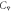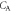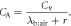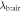Abstract
Real-time measurements of the differences in inhaled and exhaled, unlabeled and fully deuterated acetone concentration levels, at rest and during exercise, have been conducted using proton transfer reaction mass spectrometry. A novel approach to continuously differentiate between the inhaled and exhaled breath acetone concentration signals is used. This leads to unprecedented fine grained data of inhaled and exhaled concentrations. The experimental results obtained are compared with those predicted using a simple three compartment model that theoretically describes the influence of inhaled concentrations on exhaled breath concentrations for volatile organic compounds with high blood:air partition coefficients, and hence is appropriate for acetone. An agreement between the predicted and observed concentrations is obtained. Our results highlight that the influence of the upper airways cannot be neglected for volatiles with high blood:air partition coefficients, i.e. highly water soluble volatiles.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Acetone (2-propanone) is the major ketone contained in exhaled human breath and a marker of ketosis (e.g. when fasting, etc). To use this biomarker for medical diagnoses, a better understanding of the correspondence between breath and blood concentrations is essential.
In their paper [1] Španěl et al investigated the short-term effect of inhaled volatile organic compounds (VOCs) on exhaled breath concentrations. Using seven different VOCs with very different blood:air partition coefficients, including isoprene and acetone, they showed that exhaled breath concentrations depend linearly on the inhaled concentrations. This motivated our previous theoretical investigation [2] into the impact of inhaled concentrations for VOCs with low blood:air partition coefficients, i.e. compounds with exhalation kinetics that are described by the Farhi equation [3]. For these VOCs the exhaled end-tidal breath concentration equals the alveolar concentration.
In a subsequent study [4] we created a simple three compartment model which theoretically describes the influence of inhaled concentrations on exhaled breath concentrations for VOCs with high blood:air partition coefficients (e.g., acetone in Wigaeus et al [5]) where the influence of the upper airways cannot be neglected.
For such VOCs the exhaled end-tidal breath concentrations do not equal the alveolar concentrations, but the bronchial concentrations. Consider for example acetone, with typical concentrations of 1 μg/L in breath. Assuming that the exhaled end-tidal breath concentration equals the alveolar concentration and using the Farhi equation7
the blood:air partition coefficient (dimensionless Henry constant) of acetone  (from table 2 in [6]) leads to a concentration of 0.341mg/L in blood, which differs considerably from typically measured values in blood of 1 mg/L. In addition, the Farhi equation also predicts that changes of blood flow and breath flow would have no effect on breath concentrations of acetone, which can be refuted through hyperventilation or exercise experiments.
(from table 2 in [6]) leads to a concentration of 0.341mg/L in blood, which differs considerably from typically measured values in blood of 1 mg/L. In addition, the Farhi equation also predicts that changes of blood flow and breath flow would have no effect on breath concentrations of acetone, which can be refuted through hyperventilation or exercise experiments.
Hence one cannot neglect the influence of the upper airways when investigating VOCs with high partition coefficients, such as ethanol or acetone.
The models presented in our earlier work [2, 4] also link the exhaled breath concentration of systemic VOCs to physiological parameters such as endogenous production rates and metabolic rates. In addition they suggest how it is possible to take care of the contribution of ambient air concentrations when analyzing breath. They show that neither subtracting nor ignoring the inhaled concentration is a correct solution.
To confirm the theoretical investigation of our model [4] in more detail we performed a series of inhalation measurements with different ambient air concentrations of acetone, the most abundant VOC in breath, with test persons at rest and exercising under a constant workload on an ergometer. We decided to complement the study with an additional release of deuterated acetone-d6 in ambient air along with unlabeled acetone. Acetone-d6 has practically the same metabolic rate and blood:air partition coefficient as unlabeled acetone, but it is not endogenously produced. In the case of fully deuterated acetone, isotopic exchange is not facile with the methyl groups and hence the only dominant product ion observed, resulting from a reaction with H3O+, is the protonated parent, i.e., m/z 65 is used for the determination of the acetone-d6 concentration; see also [7] for the behavior of deuterated compounds when using proton transfer reaction mass spectrometry as the analysis tool.
2. Methods
2.1. Chemicals and materials
Acetone (99.9%, Sigma-Aldrich) and acetone-d6 (99.9%, Sigma-Aldrich) were released simultaneously into the laboratory air using a protocol given elsewhere [2]. Therefore, only a short description will be provided here. First, high concentration gas mixtures of the species of interest were prepared by injecting an appropriate volume (depending on the desired concentration and less than 3ppm) of liquid acetone or acetone-d6 into an evacuated 0.25 L glass bulb (Supelco, Canada) equipped with two two-way valves. The valves were then opened and the bulb was rinsed with synthetic air at the flow of 3 L/min for 2 minutes. To ensure a homogenous distribution of the released acetone in the room, the ambient air was continuously mixed with the help of a fan.
Calibration curves used to convert ion signal intensities to concentrations in ppb were obtained on the basis of triplicate analyses of 6 distinct standard mixtures of acetone and acetone-d6 each in concentrations of 50, 100, 250, 500, 1000, and 2000 ppb. These standards were prepared under dry and humid conditions, with the latter needed for the analysis of breath samples. Calibration mixtures were prepared from high purity liquid substances. A detailed description of the generation of calibration mixtures is provided elsewhere [8] and, therefore, only a short outline of the procedure will be given here. Gaseous mixtures of unlabeled acetone and acetone-d6 were produced by means of a GasLab calibration mixtures generator (Breitfuss Messtechnik, Germany). The device generates gas mixtures at different humidity levels via evaporation of liquid species. To achieve desired concentrations, pure substances were additionally diluted with water. Effectively, gas mixtures exhibiting volume fractions ranging from 50 to 2000 ppb were used for quantification of acetone and acetone-d6 in breath.
2.2. Breath sampling protocol
The test subjects freely inhaled/exhaled via a flow transducer, which was connected to a silicone mask covering both the mouth and nose [9]. From the transducer, breath samples were directed to a proton transfer reaction time-of-flight mass spectrometer (PTR-TOF-MS) via a heated (40 °C) 3m long, 1/8" diameter Teflon transfer line at a steady flow rate of 50 ml/min. Exercise tests were carried out on a supine medical ergometer at an angle of approximately 45° (eBike L, GE Medical Systems, Milwaukee, USA) operating at a constant workload mode. The main advantage of this type of ergometer is that the supporting bed stabilizes the torso of the volunteer thereby reducing the upper body movement, which in turn reduces the risk of air leaks into the mask.
2.3. PTR-TOF-MS analysis
Acetone and acetone-d6 were monitored using an Ionicon Analytik GmbH (Innsbruck, Austria) PTR-TOF 8000. The H3O+ primary ions were produced by supplying the hollow cathode with pure H2O vapor at 6 cm3/min. The settings of the ion source used in this study were as follows: ion source current 3 mA, source voltage 160 V, source-out voltage 40 V, and source valve opening 50%. At these values and a dry air sample in the drift tube operating at a reduced electric field of 130 Td, a temperature of 60 °C and a pressure of 2.4 mbar, the only other reagent ion in the drift tube is  , but this is at a very low intensity (approximately 2% of the H3O+ intensity) so that it can be safely ignored. The ion mass (m/z) calibration was based on three peaks always present in the spectra: H
, but this is at a very low intensity (approximately 2% of the H3O+ intensity) so that it can be safely ignored. The ion mass (m/z) calibration was based on three peaks always present in the spectra: H O+ (21.022), NO2+ (45.992) and C3H7O+ (59.049). The mass spectral scans ranged from approximately m/z 2.7 to 207 and were acquired in a time of 500 ms by co-adding 12 500 single 40 μs long PTR-TOF-MS extractions recorded at a sampling frequency of 10 GHz. The mass resolution of the PTR-TOF-MS 8000 for these measurements at m/z 59 and 65 was approximately 4000.
O+ (21.022), NO2+ (45.992) and C3H7O+ (59.049). The mass spectral scans ranged from approximately m/z 2.7 to 207 and were acquired in a time of 500 ms by co-adding 12 500 single 40 μs long PTR-TOF-MS extractions recorded at a sampling frequency of 10 GHz. The mass resolution of the PTR-TOF-MS 8000 for these measurements at m/z 59 and 65 was approximately 4000.
2.4. Experimental setup
For our experiments we used a closed laboratory (doors and windows shut) but with the building's ventilation system operating. Thus, the air exchange by the ventilation system caused a slow decrease of the ambient air concentration after the release of acetone and acetone-d6.
Each of our three volunteers conducted seven inhalation sessions with different ambient air concentration releases. The concentrations were released repeatedly into the room on different days. Four times approximately 2 ppm and three times approximately 500 ppb. After closing the door and preparing the volunteer (resting on the ergometer and breathing through the mask) the exact protocol of a session was:
- 6 minutes: rest
- 30 seconds: rest and simultaneous release of acetone and acetone-d6
- 9 minutes and 30 seconds: rest
- 10 minutes: exercise with a workload of 75 Watts on a supine ergometer (note: our experience is that under this moderate workload the blood flow approximately doubles and the breath flow increases by about fourfold. These new steady states are reached within one minute)
- approximately 15 minutes rest
We sampled and analyzed the air flow through the mask continuously (sampling frequency 5 Hz). Using the time profile of isoprene the periods of inhalation and exhalation were identified and distinguished. This provided a means to split the continuously measured signal into two time series for each acetone compound, where one corresponds to the inhaled concentration and the other to the exhaled concentration. Although the variability of data is considerable when displaying the individual breaths, we have refrained from downsampling to smooth the signal.
3. Results
Figure 1 shows a typical time profile of inhaled and exhaled unlabeled acetone concentrations, and figure 2 shows another typical time profile of inhaled and exhaled acetone-d6 concentrations. Immediately after the release of unlabeled acetone and acetone-d6 into the ambient air at minute 6 the concentration of unlabeled acetone and acetone-d6 is not evenly distributed which causes the peak in the signal presented in figures 1 and 2. This peak was excluded in further evaluations. However, an equilibrium between inhaled concentration and exhaled concentration is reached within less then two minutes. On such a short time scale the ambient air concentrations can be considered to be constant. Hence we compared the concentration of every single inhalation with the concentration of the following exhalation. Note that coughing or similar actions of the volunteer may lead to incomplete exhalations, producing misleading data points. Therefore we excluded exhalations which were shorter than 1 s or where the concentration of exhaled isoprene was below 5 ppb. Even with this precaution, a few outliers still remained, but their number is sufficiently low and thus have no effect on the statistics.
Figure 1. A typical time profile of inhaled (blue) and exhaled acetone (red); the vertical dashed lines mark the beginning and end of exercise.
Download figure:
Standard image High-resolution imageFigure 2. A typical time profile of inhaled (blue) and exhaled acetone-d6 (red); the vertical dashed lines mark the beginning and end of exercise.
Download figure:
Standard image High-resolution imageIn figure 3 the exhaled acetone concentration is plotted as a function of the inhaled concentration for two different concentration releases of acetone into the ambient air. In (a) the release was approximately 2 ppm and in (b) approximately 500 ppb. Measurements before the release of acetone are marked in green. Measurements after the release of acetone are marked in blue when at rest, and in red during exercise.
Figure 3. A typical dependence of exhaled acetone concentration on inhaled acetone concentration; in (a) the room concentration was approximately 2 ppm at release and in (b) it was approximately 500 ppb at release. In green are measurements before the release of acetone, in blue after release at rest and in red after release during exercise. The arrows indicate the course of time. The grey solid line marks the linear relation as predicted by our model. The dotted line indicates slope one.
Download figure:
Standard image High-resolution imageNote that the mask does not empty completely due to dead volume when exhaling, and therefore the initial values (green) are shifted to the right in the case of unlabeled acetone. The arrows indicate the course of time. Thus the inhaled concentration moves from the initial state to the maximal inhaled concentration and then the inhaled concentration decreases slowly.
In figure 4 the exhaled acetone-d6 concentration is plotted as a function of the inhaled acetone-d6 concentration for two different releases of acetone-d6 into the ambient air.
Figure 4. A typical dependence of exhaled acetone-d6 concentration on inhaled acetone-d6 concentration; in (a) the room concentration was approximately 2 ppm at release and in (b) it was approximately 500 ppb at release. In green are measurements before the release of acetone-d6, in blue after release at rest and in red after release during exercise. The arrows indicate the course of time. The grey solid line marks the linear relation as predicted by our model. The dotted line indicates slope one.
Download figure:
Standard image High-resolution imageIn all experiments we observe that the exhaled concentration  depends linearly on the inhaled concentration
depends linearly on the inhaled concentration  and hence can be described by a straight line of the form
and hence can be described by a straight line of the form  .
.
The interpretation of this model is the following: b is the concentration which is measured when the ambient air is not spiked with acetone/acetone-d6. The gradient a gives the portion of the inhaled concentration one must subtract for correction, i.e., one has to take  . The values of a and b depend on the individual metabolic and production rates of the body, and hence are different for different volunteers. b is proportional to the endogenous production rate and hence zero for acetone-d6. a depends on the metabolic rate and would equal 1 (dotted line) if the metabolic rate is zero. Table 1 summarizes the mean values of the gradients and the intercepts over all seven sessions for each volunteer. The values for b are typical values when compared with [10] (median: 559 ppb) and show the usual variations for different individuals.
. The values of a and b depend on the individual metabolic and production rates of the body, and hence are different for different volunteers. b is proportional to the endogenous production rate and hence zero for acetone-d6. a depends on the metabolic rate and would equal 1 (dotted line) if the metabolic rate is zero. Table 1 summarizes the mean values of the gradients and the intercepts over all seven sessions for each volunteer. The values for b are typical values when compared with [10] (median: 559 ppb) and show the usual variations for different individuals.
Table 1.
Individual averaged values (mean) of the seven sessions of the intersection b in ppb (i.e.,  ) for unlabeled acetone for the different volunteers and individual averaged values (mean) of the dimensionless gradient a for acetone-d6 for the different volunteers.
) for unlabeled acetone for the different volunteers and individual averaged values (mean) of the dimensionless gradient a for acetone-d6 for the different volunteers.
| person | b | sd | a | sd | |
|---|---|---|---|---|---|
| Volunteer A | rest | 411 | 105 | 0.58 | 0.03 |
| Volunteer A | exercise | 490 | 121 | 0.51 | 0.05 |
| Volunteer B | rest | 433 | 107 | 0.60 | 0.06 |
| Volunteer B | exercise | 543 | 138 | 0.52 | 0.06 |
| Volunteer C | rest | 585 | 90 | 0.53 | 0.05 |
| Volunteer C | exercise | 705 | 104 | 0.46 | 0.03 |
Hyperventilation with increased tidal volume or exercise raises the breath concentration of unlabeled acetone whereas high-frequency hyperventilation without increased tidal volume will have no effect. The first two maneuvers increase the diffusion between the bronchioles and alveoli but the third does not, for example see figure 6 in [11].
4. Discussion
Španěl et al [1] investigated the short-term effect of inhaled VOCs on exhaled breath concentrations and showed for seven VOCs, including acetone, that the exhaled breath concentration depends linearly on the inhaled concentration.
In a recent paper [4], we created a simple three compartment model which theoretically describes the influence of inhaled breath concentrations on exhaled breath concentrations for VOCs with blood:air partition coefficients sufficiently high for which the influence of the upper airways cannot be neglected. This model also predicts a linear relation between inhaled and exhaled concentrations. The measurements presented in this paper further confirm this predicted behavior.
The essential part in this model is that for VOCs with high blood:air partition coefficients, the bronchioles (upper airways) are in equilibrium with the exhaled breath and hence exhaled breath does not reflect the concentration in the alveoli. Thus for acetone the concentration in the alveoli is higher than in the bronchioles. However, when a volunteer is exercising the increase of the breath flow yields an additional contribution from the alveoli which therefore raises the exhaled acetone concentration as can be seen in figures 1 and 3.
Although the physical volume of the bronchioles is small, the effective volume is large due to the high value of acetone's blood:air partition coefficient ( ). For details about effective volumes, see appendix A.2 in [11]. Hence this compartment dominates the exhaled concentration when at rest. On the other hand, the small physical volume of the bronchiole compartment results in a quasi steady state when inhaling acetone or acetone-d6 in a short time.
). For details about effective volumes, see appendix A.2 in [11]. Hence this compartment dominates the exhaled concentration when at rest. On the other hand, the small physical volume of the bronchiole compartment results in a quasi steady state when inhaling acetone or acetone-d6 in a short time.
The concentration level of acetone-d6 in the rest of the body, however, will still be low for a much longer time and therefore the concentration of acetone-d6 in the alveoli, in contrast to unlabeled acetone, is much lower than in the upper airways (bronchioles). When a volunteer is exercising on an ergometer the increase of the breath flow yields an additional contribution from the alveoli which now lowers the exhaled concentration as can be seen in figure 2 and 4.
To take into account the room level of acetone we suggest to subtract approximately 50% of the room concentration of acetone from measured breath acetone concentrations. For VOCs with high blood:air partition coefficients we generally suggest to avoid any kind of hyperventilation or breath holding. Also we do not recommend the use of isothermal rebreathing as a possible protocol for highly water soluble VOCs. We investigated this in [12] and showed that end-exhaled breath concentrations extracted after about half a minute of rebreathing (corresponding to the common protocol of providing around five consecutive rebreaths) are still likely to underestimate the underlying alveolar level.
Acknowledgments
CA gratefully acknowledges support from the Austrian Research Promotion Agency (FFG) for the program KIRAS Security Research under the grant DHS-AS. This work has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 824986. PM, KU, and CAM gratefully acknowledges support from the EU Horizon 2020 project IMPACT, project number 674911. Finally, we thank the government of Vorarlberg, Austria for its generous support.
Footnotes
- 7
The Farhi equation [3] relates the mixed venous concentration
 with the alveolar concentration
with the alveolar concentration  by
by
Here
 is the blood:air partition coefficient and r is the ventilation-perfusion ratio which is approximately 1 at rest.
is the blood:air partition coefficient and r is the ventilation-perfusion ratio which is approximately 1 at rest.