Abstract
Despite the widespread use as platforms for various biomedical applications, engineering hydrogels to impart multifunctionality and control physical properties, while closely mimicking the native cellular microenvironment, is still a significant challenge. Herein, nanofibers consisting of hydrophilic and photocrosslinkable biopolymer and conductive polymer (i.e. PEDOT:PSS) are first fabricated via electrospinning, cut into micrometer-lengths, and chemically crosslinked to develop dispersible hybrid nanofiber (dhNF) as heteroscale reinforcing elements for developing nanocomposite hydrogels. The dhNF can be readily dispersed in aqueous precursor solutions without dissolution and incorporated into hydrogels. The resulting 'heteroscale' dhNF-infused hydrogels, consisting of molecular and nanofibrous polymeric network, more closely resembles natural extracellular matrix, and show significant improvement on both mechanical strength and electrical conductivity, by dhNF concentration as well as PEDOT:PSS content in dhNF. These properties not only directly help improve the viability and proliferation of encapsulated cells, but also more effectively relayed external electrical stimulation mediated by enhanced conductivity.
Export citation and abstract BibTeX RIS
1. Introduction
Hydrogels have become a fixture in biomedical engineering, as their tunable elasticity and high water content are highly attractive as drug delivery vehicles and cell culture platforms [1–4]. However, hydrogels generally suffer from such drawbacks as low mechanical strength and lack of optical and electrical properties, which prompted the development of several design strategies to overcome these issues. For example, mechanical properties of hydrogels can be improved or tuned by varying crosslinking density and hybridizing with different monomers/macromers via interpenetrating network formation or co-polymerization [5, 6]. However, all these methods involve irreversible changes to the original polymeric network, and therefore, the concurrent, and often unwanted, changes in various properties of the resulting hydrogels are inevitable.
Nanocomposite formation with various nanomaterials as fillers has also been widely adopted for this purpose [7–12]. Nanomaterials prepared from a variety of sources have been used, from inorganic (e.g. calcium phosphate, nanoclays), metallic (e.g. gold, silver, iron oxide) to organic (e.g. graphene oxide, carbon nanotubes, polymers). Nanocomposites have the distinctive advantage over hybridized polymeric networks in introducing unique physicochemical and biological features not generally shown for polymers, such as electromagnetic and optical properties (e.g. conductiviey, surface plasmon resonance, near IR absorption) as well as biological effects (e.g. anti-microbial effect, osteoinduction) [13, 14]. The biomedical potential of many of these nanomaterials, despite their attractive characteristics, have not been fully assessed in terms of pharmacokinetics due to their diverse nature (e.g. size, shape, source material) and relative infancy to the biomedical field.
More recently, the strategy of integrating nanofibers with hydrogel to create the composite structures has been increasingly investigated for biomedical applications [15, 16]. Nanofibers fabricated via electrospinning are in a class of their own as a popular scaffold material in tissue engineering applications, for such attributes as simple fabrication route, high porosity and surface area, diverse polymer sources, and tunable dimensions [17, 18]. Due to the miniature dimensions, however, it has inherent mechanical weakness and is not feasible to handle the nanofibers individually, and as a result, the nanofibers are generally used in a sheet (or 'mat') form as deposited on a collector during electrospinning. With these distinctive characteristics of nanofibers and hydrogels, their integration into a composite structure would be expected to combine and synergize their attributes while minimizing their weaknesses; hydrogels with imparted with multifunctionality while maintaining their polymeric network, and nanofibers with defined 3D architecture and structural integrity. Furthermore, in a physiological perspective, since the native ECM is 'heteroscaled'; consisting of fibrous collagen as structural elements crosslinked with other functional biopolymers (e.g. proteoglycan), the nanofiber infusion would more closely mimic the native ECM than conventional hydrogels only prepared from molecular-level crosslinking for their use as tissue engineering scaffolds.
Several previously published reports have introduced the concept of incorporating nanofibers into hydrogels, by simply embedding either the nanofiber mats or short nanofibers that were cut from the nanofiber mats after electrospinning into hydrogels [19–25]. However, these approaches have several limitations that need to be addressed in order to develop more scalable composite hydrogels with multifunctional properties. First, the nanofibers were made from well-known synthetic hydrophobic polymers, such as poly(lactic acid) and polycaprolactone, to prevent the dissolution of nanofibers in aqueous environment. The hydrophobic nature of the nanofibers often results in limited dispersion in aqueous media. In addition, this severely limits the material selection and formulations to create a diverse array of bioactive nanofibers, especially given the fact that many biopolymers are hydrophilic. Second, it is difficult to homogeneously disperse the nanofibers within the hydrogel network, due to their length as well as hydrophobicity preventing stable colloidal suspension. Third, nanofibers are generally made from a single type of polymer, as it is technically challenging to combine polymers with different physical properties (e.g. solvent compatibility, hydrophilic/hydrophobic balance).
In this study, multifunctional hybrid nanofibers, consisting of hydrophilic and biocompatible biopolymer and conductive polymer, were synthesized via electrospinning and then cut into a dispersible form having a micrometer-length scale in order to integrate into a hydrogel structure (figure 1). Moreover, the short nanofibers were further chemically crosslinked in order to prevent dissolution in aqueous environment. The resulting 'dispersible hybrid' nanofibers (dhNF) would readily disperse into a gel precursor solution, and stably integrate into a hydrogel network while maintaining their fibrous morphology and without phase separation, due to their hydrophilicity and crosslinking. The nanofiber-infused hydrogel would possess enhanced mechanical properties and electrical conductivity while improving the bioactivity of encapsulated cells. Furthermore, this strategy allows for the incorporation of a conductive polymer that normally cannot be chemically conjugated to the hydrogel due to the lack of reaction functional group. More significantly, dhNF-hydrogels with higher conductive polymer content could more effectively transmit electrical stimulation through the dhNF-hydrogels and influence the encapsulated cells, further highlighting the versatile nature of the dhNF-hydrogels.
Figure 1. Schematic illustration of the fabrication of composite hydrogel infused with dispersible hybrid nanofibers (dhNF). Hybrid nanofiber consisting of bioactive and conductive polymers are fabricated by electrospinning (a), then cut into micrometer-scale length and crosslinked via carbodiimide coupling to develop dhNF (b). Finally, the gel precursor solution dispersed with dhNF is crosslinked to develop dhNF-hydrogel (c).
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Synthesis of methacrylic gelatin (MGel)
Gelatin (10 g, from porcine skin, gel strength 300, Sigma Aldrich), 4-dimethylaminopyridine (1 g, Sigma Alrdich) and 4-methoxyphenol (0.1 g, Sigma Alrdich) were dissolved in 100 ml dimethyl sulfoxide at 50 °C. Glycidyl methacrylate (4 ml, Sigma Aldrich) was slowly added to the mixture, and the reaction was continued for 48 h at 50 °C under dry N2. The feed molar ratio of glycidyl methacrylate to the amino acid residues in gelatin was 0.32:1. The product was obtained by purification via dialysis against deionized water and lyophilization. The synthesis was confirmed by identifying the methacrylic peaks in 1H-NMR (400-MR DD2, Agilent) (figure S1 is available online at stacks.iop.org/BF/12/015020/mmedia) [26, 27].
2.2. Synthesis of dispersible-hybrid nanofibers (dhNF)
Methacrylic gelatin (MGel) and poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT:PSS, Sigma Aldrich) were dissolved in 2,2,2-triflouroethanol (TFE)/water (7:3 volume ratio) supplemented with 5% (v/v) ethylene glycol (Sigma Aldrich) as a dopant for PEDOT:PSS. The concentration of MGel was 18% (w/v), and the concentration of PEDOT:PSS was controlled up to 5 wt% of the total polymer content, which was the maximum concentration allowed at the given MGel content. The MGel concentration was chosen that gave optimal viscosity for stable nanofiber formation, as opposed to particulate formation at lower concentration and aggregate formation at higher concentration. Ultrasonication was applied for 1 h (output power: 65W, frequency: 20kHz, pulse: 5 s on, 5 s off, VCX130, Sonic) to fully dissolve PEDOT:PSS. For electrospinning, the solution was loaded into a syringe with 26 G needle, and ejected at a flow rate of 0.5 ml h−1 using an electronic syringe pump (NanoNC, Korea). The applied voltage and distance between the needle and the aluminum collector plate were 12 kV and 15 cm, respectively. Nanofibers were continuously deposited on the collector, and the nanofiber mat was dried in vacuo for 24 h.
The dried nanofiber mat (15 mg) was immersed in 2 ml ethanol, and ultrasonication was applied (VCX130, Sonic) in a pulse mode (output power: 65 W, frequency: 20 kHz, pulse: 5 s on, 5 s off) for various times up to 30 min, resulting in shortened nanofiber suspension. Then, the shortened nanofibers were treated with 5 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Sigma Aldriceh) and 2 mM N-hydroxysuccinimide (NHS, Sigma Aldrich) in 95% ethanol for 48 h [28, 29]. Intermittent sonication for 30 min for every 2 h was applied to prevent fiber aggregation. After crosslinking, the dhNFs were washed 3 times with isopropanol, collected by centrifugation, and dried in vacuo. The dhNFs were dispersed in a phosphate buffered saline (PBS, pH 7.4) at 60 mg ml−1 as a stock solution.
2.3. Fabrication of dhNF-infused hydrogels
A gel precursor solution, consisting of 8% MGel and dhNF (from 0% to 3%), supplemented with 0.2% Irgacure®2959, was placed in between two glass plates with a 1 mm spacer. The solution was treated with UV (800 mW cm−1) to induce the crosslinking reaction. The UV exposure time was dependent on the PEDOT:PSS content of dhNF, as longer UV exposure was required to form hydrogel with higher PEDOT:PSS content (e.g. 80 s for 0.2%, 100 s for 0.5%, 120 s for 0.8%). The hydrogel disks were cut out with a hole puncher (8 mm diameter), and incubated in PBS before further analysis. Separately, the dhNF-hydrogels were made with a lower MGel concentration at 4%, and monitored their structural integrity under PBS at 37 °C in order to confirm the covalent linkage. The MGel hydrogel at 4% became readily disintegrated in PBS due to structural weakness.
2.4. Characterization of dhNF-infused hydrogels
2.4.1. Mechanical properties
Each hydrogel disk was compressed at a rate of 1 mm min−1 using a universal testing machine (Model 3343, Instron) [30, 31]. The plot of stress versus strain was obtained, and the elastic modulus was calculated as the slope at the initial 10% strain where the plot was linear (elastic region). The swelling ratio was measured as the weight ratio of the fully swollen hydrogel to the dried polymer mesh.
2.4.2. Scanning electron microscopy (SEM)
The detailed structural features of nanofibers and nanofiber-infused hydrogels were monitored with SEM (S-4800, Hitachi). For nanofibers, the fiber mats and dhNF were dried under vacuum after fabrication. For hydrogels, they were first frozen in liquid nitrogen, fractured to expose cross-sectional area, and lyophilized. Dried samples were sputter-coated with Pt to increase the resolution of SEM image. To quantify the amount PEDOT:PSS in nanofibers, energy dispersive x-ray (EDX) analysis was performed using a built-in EDX system in SEM to measure the sulfur content. The EDX was performed under low vacuum with back scatter electron detection.
2.4.3. Impedance
Impedance measurement for nanofibers and nanofiber-infused hydrogels were conducted using impedance analyzer (4294A, Agilent). For nanofibers, the nanofiber mat, as prepared from electrospinning, was fully dried under vacuum, cut to a 1 cm × 1 cm piece, and placed in between two metal probes connected to the impedance analyzer. For nanofiber-infused hydrogels, the hydrogels were dried under vacuum for 30 min to remove access water at the surface, and placed in between two Pt-coated coverslips connected to the metal probes for the analyzer. The impedance was measured at the frequency range from 100 Hz to 1 Mhz.
2.5. In vitro studies
2.5.1. 3D cell encapsulation
The cells were first dispersed in a gel precursor solution at 1 × 106 cells ml−1, placed in between two glass plates (0.4 mm spacer), and then photocrosslinked with UV for 1 min to generate cell-encapsulated hydrogel. The hydrogel was cut into small disks (6 mm diameter) and incubated in the cell culture medium. For both 2D and 3D, the hydrogels were incubated in the cell culture media at 37 °C with 5% CO2. Here, murine marrow stromal cells ('D1', ATCC®) and fibroblasts ('NIH-3T3', ATCC®), were used. For both cell types, the cell culture medium was Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (all purchased from Thermo Fisher Scientific).
The cell viability was measured using LIVE/DEAD Cell Viability Assay (Thermo Fisher) [26, 27, 31]. Briefly, each hydrogel sample was treated with calcein-AM and ethidium homodimer-1 to fluorescently label live (green) and dead (red) cells, respectively. The cells were visualized under an inverted fluorescent microscope (XDS-3FL, Optika), and the numbers of live and dead cells were counted. The viability was reported as the percentage of live cells (mean ± standard deviation, n = 5). To quantify the cell proliferation, MTT assay was performed [31]. Briefly, each hydrogel disk was placed in a well of 96-well plate with 0.1 ml of the cell culture medium. Then, 0.01 ml of MTT solution (0.5% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Sigma Aldrich) was added and incubated for 4 h at 37 °C, during which MTT was converted to MTT formazan by living cells. 0.1 ml a MTT stop solution (20% sodium dodecyl sulfate in water/dimethylformamide 50:50) was added to dissolve MTT formazan. The amount of MTT formazan was measured by their characteristics of absorbance at 570 nm using a spectrophotometer (Multiskan GO, Thermo Fisher) (mean ± standard deviation, n = 5).
2.5.2. Electrical stimulation
Electrical stimulation was applied to the cells cultured within the hydrogel. The detailed schematic of the experimental setup for electrical stimulation is described in supplementary information (figure S2) [32, 33]. Briefly, two carbon electrodes were attached 1.5 cm apart on the bottom of a glass petri dish. The carbon electrodes were connected to a function generator (33220A, Agilent) via platinum wire. After placing the cell-laden hydrogel samples in between the electrodes immersed in the cell culture medium, pulsed electrical field (square, 2 ms, 5 V cm−1, 1 Hz) was continuously applied. At designated times, the sample was taken for further in vitro analyses (e.g. viability, proliferation, spreading).
3. Results and discussion
3.1. Fabrication of dispersible hybrid nanofibers (dhNF)
Multifunctional nanofibers were first developed via electrospinning, consisting of methacrylic gelatin (MGel) that has been increasingly explored as cell-responsive and hydrophilic polymer presenting methacrylic functional groups capable of radical co-polymerization, and PEDOT:PSS, a conductive polymer system consisting of conjugated polymer (PEDOT) and negatively charged polymer (PSS) (figure 1(a)) [26, 27]. Unlike other conjugated polymers, PEDOT:PSS possesses a certain degree of biocompatibility and hydrophilicity, owing to the presence of anionic PSS, thereby making it especially attractive for biomedical applications [34]. However, since PEDOT:PSS alone is not sufficient for imparting cell adhesive properties to nanofibers, it would be highly beneficial to incorporate cell-responsive molecules, such as gelatin, into the nanofibers. Furthermore, rather than utilizing gelatin itself, the presence of methacrylic groups on MGel would allow covalent conjugation of dhNF into the hydrogel. In order to hybridize MGel with PEDOT:PSS, the two solvent system of trifluoroethanol and water was used and their composition was optimized (7:3 ratio) to fully dissolve both polymers, maintain the adequate viscosity, and induce timely evaporation. The amount of PEDOT:PSS in nanofiber was varied to control the conductivity. The SEM images show the amount of PEDOT:PSS had minimal effect on the nanofiber formation and the thickness (figure S3(a)). The PEDOT:PSS content was readily noticeable by the characteristic dark color, and it was further confirmed by the increasing sulfur content obtained via EDX analysis (figure S3(b)).
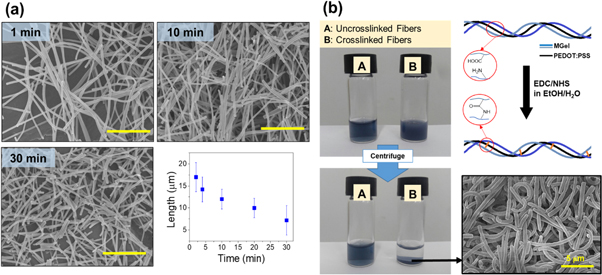
The nanofiber as fabricated by electrospinning are long, continuous fibers deposited on the collector plate. This nanofiber 'sheet' does not allow even distribution and dispersion within aqueous environment. Therefore, the nanofiber mat was further processed by cutting into short nanofibers having micrometer-scale in length via ultrasonication to the nanofiber mat immersed in a solvent (figure 2(a)). The ultrasonication readily resulted in the dispersion of short nanofibers having 17 (±3) μm in length in only 2 min, and with increasing ultrasonication time up to 30 min, the nanofiber length further decreased to 7 (±2) μm. Further processing did not show any further decrease in length, indicating it has reached the minimum length under the given ultrasonication strength.
Figure 2. (a) SEM images of short nanofiber formation by sonication cutting at different times. The plot represents the average length of short nanofibers with sonication time (scale bar: 10 μm). (b) The short nanofibers are covalently crosslinked via carbodiimide coupling to allow dispersion in aqueous solutions without dissolution. The nanofibers without crosslinking became readily dissolved.
Download figure:
Standard image High-resolution imageSince the nanofibers consisted of hydrophilic polymers, they dissolved readily in aqueous solutions, rendering unfeasible for any application involving aqueous media. Therefore, to preserve the nanofiber structure in aqueous media for stable and long-term inclusion in hydrogels as 3D cell culture platform, the short nanofibers were further chemically crosslinked to eventually develop 'dispersible' hybrid nanofibers (dhNF) (figure 2(b)) [28, 29]. Gelatin possess numerous carboxylate and amine groups, not only at the terminal but also from aspartic acid and glutamic acid, and lysine, allowing amide formation via carbodiimide coupling. The crosslinking was confirmed by dispersing the nanofibers in aqueous buffered solution, and measuring their stability after applying high shear stress (e.g. ultrasonication). For uncrosslinked fibers, they immediately disintegrate and became dissolved, whereas the crosslinked dhNF were stably dispersed in the solution, up to a month at room temperature while fully maintaining their structures, confirming the extent of crosslinking reaction was significant enough for their aqueous stability. Moreover, the PEDOT:PSS did not diffuse out of the crosslinked nanofibers even though PEDOT:PSS does not participate in the reaction further demonstrated that there is significant physical association between gelatin backbone and PEDOT:PSS.
3.2. Physical properties of dhNF-infused hydrogels
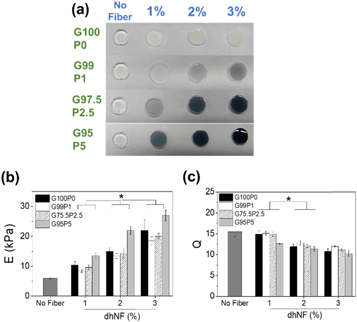
The hydrogels infused with dhNF were fabricated and their mechanical and electrical properties were measured to assess the composite effect of dhNF. At a given polymer concentration of hydrogel (i.e. 8% (w/v) MGel), the concentration of dhNF was controlled up to 3% (w/v). Also, at a given dhNF concentration, the PEDOT:PSS content was controlled up to 5 wt% from the total polymer content, denoted as G100P0, G99P1, G97.5P2.5 and G95P5 (G and P represent MGel and PEDOT:PSS contents, respectively).
The length of dhNF was kept at 7 μm, the minimum length attained by the ultrasonication process, as shorter dhNF showed more stable aqueous dispersion. The dhNF dispersed in MGel precursor solution maintained their colloidal stability for several hours, allowing the dhNF to be stably dispersed during a few minutes of photocrosslinking (figure S4). The dhNF-hydrogels showed increasing opaqueness with dhNF concentration, and increasing darkness with PEDOT:PSS content, evenly throughout the hydrogel, as expected (figure 3(a)). The SEM images of cross-sectional views of dhNF-hydrogels also confirmed the even distribution of dhNF within hydrogels (figure S5).
Figure 3. (a) Macroscopic images, (b) elastic moduli, and (c) swelling ratios of dhNF-hydrogels with varying dhNF concentration and PEDOT:PSS content (*p < 0.05, n = 6).
Download figure:
Standard image High-resolution image3.2.1. Mechanical properties
It is widely known that mechanical properties of ECM play a significant role in cellular physiology via mechanotransduction mediated by cell-ECM linkages (i.e. focal adhesion) [35–38]. Therefore, it is important to control the mechanical properties of hydrogels as 3D cell culture platform. Here, elastic moduli of dhNF-hydrogels were measured via uniaxial compression in order to demonstrate the ability of dhNF to control the hydrogel mechanics. The modulus increased with dhNF concentration up to 3%, regardless of PEDOT:PSS content (figure 3(b)), which suggested that the dhNF were successfully infused into the hydrogels without compromising the crosslinking reaction, and helped increased the mechanical properties. Interestingly, at a given dhNF concentration, the modulus showed a biphasic trend, in which the moduli decreased from G100P0 to G99P1, but increased with further increase in PEDOT:PSS content from G99P1 to G95P5. The initial decrease from G100P0 to G99P1 may have been due to the reduced amount of MGel involved with chemical conjugation, but the subsequent increase in PEDOT:PSS likely strengthened the composite structure via increased physical interaction [39–41].
The swelling ratios of the hydrogels decreased with dhNF concentration and the reverse biphasic trend with PEDOT:PSS content, as expected for elastic network (figure 3(c)) [5, 42, 43]. However, the overall decrease in swelling ratio with dhNF concentration up to 3% was much smaller than the increase in elastic modulus; 450% increase in elastic modulus and only 34% decrease in swelling ratio. As a control, the swelling ratios of MGel hydrogels with varying MGel concentration, from 8% to 15%, showed 55% decrease, despite having a similar range of elastic moduli (figure S6). This result further accentuates the value of incorporating dhNF into a hydrogel, because the conventional method of controlling the crosslinking density of the hydrogel itself usually results in much greater reduction in swelling, which severely limits the diffusion and available space within the hydrogel important for the bioactivity of encapsulated cells. The inner morphology of the hydrogels visualized via SEM revealed that dhNF were well connected to the polymeric network (i.e. dhNF did not separate from the polymeric network), and the porosity of the network was not significantly affected except at the highest dhNF concentration at 3%, in which the pores were partially covered by the increased amount of dhNF (figures 4(a) and S7). This observation further corroborates the substantial increase in mechanical properties while minimizing the change in diffusional properties.
Figure 4. (a) SEM images of dhNF-hydrogels with varying dhNF concentration (scale bar: 5 μm). (b) Elastic moduli (E) and (c) swelling ratios (Q) of dhNF-hydrogels with varying dhNF length (G100P0, 1%) (*p < 0.05, n = 6). Impedance of dhNF-hydrogels with (b) varying dhNF concentration (at G97.0P2.5) and (c) PEDOT:PSS content (at 2% dhNF).
Download figure:
Standard image High-resolution imageThe effect of dhNF length on the mechanical properties of hydrogels was also explored. The largest increase in moduli with increasing dhNF concentration was demonstrated at the intermediate dhNF length of 10 μm, especially at the highest dhNF concentration of 3%, as compared to those at the longest and shortest dhNF lengths of 17 and 7 μm, respectively (figure 4(b)). This suggested that there was an optimal length for maximizing dhNF interaction with surrounding polymeric network. maximal mechanical properties; shorter dhNF could not form extensive interaction with surrounding polymer network compared to longer dhNF, while further increase in dhNF length could disrupt the crosslinking reaction. The swelling ratios were similarly unaffected by the dhNF length, indicating that the physical dimension of dhNF, without altering the crosslinking density of hydrogel, did not have significant influence over hydrogel permeability (figure 4(c)).
To further illustrate the ability of dhNF to be covalently linked to the hydrogels, by the presence of methacrylic functional groups on MGel, dhNF were incorporated into a hydrogel having extremely low mechanical strength using lower MGel concentration, thereby becoming easily disintegrated (figure S8). The hydrogel without dhNF readily became disintegrated within a day in an aqueous solution. On the other hand, dhNF inclusion resulted in the maintenance of hydrogel structure. This result demonstrated the covalent linkage between the dhNF and the polymeric network of hydrogel, leading to improved mechanical properties.
3.2.2. Electrical conductivity
One major deficiency in current hydrogel systems for biomedical applications is the lack of electrical conductivity, since it is technically challenging to incorporate conductive polymers that are generally hydrophobic in nature (i.e. electron-rich π-conjugated system) and devoid of reactive functional groups. Incorporating conductive nanomaterials, such as reduced graphene oxide and gold nanoparticles, have been explored [44, 45], but their structural features as well as chemical nature do not accurately represent the cellular microenvironments. Utilizing biocompatible nanofibers consisting of conductive polymers, therefore, could provide a more physiologically relevant mode of imparting electrical conductivity to a hydrogel system, taking a cue from cardiac tissue physiology, in which a fibrous network, called purkinje fiber, is responsible for rapid relay of electrical signals throughout the heart [46–48].
To evaluate the electrical conductivity of dhNF-hydrogel, the impedance was measured while varying the dhNF concentration of PEDOT:PSS content in dhNF (figures 4(d) and (e)). With increasing the dhNF concentration and PEDOT:PSS content at a given dhNF concentration, the impedance of dhNF-hydrogels decreased significantly, which demonstrated that the presence of PEDOT:PSS indeed help lower the resistance of highly hydrated environment of hydrogels. Furthermore, this result further proved that the amount of dhNF within the hydrogel is significant enough to enhance the conductivity. It is also noteworthy that the impedance of dhNF itself in a dried state decreased drastically and converged to zero with increasing frequency (figure S9), but the impedance of dhNF-hydrogels showed much smaller decrease with frequency and remained high even at higher frequency, indicative of the highly resistive environment of hydrogel. Therefore, the fact that the presence of dhNF could significantly lowered the electrical resistance of the hydrogel attested to the conductivity of dhNF.
3.3. Bioactivity of dhNF-infused hydrogels
3.3.1. Cell viability and proliferation in 3D culture
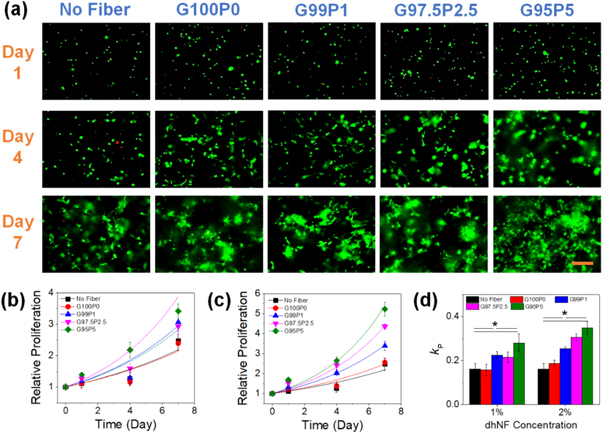
The biocompatibility of the dhNF-infused hydrogels was determined by measuring the viability and proliferation of mesenchymal stem cells (MSC), in response to the effects of dhNF concentration and PEDOT:PSS content in dhNF. Regardless of dhNF concentration, the cell viability remained high, above 80%, but the initial viability at day 1 showed a small but significant increase with PEDOT:PSS content (figures 5, S10 and S11(a)), demonstrating the biocompatibility of the dhNF-hydrogels.
Figure 5. (a) The fluorescent microscopic images of MSCs encapsulated in dhNF-hydrogels with varying PEDOT:PSS content in dhNF (1% dhNF). The cells were fluorescently labeled to visualize live (green) and dead (red) cells (scale bar: 100 μm). The cell proliferation over time was measured for dhNF-hydrogels with varying dhNF concentrations; (b) 1% and (c) 2%. (d) The plots in (b) and (c) were fitted with a power-law model to obtain the proliferation rates (kP) (*p < 0.05, n = 6). The hydrogel without dhNF ('no fiber') was examined as a control.
Download figure:
Standard image High-resolution imageWith the dhNF concentration of 2% and below, the cells within the hydrogels showed extensive spreading and proliferated over time. Compared to the hydrogel without dhNF, the cell proliferation showed significant increase with dhNF inclusion. In addition, increasing the dhNF concentration from 1% to 2% enhanced the cell proliferation at the same PEDOT:PSS content, despite having lower degree of swelling. These results were likely based on the increased mechanical stiffness of the hydrogels imparting enhanced mechanotransduction signals to the encapsulated cells [49, 50]. The presence of PEDOT:PSS in dhNF not only could increase the mechanical properties of the hydrogels, but the high electron density of PEDOT:PSS could also reduce the reactive oxygen species during the cell culture as well as excess radicals generated during the hydrogel synthesis [51, 52]. In addition, increased ion conductivity and diffusivity through the hydrogel by PEDOT:PSS could further promote the cellular activities. It should be noted that the cell proliferation was highly diminished when the dhNF concentration was further increased to 3%, which suggested that the decrease in hydrogel permeability was significant enough to limit the available space and diffusion for the encapsulated cells (figures S11(b) and (c)).
3.3.2. Effect of electrical stimulation
The application of external forces such as mechanical and electrical stimulations has been widely adopted to explore various cellular activities under dynamic conditions, such as viability, proliferation, and directional alignment [32, 53–55]. For electrical stimulation, the studies are mostly done to the cells cultured on the surface of conductive substrates. 3D studies, however, have not been extensively explored, mainly due to the deficiency of a scaffold material that encompasses tunable mechanics, adequate permeability and electrical conductivity, all crucial factors for 3D cell culture. The dhNF-hydrogels developed in this study, which possess said attributes, were expected to provide optimal 3D cellular environment for electrical stimulation.
MSCs encapsulated in dhNF-hydrogels, as presented in figure 5, were subjected to electrical stimulation (ES) by applying pulsed electrical field (figures 6, S2). Interestingly, the cell proliferation that was clearly evidently without ES was highly inhibited and the viability also decreased over time. This decrease in cellular activity was more pronounced in dhNF-hydrogels with PEDOT:PSS, which suggested that this was likely caused by the increased electrical potential generated within the dhNF-hydrogels. Previous studies have similarly demonstrated the diminished proliferative potential and increased apoptosis of MSCs in response to external ES [33, 56]. To further demonstrate the ability of dhNF-hydrogels in relaying electrical potential, a different cell type, fibroblasts, was explored. Similarly, the fibroblasts encapsulated within the dhNF-hydrogels proliferated over time, more so at dhNF with PEDOT:PSS without ES (figure S12). However, unlike MSCs, the fibroblasts showed greater proliferation at day 1, and more pronounced cell spreading over time within dhNF-hydrogels with ES. Moreover, at high PEDOT:PSS content of dhNF, there were more cells in thicker bundles that were interconnected throughout the hydrogel structure, whereas in hydrogels devoid of PEDOT:PSS, the cells mostly showed individual spreading at the vicinity without much interconnection. Taken together, these results clearly demonstrated the significantly enhanced bioactive and conductive microenvironment imparted by dhNF-hydrogels, allowing more diverse and complex investigation of cellular physiology under ES in 3D. Although it was not explored in this present study, it is also suggested that in order to enrich and diversify the utilization of dhNF, it would be beneficial to control their spacing and alignment within hydrogels to investigate the effect of anisotropic electrical stimulation on various cell types in 3D, which will be explored in future studies [57–59].
Figure 6. The fluorescent microscopic images of MSCs and fibroblasts encapsulated in dhNF-hydrogels with varying PEDOT:PSS content in dhNF (1% dhNF), subjected to electrical stimulation. The cells were fluorescently labeled to visualize live (green) and dead (red) cells (scale bar: 100 μm). The blue arrows indicate the cells in bundles that are interconnected.
Download figure:
Standard image High-resolution image4. Conclusion
In this study, a multifunctional 'heteroscaled' hydrogel consisting of hybridized networks of polymers and nanofibers were developed to impart diverse functionalities to an otherwise inert environment, with more close resemblance of a natural ECM. Unlike conventional hydrogels that are made only of polymers, this hydrogel system is infused with dispersible hybrid nanofibers (dhNF) consisting of bioactive gelatin and electrically conductive PEDOT:PSS. The dhNF were developed by (1) continuous nanofiber generation by electrospinning, (2) cutting the nanofiber to micron-scale lengths, and (3) covalent crosslinking for aqueous stability. The presence of methacrylic groups on gelatin allowed covalent incorporation of dhNF to hydrogels. The dhNF-hydrogels showed improved mechanical rigidity with dhNF concentration via composite effects, but this did not lead to significant decrease in media diffusivity that is commonly shown for hydrogels with controlled crosslinking density. The electrical conductivity, assessed via impedance analysis, showed marked increase with PEDOT:PSS content. In vitro 3D cell encapsulation studies have also demonstrated that the presence of dhNF promoted significant viability and proliferation through improved mechanical and electrical properties. dhNF-hydrogels with higher PEDOT:PSS content were more conducive towards external electrical stimulation, resulting in further alteration of cellular phenotypes. Taken all together, the dhNF-hydrogels developed herein could efficiently provide functional diversity for complex 3D cell culture platform for greater understanding of cellular physiology and eventually leading to various biomedical applications.
Acknowledgments
This study was supported by the 2019 Research Fund (1.190048.01) of UNIST (Ulsan National Institute of Science and Technology), Basic Science Research Program and Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2018R1D1A1B07048522, 2018K1A4A3A01063890).