Abstract
Although three-dimensional (3D) printing techniques are used to mimic macro- and micro-structures as well as multi-structural human tissues in tissue engineering, efficient target tissue regeneration requires bioactive 3D printing scaffolds. In this study, we developed a bone morphogenetic protein-2 (BMP-2)-immobilized polycaprolactone (PCL) 3D printing scaffold with leaf-stacked structure (LSS) (3D-PLSS-BMP) as a bioactive patient-tailored bone graft. The unique LSS was introduced on the strand surface of the scaffold via heating/cooling in tetraglycol without significant deterioration in physical properties. The BMP-2 adsorbed on 3D-PLSS-BMP was continuously released from LSS over a period of 32 d. The LSS can be a microtopographical cue for improved focal cell adhesion, proliferation, and osteogenic differentiation. In vitro cell culture and in vivo animal studies demonstrated the biological (bioactive BMP-2) and physical (microrough structure) mechanisms of 3D-PLSS-BMP for accelerated bone regeneration. Thus, bioactive molecule-immobilized 3D printing scaffold with LSS represents a promising physically and biologically activated bone graft as well as an advanced tool for widespread application in clinical and research fields.
Export citation and abstract BibTeX RIS
1. Introduction
Successful reconstruction of bony defects caused by cancer, trauma, infection, and aging is a critical challenge in orthopedics, dentistry, and otolaryngology [1–4]. Various bone grafting materials, which act as scaffolding matrices and carriers of bioactive molecules, have been widely utilized to address the unmet needs [4–8]. In recent years, the need for high-precision patient-tailored bone grafts has gradually increased to fulfill the therapeutic needs and expectations of patients. The three-dimensional (3D) printing technique, developed by Charles W Hull in the early 1980s and first used in medical field for dental implants in the early 2000s [9, 10], is accepted as the standard technology in this field. The 3D printing technique is a method used for additive manufacturing of metals, ceramics, and polymers, which does not limit the shape and size of the desired 3D objects [11, 12]. Further, the 3D printing technique can morphologically mimic macro- and micro-structures and multi-scale structures of human tissues for rapid and efficient regeneration [12–16]. Using techniques such as computed tomography (CT), magnetic resonance imaging, ultrasound, and x-rays, it is possible to manufacture customized matrices for target tissue regeneration and surgical models for each patient [17, 18]. For tissue regeneration, metals, synthetic polymers, and hydrogel-based bio-inks have been adopted as representative base materials for 3D printing [15]. However, the long-term degradation and toxicity of ions during corrosion of metals [19], poor mechanical strength (difficulty in maintaining shape and size), and chemical modification of base materials/UV irradiation to solidify hydrogel, which is unfavorable to cellular environment [20], still hinder widespread use in clinical practice. Despite hydrophobicity and lack of specific interaction with cells, which decrease the efficiency of cell adhesion, proliferation, and differentiation [21], synthetic polymers have been predominantly used in 3D printing for tissue engineering due to their reliable mechanical strength, reproducible mechanical and chemical properties, and technical feasibility [19, 22–25]. Synthetic polymers approved for human use include polycaprolactone (PCL), polylactic acid, and poly(D,L-lactic-co-glycolic acid) [19, 22–25]. PCL is the most frequently used synthetic polymer for extrusion-based 3D printing scaffolds, due to their low melting temperature and high crystallinity, which facilitate practical application [26]. To compensate for the low bioactivity of synthetic polymer-based 3D printing scaffolds, surface modifications have been routinely implemented, including adsorption or coating of bioactive molecules (e.g. growth factors, peptides, platelet-rich plasma, biomimetic apatite, and polydopamine) [25, 27–29], altered topographical features (e.g. etching by alkaline solution or solvent [30, 31]), and modified chemistry (e.g. plasma or highly corrosive chemical treatment [32–34]). Bulk modifications including mixing with bioactive particles, such as hydroxyapatite, bioactive glass, β-tricalcium phosphate, calcium silicate, and alumina, have also been used to enhance the interaction with cells and tissues [15, 16, 25, 33, 35]. However, such modifications are hampered by toxic chemical reactions, limited penetration depth of plasma, transient surface rearrangement, complex manufacturing procedures, unstable adsorption of bioactive molecules (i.e. rapid desorption and/or release from matrix as well as surface modification), insufficient surface exposure of bioactive particles by polymeric skin layer, uneven distribution of bioactive particles in polymers due to the high viscosity, and weak mechanical strength due to low interaction between polymer (organic) and particles (inorganic) [16, 33].
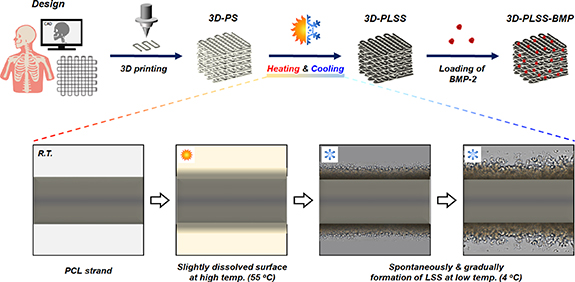
As reported previously [36–40], we developed PCL-based porous matrices including sheets and microparticles with leaf-stacked structure (LSS) using biocompatible materials (PCL and tetraglycol) via dissolution (at high temperature) and precipitation (at low temperature) techniques such as heating and cooling. PCL dissolved in tetraglycol at high temperature (90 °C) and spontaneously aggregated into LSS at low temperature (4 °C). Tetraglycol is not a good solvent for PCL at room temperature. Based on previous studies, it was hypothesized that exposure of the PCL-based 3D printing scaffold to tetraglycol that slightly dissolves the surface at appropriate temperature, followed by cooling, an LSS is generated spontaneously on the surface (figure 1). The unique LSS induced regeneration of target tissues, such as bone and cartilage. The regeneration was facilitated by the large surface area/complex pore structure, resulting in sustained release of bioactive molecules. The microtopography facilitated the differentiation of stem cells into target cell types, such as osteoblasts. A unique 3D printing scaffold with LSS may be an elegant solution to overcome the limitations of conventional synthetic polymer-based 3D printing scaffolds for target tissue regeneration. In this study, the changes in morphology and mechanical strength based on dissolution and precipitation were investigated to incorporate LSS onto the 3D printing scaffold. The release of bone morphogenetic protein-2 (BMP-2), cell adhesion, proliferation, and osteogenic differentiation, as well as new bone formation (seen in rat models of calvarial and canine mandibular defects) of BMP-2-immobilized 3D printing PCL scaffold with LSS (3D-PLSS-BMP) were also compared with conventional 3D printing PCL scaffold with smooth surface and commercially available bone graft.
Figure 1. Schematic diagram outlining the introduction of physical and biological stimuli into conventional PCL 3D printing scaffold for effective bone regeneration (3D-PS, PCL 3D printing scaffold; 3D-PLSS, PCL 3D printing scaffold with LSS; 3D-PLSS-BMP, BMP-2-immobilized 3D-PLSS).
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Materials
PCL (M.W., 80 000 Da; Evonik) and tetraglycol (glycofurol; Sigma-Aldrich) were used to design a 3D printing scaffold with LSS. Recombinant human BMP-2 (M.W., 13 000 Da; R&D Systems) was used as a bioactive molecule to promote osteogenic differentiation of stem cells and induce new bone formation. Ethylene oxide was used to sterilize 3D printing scaffolds in in vitro cell cultures and in vivo animal studies.
2.2. Preparation of PCL 3D printing scaffold
PCL 3D printing scaffolds (3D-PS) were prepared using a custom-designed printing system (Laboratory system, Korea Institute of Machinery and Materials) [41]. Briefly, PCL was melted in a stainless-steel syringe until completely transparent at 100 °C for stable extrusion from the syringe. The scaffold was designed using a laboratory-made software to ensure a strand size of 300 μm and a distance of 600 μm between the strands. The strand fabrication was mechanically regulated based on the speed of the printing head and pressure controller to optimize the scaffold design. To fabricate a strand measuring 300 μm in size, a precision nozzle with similar inner diameter was selected. Scaffolds with appropriate sizes for mechanical properties, in vitro cell culture (4 mm × 4 mm × 4 mm) and animal experiments (same dimension as bone defects) were prepared at a printing speed of 200 mm min−1 and a syringe pressure of 600 kPa on a sterilized bench at room temperature.
2.3. LSS formation on 3D-PS
A modified heating–cooling method [37, 38, 40] was used to load the LSS on the surface of 3D-PS. A 3D-PS was immersed in tetraglycol at 55 °C for 40 s to slightly dissolve the surface of strands. This 3D-PS was transferred to tetraglycol (25 °C) and immediately refrigerated (4 °C) for 10 min to induce polymeric precipitation of LSS on the strand surface. The scaffold was centrifuged (1500 rpm, 3 min) to remove residual tetraglycol and unprecipitated PCL solution, washed with excess water, and freeze-dried (3D-PS with LSS (3D-PLSS)). To demonstrate the formation of LSS on the strand surface in 3D-PS during precipitation, the 3D-PS stored at 4 °C after heat treatment at 55 °C was monitored under a light microscope (BX51; Olympus). Morphologies of 3D-PS and 3D-PLSS were analyzed under a scanning electron microscope (SEM; S-4300; Hitachi) at the Center for Bio-Medical Engineering Core Facility (Dankook University). Porosity of the strands (length, 4 mm) comprising 3D-PS and 3D-PLSS was analyzed with a porosimeter using mercury (PoreMaster 33GT; Quantachrome).
2.4. Mechanical properties of 3D-PS
A compressive strength test was performed to analyze the changes in mechanical strength following the modified heating–cooling of 3D-PS in tetraglycol (3D-PS vs. 3D-PLSS). Each 3D printing scaffold (4 mm × 4 mm × 4 mm) was placed between a cylindrical load of cells (10 kN) with a ø 30 mm metal plate. The specimen was compressed at a crosshead speed of 1 mm min−1 until failure and the stress–strain curve of each scaffold was obtained.
2.5. Fabrication of BMP-2-loaded 3D-PS
The 3D-PS and 3D-PLSS (4 mm × 4 mm × 4 mm) were immersed in 1 ml of BMP-2 solution (1 μg ml BMP-2 in phosphate-buffered saline (PBS; pH ∼ 7.4) containing 1% bovine serum albumin) and stored under positive pressure for 3 h (4 °C). To obtain BMP-2-immobilized 3D printing scaffolds (3D-PS-BMP and 3D-PLSS-BMP), the scaffolds were washed with PBS, and then freeze-dried. The BMP-2 load on the scaffolds was determined directly using ELISA [42]. To investigate the release behavior of BMP-2 loaded on the 3D-PS-BMP and 3D-PLSS-BMP, the scaffolds were incubated in 1 ml PBS at 37 °C for 32 d. The incubated whole medium was collected daily and replaced with 1 ml of PBS prepared afresh. The quantity of BMP-2 in medium was detected via ELISA (Duoset; R&D Systems).
2.6. In vitro cell culture
Human periosteum-derived cells (hPDCs) were obtained from the periosteal tissues of patients, and used to determine whether the unique morphology of LSS on 3D-PLSS and continuously released BMP-2 from 3D-PLSS-BMP enhanced the osteogenic differentiation compared with 3D-PS (without LSS) and 3D-PLSS (without BMP-2), respectively. The cell harvesting technique was approved by the Ethics Committee of Gyeongsang National University Hospital (study code: GNUH 2014-05-012). Before the study commenced, informed consent for the collection of periosteal tissues was obtained from the patients, as required by the ethics committee. The experiment was also performed in accordance with the principles set out in the Helsinki Declaration and local legal requirements. All scaffolds were laid in 24 well plates, pre-wetted with 70% ethanol and washed five times with PBS to ensure uniform cell seeding into pore structure [43]. Cell suspension (passage 5; 4.0 × 105 cells (in 80 μl; Dulbecco's modified Eagle's medium (DMEM) (Welgene)-based standard cell culture medium)) was seeded on each scaffold and incubated for 12 h to facilitate cell adhesion. The scaffolds were transferred to a new set of 24 well plates filled with osteogenic differentiation medium [44] and incubated for 4 weeks. The number of adhered and proliferated cells on scaffolds were estimated using a cell counting kit-8 (Dojindo) at 0, 1, 2, and 4 weeks. Live/dead staining of initial cell seeding at 0 week was also performed in all groups. For morphological analysis, cells adhering on the scaffolds were fixed with 5% glutaraldehyde and observed via SEM at 0, 1, 2, and 4 weeks.
2.7. Focal adhesion analysis
2.7.1. Quantitative real-time polymerase chain reaction
To determine the morphology effects of scaffolds on cell adhesion (LSS surface vs. smooth surface), focal adhesions (cell–extracellular matrix (ECM) adhesion [45]) of hPDCs on the 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP were investigated. After 6, 24, and 72 h of cell culture on each scaffold, the total RNA was extracted using TRIzol reagent (Invitrogen, Life Technologies) and converted into complementary deoxyribo nucleic acid (cDNA) using a PrimeScriptTM RT Master Mix (TaKaRa). The polymerase chain reaction (PCR) was performed by mixing the cDNA templates, probes (vinculin, paxillin, talin, actinin alpha 1 (ACTN 1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ThermoFisher Scientific)) with TaqMan® Universal PCR Master Mix (Applied Biosystems Inc.), according to the manufacturer's protocol [37]. Quantitative gene expression data were normalized to the expression levels of GAPDH values.
2.7.2. Immunocytochemical analysis
The hPDCs adhering to each scaffold were fixed, permeabilized, and blocked after 6, 24, and 72 h of cell culture. The cells were incubated with anti-vinculin antibody (vinculin monoclonal antibody, Alexa Fluor® 488 (ThermoFisher Scientific)) and rhodamine-phalloidin (ThermoFisher Scientific). A confocal laser microscope (LSM 700; Carl Zeiss) was used to acquire immunofluorescence images.
2.8. Osteogenic differentiation
2.8.1. Alkaline phosphatase activity
After 0, 1, 2, and 4 weeks of cell culture, the alkaline phosphatase (ALP) activity of the hPDCs adhering to 3D-PS, 3D-PS-BMP, 3D-PLSS, and 3D-PLSS-BMP was determined via para-Nitrophenyl phosphate (p-NPP) hydrolysis using an ALP enzyme kit (Abcam) [38].
2.8.2. Calcium deposition
After 0, 1, 2, and 4 weeks of cell culture, the calcium levels in hPDCs adhering to 3D-PS, 3D-PS-BMP, 3D-PLSS, and 3D-PLSS-BMP were determined using a colorimetric calcium assay (Wako Pure Chemical Industries) [38].
2.8.3. qRT-PCR analysis
After 0, 1, 2, and 4 weeks of cell culture, the expression of specific markers associated with osteogenic differentiation (Runx2, type Ⅰ collagen, osteocalcin, osteopontin, and GAPDH (all ThermoFisher Scientific)) was analyzed using the same procedures described for focal adhesion.
2.8.4. Immunocytochemical analysis
After 0, 1, 2, and 4 weeks of cell culture, immunocytochemical staining was performed to visualize the expression of osteogenic markers (Runx2 and osteocalcin). The hPDCs adhering to 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP were fixed, permeabilized, and blocked. The cells on each scaffold were incubated with primary antibodies (Runx2 (1:100 dilution; Abcam) and osteocalcin (1:100 dilution; Abcam)) for 12 h at 4 °C, followed by incubation with secondary antibodies (DyLight 488 (Abcam; for Runx2) and Northern Lights 557 (Abcam; for osteocalcin)). The immunofluorescence images were obtained using a confocal laser microscope.
2.9. Animal study
2.9.1. Rat model of calvarial defect
The rat study was approved by the Animal Care Committee of Dankook University of Korea (approval No.: DKU-19-030) and performed according to the institutional guidelines. A total of 24 male Sprague–Dawley (SD) rats, 6 weeks old and weighing 200–250 g each were used for this experiment. SD rats were divided into four groups (three rats/group): (i) blank (without scaffold), (ii) 3D-PS (implantation of 3D-PS w/o BMP-2), (iii) 3D-PLSS (implantation of 3D-PLSS without BMP-2), and (iv) 3D-PLSS-BMP (implantation of 3D-PLSS with BMP-2). Under general anesthesia with 2% isoflurane (Hana Pharmaceutical Co.), full-thickness calvarial defects with a diameter of 8 mm were created using a trephine bur. Each defect was treated with 3D-PS, 3D-PLSS and 3D-PLSS-BMP (8 mm in diameter, thickness in 700–800 μm). The periosteum and skin were then sutured with a 5-0 nylon (Ethicon). At 4 and 8 weeks after surgery, the SD rats were sacrificed with an overdose of CO2 gas, and their defect sites containing the graft implants were harvested. Tissue samples containing host and regenerated bones were fixed with 4% paraformaldehyde and scanned via micro-CT (μ-CT; SkyScan-1176, Bruker). The scanned CT images were 3D reconstructed using NRecon (Bruker). The bone volume, bone fractional area, and bone density profiles in the defect site were analyzed using a CT analyzer program (CTAn, Bruker) and a 3D visualization program (CTVox, Bruker). Histological analysis was conducted by decalcifying tissue samples with a decalcifying solution (Sigma-Aldrich), and embedded in paraffin, followed by cutting into 8 μm transverse sections, sequentially. Tissue sections were stained with Masson's trichrome for visualization using a slide scanner (MoticEasyScan Pro; Motic).
2.9.2. Canine model of mandible defect
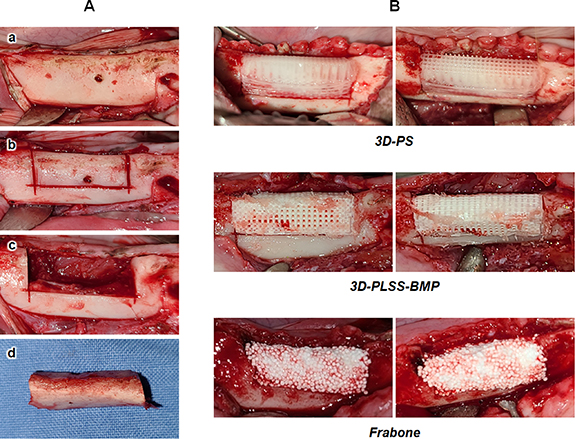
The beagle study was approved by the Animal Center of Biomedical Experimentation at Gyeongsang National University (study code: GNU-191015-D0053) and performed according to the institutional guidelines. Mandibles from six beagles were divided into three groups (two beagles/group): (i) 3D-PS (implantation of 3D-PS without BMP-2), (ii) 3D-PLSS-BMP (implantation of 3D-PLSS with BMP-2), and (iii) Frabone (commercially available bone graft (hydroxyapatite (60 ± 5%) and β-tricalcium phosphate (40 ± 5%) coated with hyaluronic acid, FRABONE-I®, Inobone)). Beagles were aged 8–10 months and weighed approximately 9–10 kg. Each animal was maintained in an individual cage and fed with commercial diet by a veterinarian and a standard laboratory pellet diet (Purina Canine LabDiet, Cargill). The beagles were sedated with acepromazine (0.05 mg kg−1, SEDAJECT injection, Samwoo median) and medetomidine (0.02 mg kg−1, Domitor®, Zoetis Pharmaceutical Inc.) administered intravenously. General anesthesia was induced with alfaxalone (2 mg kg−1, Alfaxan®, Jurox Pty Ltd) injected intravenously and maintained on isoflurane (Ifran®, Hana Pharm) in 100% oxygen via an endotracheal tube. All beagles received intravenous fluid for the duration of anesthesia. Once the animal was anesthetized, intrasulcular incision was performed and mucoperiosteal full-thickness flap was reflected from the underlying bone in order to facilitate tooth extraction. Four premolars and three molars were then extracted with minimal trauma, and the wounds were closed using 4-0 vicryl sutures. Sedation was reversed using atipamezole (0.02 mg kg−1, AntisednTM, Pfizer) injected intramuscularly. First-generation cephalosporin was injected intramuscularly twice a day for 7 d. Three months after the extraction of the premolars and molars, bone defects were created on the right side of mandible in all beagles. Under general anesthesia as described above, local anesthesia was administered to the edentulous area with 2% lidocaine mixed with 1:100 000 epinephrine (Lidocaine HCl, Huons Co.). The crestal incisions were placed in the edentulous ridge of premolars and molar regions. Buccal-lingual full thickness flaps were elevated and a standardized rectangular bone defect measuring 10 mm × 25 mm × 8 mm in size was created on the alveolar ridge using a reciprocating and sagittal saw (figure 2(A)). Inferior alveolar nerve and vessels were well preserved. The bone defects were irrigated with sterile saline copiously. The wound was closed using 4-0 vicryl sutures. Immediately after the surgery, the animals were examined via plain radiography and CT under general anesthesia. The 3D reconstruction of the defect was transferred as a stereolithographic (STL) file to a 3D CAD/CAM program. The geometric data were extracted from the STL files, and the 3D-PS with the same dimension as the defect was printed using a pneumatic dispenser. At 1 week post-surgery, surgical re-entry was performed to facilitate the implantation of the bone grafts. Some inflammatory tissue was removed from the defects, and the 3D printing scaffolds (3D-PS and 3D-PLSS-BMP) designed to ensure adequate contact with surrounding bones were implanted (figure 2(B)). Commercially available bone graft (Frabone) was also implanted into the defect. No stabilization with internal fixation devices, including osteosynthesis plates and screws, was performed. The wound was closed using 4-0 vicryl sutures. During the closure, electrocautery was used in cutting mode for better approximation of wound edges. Radiographic images were evaluated at 0, 4, 8, 12, and 16 weeks after implantation. The beagles were sedated with acepromazine and medetomidine to acquire plain radiographs and CT scans. The CT scan was divided into five sections based on the size of the defect. 3D CT images were also used to evaluate the healing of the bony defect in the mandible. The bone mineral density of the regenerated bone area was measured in Hounsfield units (HU) based on CT scans. The pixel data with a DICOM CT image was used to calculate the HU by placing an elliptical region of interest (ROI) that was mainly confined to the regenerated bone area to reduce the potential for volume averaging from the adjacent cortical bone. Depending on the growth of beagles and the regenerated bone area, HU values of the elliptical ROIs (0.2636 cm2) in CT images were determined. Sixteen weeks after implantation, the beagles were sacrificed and the tissue samples were obtained for histological analysis via hematoxylin and eosin (H&E) staining.
Figure 2. Animal model and surgical procedures. (A) Standardized rectangular bone defect (size, 10 mm × 25 mm × 8 mm) was created (shown sequentially from top to bottom, a to d), and (B) 3D printing scaffolds (3D-PS and 3D-PLSS-BMP) and Frabone were implanted in each defective site.
Download figure:
Standard image High-resolution image2.10. Statistical analysis
All data were expressed as means ± standard deviation. Statistical analyses were performed via one-way analysis of variance (ANOVA) via Tukey's multiple comparison using IBM SPSS Statistics 22 (IBM). Comparisons with a p < 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Characterization of 3D-PS
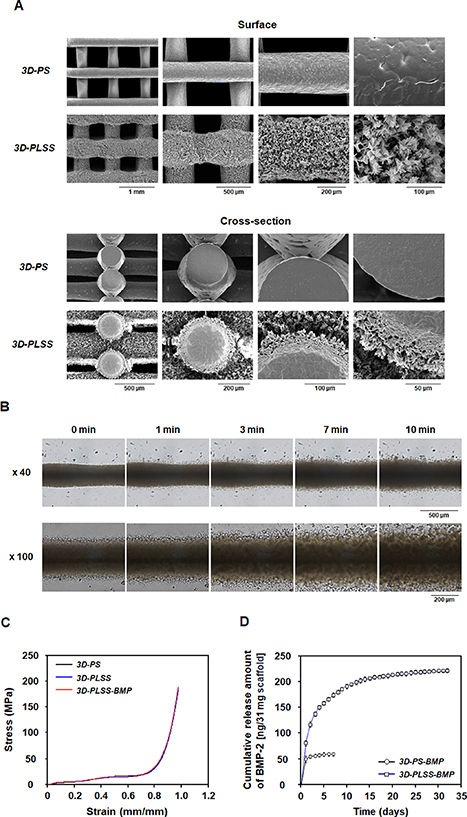
The morphology of 3D-PS (and 3D-PLSS) is illustrated in figure 3(A). The average strand diameter and distance between strands of 3D-PS were 300.2 ± 2.25 μm and 600.94 ± 1.44 μm, respectively. The average strand diameter and strand distance of 3D-PLSS changed to 457.41 ± 6.36 μm and 457.17 ± 1.98 μm after the heating–cooling procedure, indicating expansion of strand diameter due to morphological changes of strand surface from dense to porous structure. The porous surfaces appear to be uniformly covered by flat and elongated (leaf-shaped) PCL (thickness of LSS layer, ∼50 μm). This phenomenon can be explained by slight dissolution of PCL around the strand surface at high temperature (55 °C) and the spontaneous formation of LSS on the strand surface during precipitation of dissolved PCL around the strand at low temperature (aggregation/growth of the PCL on strand) (figure 3(B) and movie 1) [36–40]. Studies suggest that this LSS introduced on the surface of the PCL strand may facilitate the sustained release of growth factors [36–40] and stimulate cells physically [30, 31, 39, 40], resulting in osteogenic differentiation and bone regeneration. The dense structure in the central region of strand after heat treatment can be explained by the insufficient time/temperature for dissolution of the PCL strand during the heating and cooling step. Kosik-Kozioł et al [30] reported that surface etching of a 3D-PS in acetone/NaOH solutions under ultrasound resulted in a microtextured surface, which induced early osteogenic differentiation of mesenchymal stem cells (MSCs). After the etching process, the strand diameter decreased by ∼28% and the depth of the microtexture layer formed was ∼0.5 μm. However, the sustained release of BMP-2 from the rough surface and the osteogenic differentiation to ensure adequate bone regeneration via long-term biostimulation with growth factor were not observed, probably due to the rapid release of BMP-2 from the thin-layered open pore structure. In comparison, the LSS has an 11 fold porosity (strand in 3D-PS, 0.92% vs. strand in 3D-PLSS, 9.86%) and a substantially more complex pore structure, which provides sufficient surface area for adsorption of growth factors and a 3D path for sustained release, as mentioned earlier. To the best our knowledge, a 3D printing scaffold with both physical and biological stimulation has yet to be reported.
Figure 3. Characterization of 3D printing scaffolds. (A) SEM images showing the gross appearance, surface, and cross-sectional morphology of 3D-PS and 3D-PLSS. (B) Formation of the leaf-stacked structure (LSS) on the 3D-PS during PCL precipitation at 4 °C. (C) Mechanical properties of 3D-PS and 3D-PLSS (n= 3). (D) Release behavior of BMP-2 from 3D-PS-BMP and 3D-PLSS-BMP (n = 3).
Download figure:
Standard image High-resolution imageA compressive strength test was conducted to analyze the mechanical strength of 3D-PS (original scaffold with dense strand) compared with 3D-PLSS (heat-treated scaffold with porous LSS strand). As shown in figure 3(C), the stress–strain curves of both groups almost overlapped. At 70% of the maximum strain of the stress–strain curves [46, 47], the compressive modulus of 3D-PS and 3D-PLSS were 27.85 ± 0.59 MPa and 24.81 ± 1.38 MPa, respectively. The differences were not statistically significant, indicating that the formation of porous LSS on the 3D printing scaffold does not substantially change the mechanical properties. It was also observed that the immobilization (adsorption) of BMP-2 on the strand does not significantly affect the mechanical property of the 3D-PLSS scaffold. Based on the observations, we expected that the 3D-PLSS exhibits the unique properties of the LSS, including sustained release of growth factors to preserve bioactivity and microtopographical cues to induce osteogenic differentiation of stem cells, on the 3D printing scaffold without critical loss of mechanical strength. Thus, the drawbacks of conventional PCL-based 3D printing scaffolds in terms of bioactivity (i.e. bone regeneration) were addressed.
3.2. Release behavior of BMP-2
To determine whether the growth factor was continuously released from the LSS on 3D-PLSS, the BMP-2 was immobilized (adsorbed) on 3D-PS and 3D-PLSS in a BMP-2 solution. The loading amount of BMP-2 in the 3D-PLSS (265.89 ± 0.09 ng), as determined by direct ELISA, was significantly greater than in the 3D-PS (94.55 ± 1.32 ng). The higher loading amount in 3D-PLSS compared to 3D-PS can be explained by the larger surface area on which BMP-2 can be immobilized (adsorbed). The release behaviors of BMP-2 from the 3D printing scaffold are illustrated in figure 3(D). In case of 3D-PS-BMP, the BMP-2 equivalent to ∼73% of the loading amount was rapidly released within 7 d. However, the 3D-PLSS-BMP showed a sustained release of BMP-2 above the effective concentration (0.1–800 ng ml−1 [48]) over 32 d (∼83% of loading amount). This finding suggested that the LSS on the strand of 3D printing scaffold prevents direct diffusion (burst release) of the BMP-2 in the porous structure similar to the LSS in particles and sheets reported previously [37–40]. The sustained release profile can be understood by repeated adsorption–desorption of BMP-2 on LSS with a large surface area and delayed diffusion. Thus, the 3D-PLSS represents a potential high-precision personalized bone graft with long-term bioactivity for improved bone regeneration. The less release amount of BMP-2 compared to initial loading amount can be understood by the inherent unstable characteristics of BMP-2 in the physiological environment (half-life of BMP-2 in vivo, 7–16 min) [49–51].
3.3. Analysis of hPDC adhesion
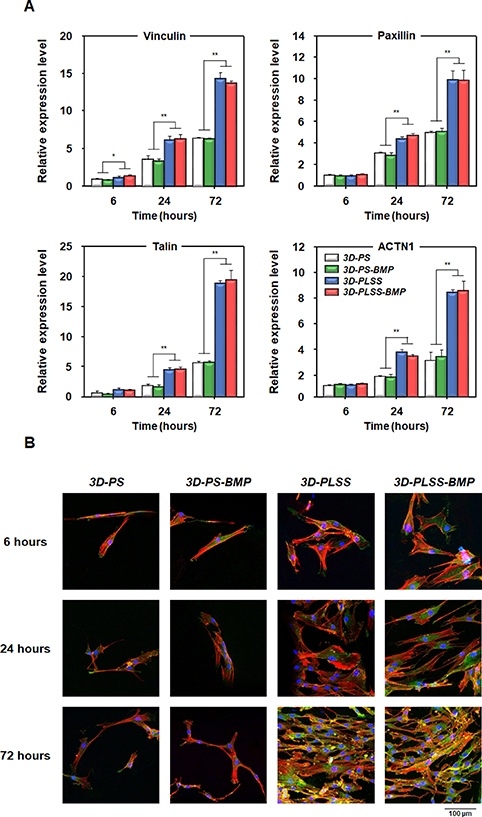
It is well known that the surface of biomaterials, which affects cell adhesion, proliferation, and differentiation, plays a crucial role in successful engraftment of biomaterials under the physiological environment for induction of target tissue regeneration [52–55]. To determine whether the LSS on the 3D-PLSS is a microtopographical cue for cell adhesion, the cell number and the expression of the focal adhesion markers of hPDCs incubated on LSS surfaces (3D-PLSS and 3D-PLSS-BMP groups) and smooth surfaces (3D-PS and 3D-PS-BMP groups) were compared. As shown in figures 4 and S1, the adhesion and proliferation behavior of hPDCs on the 3D printing scaffolds was altered. Despite seeding the cell suspension with similar cell numbers on each scaffold, a significantly larger number of cells adhered to the LSS surface (3D-PLSS, ∼2.0 × 104 cells/scaffold; 3D-PLSS-BMP, ∼2.0 × 104 cells/scaffold) than to the smooth surface (3D-PS, ∼0.3 × 104 cells/scaffold; 3D-PS-BMP, ∼0.3 × 104 cells/scaffold), indicating that the rough surface of LSS groups facilitated cell adhesion better than the smooth surface. Live/dead staining and SEM observations at week 0 (12 h after cell seeding) revealed no clear adherence of cells to the smooth surface. However, a large number of cells on LSS surface were observed, which were enough to cover the LSS structure. The initial high cell density may improve cell–cell interaction and thus promote differentiation of cells into target cells and tissues [56]. The number of cells in all groups increased over 4 weeks of culture. The BMP-2 released from the scaffolds had no significant effect on hPDC proliferation in the osteogenic medium. This finding was consistent with the study of Lecanda et al [57], who reported that BMP-2 affected the differentiation of human MSCs in osteogenic medium, but was not significantly involved in proliferation. The rapid cell proliferation on the smooth surface than on LSS surface after 1 week of cell culture can be attributed to the change of PCL surface into an interactive surface by the adsorption of cell adhesive proteins in the cell culture medium and accelerated migration and proliferation to cell-free surface via interaction with surrounding cells [58–61]. To further evaluate the topographical effects of LSS on cell adhesion, the expression of focal adhesion markers of hPDCs adhering to the LSS surfaces (3D-PLSS and 3D-PLSS-BMP) was compared with those adhering to smooth surface groups (3D-PS and 3D-PS-BMP) (figures 5 and S2). The expression of vinculin, paxillin, talin, and ACTN1 (known as focal adhesion markers [55, 62]) was increased over time in all groups. However, the expression of all markers in the LSS surface groups was significantly greater than in the smooth surface groups. The immunocytochemical analysis of vinculin revealed a green color in LSS surface groups after 24 h of cell culture. The color gradually increased, indicating that the porous LSS enhanced focal cell adhesion compared with the smooth substrate. These results are consistent with previous studies, which reported that the porous rough surfaces facilitated enhanced cell adhesion and increased focal adhesion compared with smooth surfaces [63, 64]. Focal adhesion plays an important role in cell adhesion and enables transmission of external mechanical signals into cells to initiate and regulate signaling pathways for osteogenic differentiation [65, 66]. Therefore, the porous LSS per se on 3D printing scaffold (fabricated with synthetic polymer carrying insufficient moieties to interact with cells [67]) may enhance osteogenic activity in cells via physical stimulation. Based on insignificant changes in mechanical strength after incorporation of a porous structure (LSS) on the strand surface, sustained release of BMP-2 from LSS, and improved focal adhesion on LSS, we postulate that the 3D-PLSS-BMP represents an elegant 3D printing scaffold system for osteogenic differentiation and bone regeneration. To validate these claims, further in vitro cell culture and in vivo animal studies were performed.
Figure 4. Adhesion and proliferation of hPDCs on the 3D printing scaffolds. (A) Graph, live/dead staining (green, live cells; red, dead cells) and (B) SEM images showing the number, viability and morphology of hPDCs on 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP during cell culture, respectively.
Download figure:
Standard image High-resolution imageFigure 5. Characterization of focal adhesions in hPDCs adhering to 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP. (A) qRT-PCR analysis of the expression of vinculin, paxillin, talin and ACTN1 (n = 3; *p < 0.05, **p < 0.01). (B) Immunocytochemical analysis of vinculin expression (green, vinculin; red, actin; blue, nuclei).
Download figure:
Standard image High-resolution image3.4. Osteogenic differentiation
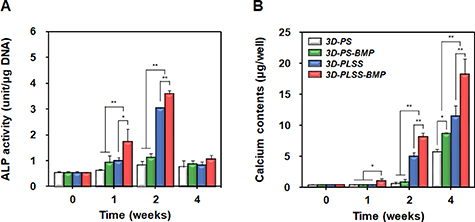
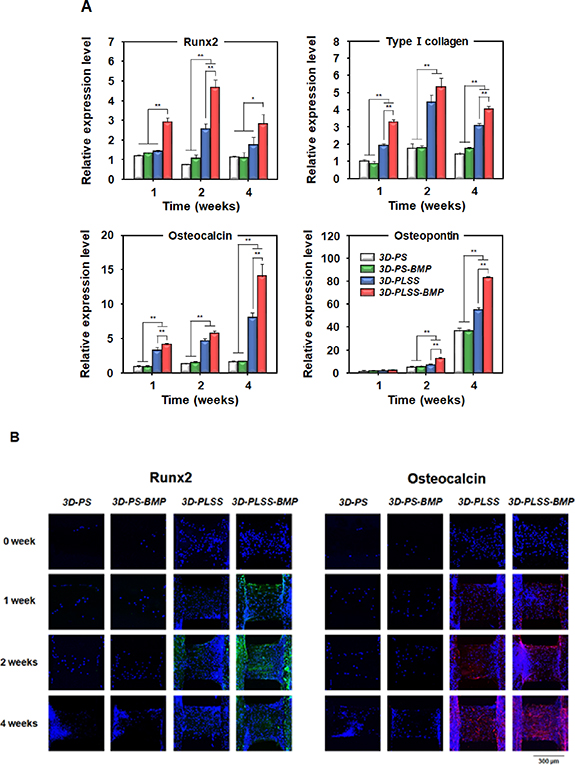
To estimate the effects of porous LSS and BMP-2 released from LSS on osteogenic activity, the osteogenic differentiation of hPDCs adhering to 3D printing scaffolds was investigated. The ALP activity (a marker of bone mineralization [68]) initially increased for 2 weeks in all the groups and then declined (figure 6(A)), suggesting that osteogenic differentiation was induced in cells [69]. However, the ALP activity of hPDCs was significantly higher in the LSS surface groups than in the smooth surface groups. The 3D-PLSS-BMP showed the highest ALP activity during cell culture. The calcium accumulation in cells induced by osteogenic differentiation [70] was also analyzed via calcium colorimetric assay (figure 6(B)). The calcium levels in all groups increased gradually during the cell culture period. After 2 weeks in cell culture, the calcium levels in hPDCs were significantly higher in the LSS groups than in the smooth surface groups. The 3D-PLSS-BMP group showed significantly higher levels of calcium compared with the other groups, suggesting that the combination of physical (LSS) and biological (BMP-2 continuously released from LSS) stimulation most effectively promote osteogenic differentiation of hPDCs. The rapid increment in calcium levels in the smooth surface groups at 4 weeks may be attributed to the increased number of cells adhering to the 3D printing scaffold (figures 4 and S1) and enhanced cell–cell interaction, which promoted differentiation into bone cells [71, 72]. The expression of marker genes during osteogenic differentiation was analyzed via quantitative real-time-PCR. Runx2 and type Ⅰ collagen (expressed at early stages of osteogenic differentiation of stem cells); osteocalcin and osteopontin (expressed during the late stage of osteogenic differentiation and bone formation) were used as specific markers [73–75]. As shown in figure 7(A), each of the markers in different groups was expressed according to a pattern generally observed during osteogenic differentiation of stem cells, indicating that the experimental hPDCs potentially differentiate into osteocytes, and thus represent appropriate cell types for evaluation of the role of physical and/or biological stimuli in osteogenic differentiation. The expression of early markers (Runx2 and type I collagen) and late markers (osteocalcin and osteopontin) during cell culture were consistent with the results of ALP activity and calcium deposition, respectively. Further, the expression of Runx2 and osteocalcin in hPDCs cultured on 3D printing scaffolds was visualized and compared with the immunocytochemical results (figure 7(B)). During cell culture, cells (blue) proliferated through the strand of 3D printing scaffold in all the groups.
Figure 6. Osteogenic differentiation of hPDCs cultured on 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP. (A) ALP activity and (B) calcium deposition in hPDCs cultured for up to 4 weeks (n = 3; *p < 0.05, **p < 0.01).
Download figure:
Standard image High-resolution imageFigure 7. Expression of osteogenic markers in hPDCs cultured on 3D-PS, 3D-PS-BMP, 3D-PLSS and 3D-PLSS-BMP. (A) qRT-PCR analysis of the expression of Runx2, type Ⅰ collagen (early marker) and osteocalcin, osteopontin (late marker) (n = 3; *p < 0.05, **p < 0.01). (B) Immunocytochemical analysis of Runx2 and osteocalcin expression (green, Runx2; red, osteocalcin; blue, cell nuclei).
Download figure:
Standard image High-resolution imageHowever, the expression of Runx2 (green) and osteocalcin (red) was distinct in LSS groups than in smooth surface groups. The 3D-PLSS-BMP exhibited the highest intensity of fluorescence of both markers during the period of interest [73, 76, 77]. These findings were supported by previous studies, suggesting that (i) continuous stimulation of BMP-2 with adequate bioactivity promotes osteogenic differentiation of stem cells and (ii) micro-rough structure not only affects the differentiation of stem cells but also induces the regeneration of bone tissue. It is well known that BMP-2 binds to cell membrane and activates osteogenic genes, thus promoting bone formation via cellular differentiation into osteoblasts/osteocytes, resulting in the synthesis of bone ECM [78]. Vlacic-Zischke et al [79] demonstrated that titanium-based dental implants with micro-rough surface promoted bone formation at the contact region. It was also reported that the 3D or 2D substrate with micro-rough surface induced the expression of osteogenic markers, including ALP and osteocalcin in cell culture experiments [79–81].
Based on in vitro cell culture studies, 3D-PLSS-BMP appears to facilitate cell adhesion, especially during the cell seeding stage, and osteogenic differentiation of hPDCs via pillar-like structures in the presence of growth factors.
3.5. In vivo bone regeneration (rat model)
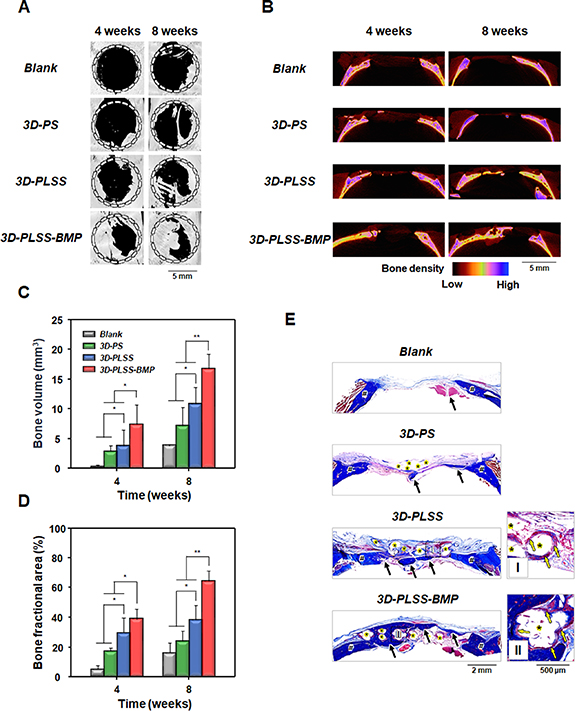
To compare bone regeneration behavior of the 3D printing scaffolds (smooth surface vs. LSS surface and without BMP-2 vs. with BMP-2), SD rats (with calvarial defect) were used as the small animal models. New bone formation in the bony defect after implantation of the 3D printing scaffolds was estimated by μ-CT at weeks 4 and 8. The new bone was gradually reconstructed from the borders of the defect to the central region in all groups (figures 8(A) and (B)). The bone formation was faster in the 3D printing scaffold groups than in the blank group. New bone appeared to regenerate along the PCL strands, indicating that the scaffolding guided bone regeneration. However, the bone regeneration behavior (qualitative analysis for regenerated bone: gray in dot circle (figure 8(A)), blue–magenta–green (figure 8(B)), and blue (figure 8(E)); quantitative analysis for regenerated bone: bone volume (figure 8(C)) and bone fractional area (figure 8(D))) did not differ significantly between blank and 3D-PS groups, indicating that the PCL strand with smooth surface may not facilitate bone regeneration, probably due to insufficient (physical/biological) bioactivity for the recruitment, proliferation, differentiation, and bone formation by osteogenic cells in the defect site. The rates of bone regeneration in the different 3D printing scaffold groups were: 3D-PLSS-BMP > 3D-PLSS > 3D-PS (figure 8). Interestingly, immature and mature bone tissues (represented by blue and red colors indicated by yellow arrows, respectively) infiltrated into porous LSS on the surface of PCL strand (3D-PLSS and 3D-PLSS-BMP groups; figure 8(E)), suggesting that the LSS served as an anchor for stable integration with host bone tissue. The blue and red colors in Masson's trichrome staining indicate mineralized and non-mineralized bone, respectively [82]. A matrix with a rough surface promotes osteoinduction, osteoconduction, and osteointegration compared with a smooth surface matrix [83, 84]. Zhang et al [85] also reported that micro-porous structures induced higher adsorption of protein including growth factors due to the large surface area and enhanced (bone-related) cell adhesion via capillary force generated by the microporsity and thus accelerated bone regeneration.
Figure 8. Analysis of bone formation in a rat model of calvarial defect after implantation of 3D-PS, 3D-PLSS and 3D-PLSS-BMP. (A) Reconstructed 3D-CT images, (B) bone density profile, (C) bone volume, and (D) bone fractional area at weeks 4 and 8 (n = 3; *p < 0.05, **p < 0.01). (E) Masson's trichrome staining at 8 weeks (#, host bone; *, PCL strand; black arrow, regenerated bone; yellow arrow, penetrated collagen tissues into leaf-stacked structure).
Download figure:
Standard image High-resolution image3.6. In vivo bone regeneration (beagle model)
In vivo studies of biomaterial-based bone tissue engineering using appropriate animal models are crucial for understanding the complex relationships underlying bone regeneration. An appropriate animal model should simulate the bone defects in human to obtain reliable results. Among many different types of animal models, dogs have been found to carry a bone composition similar to that of humans. Canine bone resembles human bone in terms of bone density and shows comparable intracortical remodeling [86–88]. Several bone sites have been used to demonstrate bone defects, including crania, femora, tibia, and mandibles. A model of mandibular defect is the most widely used to evaluate the effect of biomaterials, cells, and bioactive molecules on bone regeneration [89–91]. Bone regeneration in defective mandible, which is the largest bone in the facial skeleton and has relatively poor blood supply and highly developed masticatory system, can yield a clinically feasible outcome [92, 93].
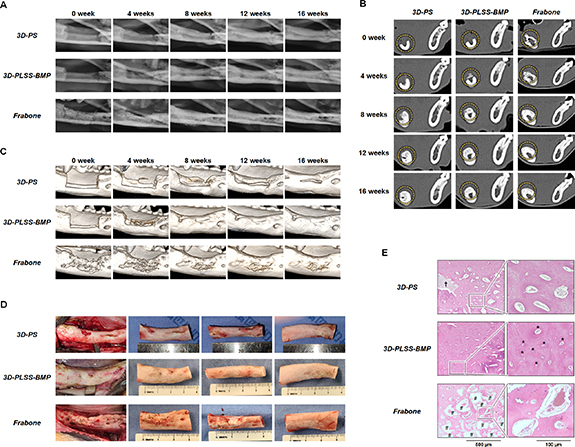
Plain radiographs (figure 9(A)) and CT scans (figures 9(B) and S3) revealed no clinically meaningful bone formation in all the defects at 4 weeks after implantation. Some resorption was observed in commercial bone grafts (Frabone) at 4 weeks after implantation. However, the higher number of HUs in the defect filled with Frabone than in defects implanted with 3D printing scaffolds, may be attributed to the radiopacity of hydroxyapatite. A newly formed immature bone was clearly observed in all defects starting at week 8 after implantation (figures 9(A)–(C) and S3). HUs were increased from week 4 after implantation in defects containing 3D printing scaffolds, in a time-dependent manner (table 1 and figure S4). CT images and HUs showed clearer new bone formation in the defect filled with 3D-PLSS-BMP than 3D-PS. The 3D-reconstructed CT images also revealed distinct new bone formation during the healing process in all the defects starting at week 8 after implantation (figure 9(C)). However, a higher bone (mineral) volume was observed in the 3D-PLSS-BMP and Frabone groups than in the 3D-PS group. The defect filled with 3D-PLSS-BMP appeared to be completely replaced by new bone, while the new bone in defects treated with Frabone was partially replaced and somewhat disordered. Although new bone formation was most apparent in all the defects, a portion of Frabone remained without being replaced by new bone tissue during gross clinical examination of the post-mortem mandible at 16 weeks (figure 9(D)). Histological analysis showed that new bone almost filled the defects in all three groups (figure 9(E)); however, substantial bone formation occurred in the defect containing 3D-PLSS-BMP, whereas the new bone was less evident in the defect treated with 3D-PS or Frabone. In addition, dense and compact bone formation and highly organized Haversian system were distinct in the defect treated with 3D-PLSS-BMP implant. A major portion of commercially available bone graft substitute remained intact at the bony defect, and was not replaced by bone tissue.
Figure 9. Analysis of bone regeneration in beagle mandible defects after implantation of the 3D-PS, 3D-PLSS-BMP and Frabone. (A) Representative plain radiographs, (B) CT scans (circles: bone defects or regenerated bone; position of section 3 (center of defect; refer to figure S3)), and (C) reconstructed 3D CT images for up to 16 weeks. (D) Gross anatomical appearance of post-mortem beagle mandibles and (E) histological sections of tissue specimen at 16 weeks (*, Haversian system; †, PCL strand; #, hydroxyapatite from Frabone).
Download figure:
Standard image High-resolution imageTable 1. HU values of regenerated bone defects after implantation of 3D-PS, 3D-PLSS-BMP and Frabone grafts [position of section 3 (center of defect; refer to figure S3)].
| Weeks | ||||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 3D-PS | ||||||
| Animal 1 | Mean | 34.37 | 171.28 | 528.13 | 814.88 | 930.07 |
| SD | 107.56 | 283.13 | 567.17 | 621.28 | 686.26 | |
| Animal 2 | Mean | −35.16 | 185.79 | 534.01 | 871.05 | 955.55 |
| SD | 231.92 | 395.38 | 628.22 | 700.20 | 692.85 | |
| 3D-PLSS-BMP | ||||||
| Animal 1 | Mean | 17.05 | 253.55 | 687.65 | 910.61 | 1029.31 |
| SD | 126.69 | 412.56 | 686.01 | 694.66 | 716.28 | |
| Animal 2 | Mean | 36.30 | 253.48 | 666.57 | 948.73 | 1064.11 |
| SD | 122.07 | 474.77 | 732.54 | 697.21 | 686.36 | |
| Frabone | ||||||
| Animal 1 | Mean | 638.22 | 822.06 | 1452.70 | 1547.50 | 1354.98 |
| SD | 907.16 | 616.02 | 571.92 | 561.95 | 588.27 | |
| Animal 2 | Mean | 560.05 | 558.70 | 1284.06 | 1282.90 | 1212.92 |
| SD | 620.57 | 554.78 | 349.58 | 273.23 | 437.43 | |
The findings based on animal studies are supported by cell culture studies, suggesting that the bioactive BMP-2 released from the 3D-PLSS-BMP stimulates osteogenic activity of stem cells (as a biological stimulus) and the LSS on the 3D-PLSS-BMP activates signaling pathways associated with osteogenic differentiation of cells (as a physical stimulus) by providing an appropriate environment for osteogenic differentiation of cells. Therefore, the new matrix (3D-PLSS-BMP) with advantages of both 3D printing and LSS, represents an alternative as a bioactive high-precision patient-tailored bone graft to overcome the intrinsic limitations of conventional synthetic polymer-based 3D printing scaffolds. It serves as a technical platform for widespread use of 3D printing scaffolds in clinical practice.
4. Conclusion
We developed a 3D-PLSS-BMP as a bioactive patient-tailored bone graft. The unique LSS was introduced on the strand surface of the scaffold via a heating–cooling technique in tetraglycol without significant deterioration of physical properties. The BMP-2-loaded 3D-PLSS-BMP was continuously released from LSS for 32 d. Further, the LSS provides a microtopographical cue for improved focal cell adhesion, proliferation, and differentiation. In vitro cell culture and in vivo animal studies demonstrated the biological (bioactive BMP-2) and physical (microrough structure) mechanisms of 3D-PLSS-BMP for accelerated bone regeneration. Thus, the bioactive molecule-immobilized 3D printing scaffold with LSS represents a promising physically and biologically activated bone graft as well as an advanced tool for wide-spread use of 3D printing technique in clinical and research applications.
Acknowledgments
The authors gratefully acknowledge the Center for Bio-Medical Engineering Core Facility at Dankook University for providing equipment.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
Author contributions
Min Ji Kim, Jin-Ho Park and Ji Min Seok contributed equally to this work. The manuscript was written with contributions of all authors. All authors have approved the final version of the manuscript.
Funding information
This study was supported by a National Research Foundation of Korea grant awarded by the Korean government (Ministry of Science and ICT, 2019M3A9E2066347 and 2021R1A2C1093441; Ministry of Education, 2020R1A6A1A03043283) and a grant from the Korean Fund for Regenerative Medicine funded by Ministry of Science and ICT, and Ministry of Health and Welfare (RS-2022-00070460).
Supplementary data (18.0 MB PPTX)
Supplementary data (0.8 MB PDF)