-plane C-H bend, and C=C stretching modes of coronene is reported in Figure 2. Figure 3 shows the evolution of the C-H and C-D modes of the CHD and CD2 groups. Unlike τ of the C-D bands, which monotonically increases during irradiation, the band intensity of the C-H mode of the CHD group reaches a plateau value around FD = 5 × 1016 H atoms cm−2 and then decreases at higher fluences. The experimental data of Figure 2 are well fitted with an initial fast decay rate, and a slow decay rate, which is a factor of at least 30 times smaller than the corresponding fast rate cross section, rather than a single cross section. A similar result has been obtained for the trend of the aliphatic CH group concentration in hydrogenated carbon films and grains (Biener et al. 1995; Mennella 2008). The fast rate cross sections of the three considered bands are equal within the errors, suggesting that C-H and C=C bonds are "destroyed" simultaneously during irradiation (see Figure 2(a)). The weighted average of the cross section is 1.1 ± 0.1 and 1.1 ± 0.2 Å2 for the films investigated. This hydrogenation cross section compares well with the values of 1.3 and 4.5 Å2 obtained from the analysis of the C-H stretch band in two D irradiation experiments of hydrogenated carbon films (Küppers 1995). Similarly, we have estimated the cross section for the formation of the aliphatic CD2 group from the increase of the sp3 C-D bands of this group with FD (see Figure 3). We note that the integrated optical depths in Figure 3 include the contribution of CD2 groups produced by D atom irradiation of contaminant hydrocarbon molecules. The integrated optical depths obtained during D atom irradiation of the blank CsI substrate are at most 14% of the values obtained for two coronene samples (see Figure 2(b)). Contamination does not affect the estimate of the formation cross section of the CD2 groups. Fits to the difference τ(coronene)−τ(blank CsI) provide cross sections (0.7 ± 1 and 0.9 ± 1 Å2, for COR01 and COR02 films, respectively), which are equal, within the errors, to those reported in Figure 2(b) without this correction. The small values of all the estimated cross sections indicate that Eley–Rideal type processes4 drive the spectral changes produced by D atom irradiation of coronene.
Figure 2. Evolution with D atom fluence of the band integrated optical depth of the out-of-plane (op) and in-plane (ip) C-H bend, and C=C stretch bands for COR01(circles) and COR02 (squares) samples. The different data sets are offset. The best fit to the data of the relation  is also shown. The constant c represents the band optical depth of the coronene deeper layers not interacting with D atoms.
is also shown. The constant c represents the band optical depth of the coronene deeper layers not interacting with D atoms.
Download figure:
Standard image High-resolution imageFigure 3. Evolution with D atom fluence of the band integrated optical depth of the C-H and C-D bands, respectively, of the CHD and CD2 aliphatic groups for COR01 (circles) and COR02 (squares) samples. The different data sets are offset for clarity. The intensity of the C-D bands of the CD2 groups for the blank CsI substrate (triangle) is also plotted. The best fit to the data of the relation  for the C-D mode is also shown.
for the C-D mode is also shown.
Download figure:
Standard image High-resolution image3. DISCUSSION
The spectral changes of coronene films indicate that both addition of D atoms (deuteration) to coronene and exchange reactions take place after exposure to 300 K D atoms. Addition reactions lead to the formation of CHD groups on the outer edge carbon sites of coronene, which are bonded to hydrogen atoms, through the transformation of the aromatic C=C double bond into a single C-C bond and a C-D bond. An aromatic C-H bond is transformed into an aliphatic CHD group, with the formation of a radical C site on the adjacent outer edge C atom. This reaction is exothermic since the gain of ∼90 kcal mol−1 produced by the formation of a C-H bond exceeds the energy loss of 20 kcal mol−1 due to the transfer of the aromatic C=C double bond to a C-C single bond (Küppers 1995). DFT calculations on the hydrogenation of the coronene molecule show that the addition reaction of a single H atom to the outer edge sites has an energy barrier of 60 meV (Rauls & Hornekær 2008). Addition of a second D atom takes place in the outer edge site adjacent to that already deuterated through a barrierless reaction. The observed intensity decrease of the C-H and C=C bands of coronene is evidence for hydrogenation of the outer edge sites of coronene.
It is evident that addition reactions alone cannot explain the formation of the aliphatic CD2 groups and the decrease in the number of the CHD groups at high FD. Rather, the observed trends show that exchange reactions take place on the CHD groups during irradiation. Exchange reactions are expected to occur in a two step process where the first abstraction of an H (D) atom, leading to HD (D2) formation, is followed by D addition, forming CD2 (CHD). A continuous series of addition and abstraction reactions will therefore result in the formation of CD2 groups at the outer edge sites. Therefore, the interaction of D with coronene forms a CHD group via addition of D atoms to the outer C sites and destroys them (transforms into CD2) via H abstraction reaction. At low fluences, when many sites can be deuterated, competition between the two processes leads to an increase in the number of the CHD groups with increasing FD. Equilibrium is reached for a relatively small D atom fluence range of around 5 × 1016 D atoms cm−2. For high fluences, destruction prevails since the number of aromatic coronene CH sites that can be deuterated tends to zero and additional CHD groups cannot be formed. The optical depth of the C-H stretching mode of the CHD group at the end of D irradiation is 25% and 1% of its maximum value at 5 × 1016 D atoms cm−2, respectively, for the COR01 and COR02 samples. The observed experimental trends suggest that an almost complete deuteration of coronene with an almost complete D/H exchange on the outer edge sites of coronene occurs during D irradiation.
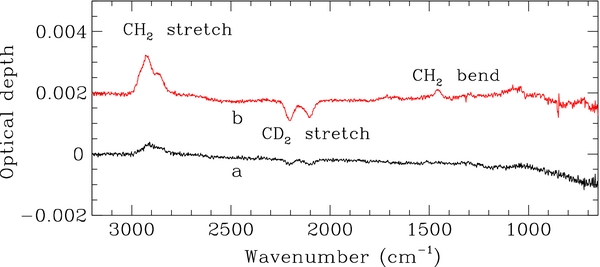
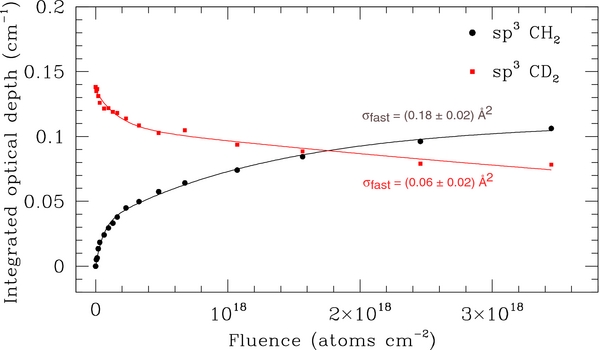
Formation of HD and D2 molecules takes place when the D atom irradiates a coronene. However, at high fluences, only a continuous exchange D/D, which does not produce spectral variations, remains active with the formation of D2. To confirm this, at the end of D atom irradiation of COR02, we irradiated the sample with H atoms at 300 K. The resulting spectral variations are shown in Figure 4. H atom exposure results in a decrease of τ for the aliphatic C-D stretch bands of the CD2 group and an increase of the corresponding C-H stretching bands. The intensity of the C-H bend mode of the CH2 group at 1456 cm−1 also increases. A small decrease of the in-plane and out-of-plane C-H coronene bands is also observed, indicating that a residual H addition to coronene, with a contribution to the formation of the CH2 groups, also takes place. The evolution with H atom fluence of the C-H and C-D stretching bands is reported in Figure 5. The estimated abstraction cross section of 0.06 Å2 compares well with the values of 0.03 and 0.05 Å2 obtained, for D irradiated hydrogenated carbon grains (Mennella 2008) and hydrogenated carbon films (Biener et al. 1995), respectively. The formation cross section of the CH2 groups is larger than the abstraction cross section derived from the intensity decrease of the aliphatic C-D bonds. This in contrast to what would be expected for exchange reactions alone: the abstraction and addition cross sections obtained during H atom irradiation of deuterated aliphatic carbon grains were indeed equal within experimental errors (Mennella 2008). Therefore, the estimated formation cross section of the CH2 groups is the result of two processes: residual hydrogenation of coronene outer edge C sites and H atom addition to the deuterated aliphatic CD2 groups via abstraction. Note that the hydrogenation cross section (1.1 Å2) is larger than the exchange cross section (necessarily less than or equal to the abstraction cross section of 0.06 Å2) and can therefore give rise to a higher estimated value for the CH formation cross section. This result suggests that COR02 film was not fully deuterated during the initial exposure to the D atom beam.
Figure 4. Evolution of the IR spectrum during H irradiation of COR02 film after exposure to the initial D atom fluence of 3.4 × 1018 D atoms cm−2. The spectra after irradiation (shown after subtraction of the spectrum at the end of D irradiation) of 9.6 × 1016 (a) and 3.4 × 1018 H atoms cm−2 (b) are plotted. The spectra are offset for clarity.
Download figure:
Standard image High-resolution imageFigure 5. Evolution with H atom fluence of the aliphatic C-H (filled circles) and C-D (squares) stretch band integrated optical depth. The best fit to the data of the relations  and
and  , respectively, for the C-H and C-D modes is also shown.
, respectively, for the C-H and C-D modes is also shown.
Download figure:
Standard image High-resolution imageConcerning deuteration of the inner carbon sites, DFT calculations indicate that addition of a D atom to a coronene center site has a barrier of about 150 meV. The barrier is lowered at 30 meV when four edge sites are already deuterated (Rauls & Hornekær 2008). Therefore, we expect that inner sites are also deuterated; however, it is not possible to discriminate them from the deuterated edge sites with IR spectroscopy. In fact, as indicated by preliminary DFT calculations, the frequencies of the inner C-D modes fall in a frequency range overlapping that of the C-D edge sites. It is worth noting that mass spectroscopy performed during thermal programmed desorption of coronene irradiated with D atoms at 1800 K provides clear evidence for deuteration of inner coronene sites (J. D. Thrower et al. 2012, in preparation).
Finally, we note that the estimated cross sections refer to the interaction of D/H atoms with coronene in solid state. However, since the coronene molecules only weakly interact and since H atoms impact and directly interact with coronene without diffusion and/or absorption, the cross sections are also representative of the interaction of H atoms with coronene in gas phase.
4. CONCLUSIONS
The IR spectral changes induced by D atom impact at the surface of coronene films give clear evidence for the operation of hydrogenation and exchange reactions, with the formation of HD and D2 molecules, via Eley–Rideal processes. The formation mechanism is similar to that proposed by Bauschlicher (1998) for cations and the results are in good agreement with DFT calculations of the interaction of D atoms with the coronene molecule. The present results show that, like hydrogenated carbon grains, hydrogenated PAHs play a role as catalysts for H2 formation at temperatures where H atom recombination on grain surface is not active.
It has been proposed that hydrogenated PAHs can be present in space (Schutte et al. 1993; Bernstein et al. 1996). Using the C-H stretching spectral region as a probe for the hydrogenation of PAHs, Bernstein et al. (1996) compared interstellar emission spectra of associated objects representative of high and low excitation environments with laboratory spectra of hydrogenated PAHs. They concluded that hydrogenated PAHs likely contribute to the observed emission of the aliphatic bands observed beside the 3.3 μm feature and suggested formation of hydrogenated PAHs by H atom addition.
H addition reactions could be important for neutral PAHs in low UV flux environments where large PAHs can retain excess H atoms. Considering the low excitation PDR in ρ Oph, Habart et al. (2003) suggested that H2 forms by abstraction of H atoms of PAHs. They proposed that the H2 emission can be explained with an H2 formation rate, R = 1 × 10−16 cm3 s−1, at Tgas = 330 K and an abundance of carbon locked in PAHs relative to hydrogen of nC/n = 7.5 × 10−5. The H2 formation rate due to abstraction of H from hydrogenated PAHs can be expressed as  (cm3 s−1), where vH (TH) is the H atom velocity (temperature), σ is the abstraction cross section, and nCH/n is the number of H atoms locked in the aliphatic CH2 groups of hydrogenated PAHs per H in gas phase. Substituting in the previous relation the value of the abstraction cross section, 6 × 10−18 cm2, estimated in the present work for HD formation on coronene, the gas temperature and the carbon locked in PAHs reported by Habart et al., one obtains R = 1.2 × 10−16 cm3 s−1, comparable to the rate inferred from observations. However, this value is an upper limit since it assumes that all PAHs are fully hydrogenated and hence contribute to H2 formation. Their contribution to R scales as the fraction of the carbon atoms bonded to hydrogen in hydrogenated neutral PAHs, nCH/nC. To constrain the contribution of hydrogenated PAHs to R, knowledge of their UV photodehydrogenation cross section is necessary. In particular, experimental cross sections on hydrogenated coronene would, in combination with the cross sections measured here, finally enable an estimation of the degree of (super-)hydrogenation of coronene as a sample PAH under PDR and general ISM conditions, giving a tight estimation of the contribution of PAHs to interstellar H2 formation.
(cm3 s−1), where vH (TH) is the H atom velocity (temperature), σ is the abstraction cross section, and nCH/n is the number of H atoms locked in the aliphatic CH2 groups of hydrogenated PAHs per H in gas phase. Substituting in the previous relation the value of the abstraction cross section, 6 × 10−18 cm2, estimated in the present work for HD formation on coronene, the gas temperature and the carbon locked in PAHs reported by Habart et al., one obtains R = 1.2 × 10−16 cm3 s−1, comparable to the rate inferred from observations. However, this value is an upper limit since it assumes that all PAHs are fully hydrogenated and hence contribute to H2 formation. Their contribution to R scales as the fraction of the carbon atoms bonded to hydrogen in hydrogenated neutral PAHs, nCH/nC. To constrain the contribution of hydrogenated PAHs to R, knowledge of their UV photodehydrogenation cross section is necessary. In particular, experimental cross sections on hydrogenated coronene would, in combination with the cross sections measured here, finally enable an estimation of the degree of (super-)hydrogenation of coronene as a sample PAH under PDR and general ISM conditions, giving a tight estimation of the contribution of PAHs to interstellar H2 formation.
This work has been supported by ASI research contracts and the European Research Council under ERC starting grant HPAH (No. 208344) and ITN network LASSIE (No. 238258).
Footnotes
- 4
H atoms impact at the coronene film surface and directly react with no diffusion and/or adsorption.