Abstract
Hard tissues, especially teeth and bones, are highly mineralized and the large-scale defect or total loss of them is irreversible. There is still no ideal strategy for the reconstruction of various hard tissue defects that can achieve the balance between biological and mechanical properties. Polyether ether ketone (PEEK) has the potential to substitute for natural hard tissue in defect areas but is limited by its biological inertness. The addition of hydroxyapatite (HA) can significantly improve the osteogenic properties and osteointegration of PEEK materials. But the mechanical properties of HA/PEEK scaffolds are far from satisfaction making scaffolds easy to fracture. We put forward a strategy to balance the mechanical and biological properties of HA/PEEK scaffolds via the regulation of the inner crystallinity and HA mixing ratio and we systematically evaluated the modified HA/PEEK scaffolds through material characterization, in vitro and in vivo experiments. And we found that the 20%HA/PEEK scaffolds with low crystallinity achieved the required strength and elasticity, and exhibited the characteristics of promoting the proliferation, migration and osteogenic differentiation of bone marrow mesenchymal stem cells. The results of the implantation of beagles' teeth, mandible and rib showed that the 20%HA/PEEK scaffold with low crystallinity could well withstand the local complex force in the defect area and combine well with natural bone tissue, which made it a candidate for a practical versatile hard tissue engineering scaffold.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Hard tissues, including bones, teeth and cartilage, provide support and protection for other organs of the human body, and their capacity to new-renew is limited [1]. Large scale of bone defect, as well as tooth loss is irreversible and always needs autologous or alloplastic materials for reconstruction. Although autologous bone grafts are always viewed as an ideal way, their clinical application is much limited for the strict accessibility, unpredictable resorption rate and poor plasticity [2, 3]. Hard tissue engineering provides another promising strategy to reconstruct hard tissue defect [4, 5]. The porous scaffolds provide support for the defect and platform for cell growth. The bioactive factors of the scaffold promote the regeneration [6]. Hard tissue engineering puts forward higher requirements for mechanical properties of scaffolds than soft tissues, especially in terms of bones and teeth which are highly mineralized, that the scaffolds need enough stiffness to withstand pressure and weight [7]. But inappropriate stiffness of scaffolds like metal materials represented by titanium alloy causes stress-shielding and further leads to bone fracture [8]. Due to the pressure from the muscle movement and mastication, the hard tissue engineering scaffolds for bones and teeth are required both stiffness and ductility to avoid breaking itself and bone fracture. Polyether ether ketone (PEEK) as a common-used implants in spine surgery, orthopedic surgery and maxillo-facial surgery, has excellent physical and chemical properties [9, 10]. However, its bio-inertia makes it difficult to produce effective combination with tissues and easy to form fiber wrapping as an implant material [11].
Hydroxyapatite (HA) is a commonly used bioactive material for its osteogenic induction ability [12, 13]. Previous studies have found that mingling HA with the PEEK tissue engineering scaffolds can enhance their osteogenic activity and tissue binding ability [14]. HA/PEEK composites seem a potential material for ideal hard tissue engineering scaffolds. However, composite HA will affect the mechanical properties of PEEK itself, bringing adverse influence to the application of PEEK scaffolds [15]. In latest studies trying to improve the biological activity of PEEK by mixing HA, it was found that the modified PEEK scaffolds tend to be more brittle and have a higher risk of fracture [16, 17]. Several efforts have been made to balance the weakening of mechanical properties of PEEK scaffolds caused by the addition of HA. Yu et al ameliorated the HA/PEEK composites by adjusting the content of nano HA and showed that the tensile, compression, bending and impact strength of the composites were improved compared with pure PEEK [18]. Converse et al found that there was a reinforced interface between PEEK matrix and HA whisker by using powder processing and molding technology, and its elastic modulus and tensile strength were similar to those of human cortical bone [19]. Even so, HA/PEEK mixtures prepared by different preparation processes have great uncertainty and poor repeatability. To achieve the balance between the mechanical and biological properties of HA/PEEK scaffolds would be one of the breakthroughs in constructing an ideal hard tissue engineering scaffold based on PEEK.
Additive manufacturing (AM) is a promising strategy to solve the problem of the poor repeatability of HA/PEEK composites and is potential to accurately control the mechanical properties and bioactivity. Fused deposition melting (FDM) is a representative technology of three-dimension (3D) printing [20]. In terms of macro morphology, it can customize the corresponding clinical samples of different defect models. As for microstructure, it is expected to accurately control the internal structure of the material by adjusting the printing parameters [21], and affect the physical and chemical properties of the material based on the change of the internal structure of the material, that is, the crystallinity [22].
Crystallization is that the molecular chains of materials are arranged according to the ordered structure, and the crystallinity is the proportion of the crystalline region in the polymer to the total material. The degree of crystallinity directly affects the physical and chemical properties of the material [23, 24]. The properties of HA/PEEK composites constructed by different preparation processes are obviously different due to different internal crystallinity. The crystallinity of HA/PEEK composites prepared by traditional manufacturing process is affected by preparation temperature, forming pressure, auxiliary nucleating agent and other factors and is uneasy to control, which is manifested in unstable material properties, uneven internal structure and poor repeatability between batches. By adjusting the printing parameters, 3D printing technology can accurately regulate the crystallinity of HA/PEEK composites to achieve the purpose of regulating their physical and chemical properties [25].
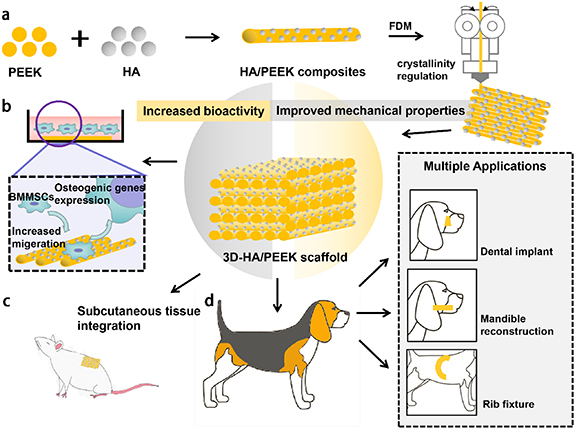
We put forward a strategy to fabricate an HA/PEEK scaffold whose mechanical properties were precisely controlled by modified internal crystallinity and biological properties by HA mix ratio. The HA/PEEK hard tissue engineering scaffolds were further applied in three chosen sites in beagles, which were rib, mandible and tooth, to prove the practical performance (figure 1).
Figure 1. Schematic illustration of the preparation, in vitro study and in vivo study of the HA/PEEK scaffolds with different crystallinity. (a) The addition of HA would increase the bioactivity of PEEK scaffolds. By adjusting the printing parameters of FDM, HA/PEEK scaffolds could demonstrate different crystallinity, which largely affected their mechanical properties. The balance between the bioactivity and mechanical properties of HA/PEEK scaffolds was achieved by the regulation of crystallinity and additional ratio of HA. (b) By co-culturing with BMMSCs, the properly manufactured HA/PEEK scaffolds could increase the cell migration and osteogenic genes expression. (c) The subcutaneous implantation showed the excellent integration between scaffolds and surrounding tissue. (d) Beagles were used for further evaluation of the application of HA/PEEK scaffolds in different kinds of bone defects.
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Fabrication and characterization of HA/PEEK scaffolds
2.1.1. Fabrication of HA/PEEK scaffolds
The HA/PEEK scaffold mold was designed and connected to the PEEK 3D-printer (Jugao Surgeon Pro, JUGAO-AM, China) to initial the 3D printing process. Turn on the temperature control system to heat the working cavity, the air circulation system to complete the internal air circulation in the working cavity of the 3D printer and the thermal insulation system to ensure the stability of the temperature field in sequence. The local temperature measurement system on the 3D printer head could form a local cooling system by air cooling, liquid cooling or semiconductor cooling, and cooperate with the local temperature disperser to form a real-time controllable local rapid cooling field near the 3D printer head. The motion of the 3D printer head was driven and the PEEK or HA/PEEK composites were extruded according to the preset model information. The printing temperature was maintained 420 °C–430 °C and the temperature of the working cavity was maintained 15 °C. The printing speed maintained 40 mm s−1.
The crystallinity is controlled by adjusting the temperature and distribution of local temperature field. According to the crystallinity information required by different parts, the material can experience different cooling speed and steady-state temperature during extrusion, so that the designed crystallinity can be formed. For high crystallinity groups, the local temperature cooling speed was maintained 200 °C–300 °C per hour. And for low crystallinity groups, the local temperature was rapidly dropped to 22 °C.
2.1.2. Characterization of HA/PEEK scaffolds
The chemical constituents were determined by x-ray photoelectron spectroscopy (XPS). Prepared PEEK, 10% (w/w) HA/PEEK, 20% (w/w) HA/PEEK and 30% (w/w) HA/PEEK scaffolds with different crystallinity were resized to less than 10 mm × 10 mm × 3 mm (figure S1). All samples were fabricated by orthogonal struts with the diameter = 400 µm. The distance between struts was 400 µm and formed porous structure. The pretreatment of incoming samples included solvent cleaning, vacuum heating or sandpaper rubbing the sample surface to remove pollutants on the material surface. The samples were then placed on the sample holder of the x-ray photoelectron spectrometer (Scientific NEXSA, ThermoFisher, USA) for testing. The spectral peaks of C, N and O were drawn and analyzed.
The crystallinity was identified using thermogravimetry differential scanning calorimetry (TG-DSC). Prepared scaffolds were resized to less than 6 mm × 6 mm × 3 mm. The samples were then analyzed by simultaneous thermal analyzer (DSC1, METTLER TOLEDO, Switzerland). The differential thermal gravimetric diagram was drawn and analyzed.
2.1.3. Mechanical evaluation
The standard samples were prepared and tested according to China National Standard for testing tensile properties of plastics (GB/T 1040.2-2006/ISO 527.2-1993) for the properties including the yield stress, breaking stress and elongation at break of the materials (figure S2). The test of the impact strength was carried out under the guidance of the Chinese national standard for testing the impact properties of plastics (GB/T 1843.1-2008/ISO 180:2000). The bending strength and bending modulus of materials were tested according to the Chinese national standard for testing the bending properties of plastics (GB/T 9341-2000/ISO 604-2002). The compression strength and modulus of materials were tested according to the Chinese national standard for testing the compressive properties of plastics (GB/T 1041-2008/ISO 604-2002). All the tests are carried out on the electronic tensile testing machine (CMT4204, MTS, China).
2.2. Cytocompatibility of HA/PEEK scaffolds
2.2.1. Isolation and culture of rat bone marrow mesenchymal stem cells (BMMSCs)
Rat BMMSCs were isolated from femurs bone marrow of male Sprague–Dawley (SD) rats aged between 3 and 4 weeks. All animals were purchased from Dashuo Experimental Animal Co. Ltd (Chengdu, China), and the animal experiments were approved by the Research Ethics Committee of West China Hospital of Stomatology, Sichuan University (Permit No. WCHSIRB-D-2021-048). The rat femur bones were isolated, cut off both ends to expose the bone cavity. The bone cavity was rinsed ten times with modified eagle's medium of alpha type medium (α-DMEM, HyClone, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Invitogen, USA) to obtain passage 0 BMMSCs. Passages 2–4 were used in the following experiments.
2.2.2. Cell seeding
All the scaffolds were ground into powder, and mixed with modified eagle's medium of alpha type medium (α-DMEM, HyClone, USA) containing 10% FBS, (Gibco, USA) with the concentration of 0.2 g ml−1 at 37 °C to achieve the extraction liquid.
2.2.3. Live/dead staining
The cytotoxicity of the HA/PEEK scaffolds was evaluated by a live/dead assay kit (KeyGEN BioTECH, China). The working solution was composed of 2 µm calcein AM and 8 µm propidium iodide. A sufficient amount of configured working solution was added on the cells, and incubated at room temperature for 30–45 min. The living and dead cells were observed under 530 ± 12.5 nm and 645 ± 20 nm excitation light respectively.
2.2.4. Cell proliferation
BMMSCs were incubated with 5% CO2 at 37 °C for 12 h, 24 h, 48 h, 72 h, 5 d, 7 d and 9 d respectively. Using a cell counting kit-8 (CCK8, Dojindo, Japan) the CCK8 cell proliferation curves of different scaffolds were obtained.
Ki67 was used as a maker to evaluate the proliferating potential of BMMSCs. After culturing, BMMSCs were blocked with 5% bovine serum albumin (BSA) solution for 30 min and dyed with prepared primary antibody Ki67, prepared fluorescent secondary antibody and prepared 5 μg ml−1 4',6-diamidino-2-phenylindole (DAPI) dye solution. The BMMSCs were observed under fluorescence microscope.
2.2.5. Cell migration
The scratch test and Transwell migration experiment were used to test the cell migration. BMMSCs were co-cultured with different scaffolds until they reached 70% of the well. The scratches with uniform and equal width were drawn straight along the center of the six well plate. BMMSCs were photographed under the inverted microscope and the scratch width was calculated to see the cell migration.
BMMSCs were evenly inoculated on the filter membrane with 8 μm pores of the upper chamber of Transwell plate (Corning, USA), with the inoculation density of 1 × 105 BMMSCs per well. The migrated cells were observed under inverted microscope, and count.
2.3. Osteogenic differentiation induction of HA/PEEK scaffolds in vitro
2.3.1. Alizarin red staining
BMMSCs were co-cultured with different materials in the osteogenic induction fluid. After 14 d, BMMSCs were fixed with 4% paraformaldehyd. The 2–3 ml 0.1% alizarin red-Tris HCl (Sigma, USA) was added to each well. The red calcified nodules were observed under inverted microscope.
2.3.2. Immunofluorescence staining of osteogenesis related genes
After co-cultured with different materials, BMMSCs were fixed by 4% paraformaldehyd. The prepared primary antibody including alkaline phosphatase (ALP) (ab95462, Abcam, USA), COL-1 (ARG52247, Arigo, China), OCN (614487, Zenbio, China), OPN (ab8448, Abcam, USA), RUNX-2 (ab76956, Abcam, USA) were diluted with 1% BSA in the ratio of 1:200. The fluorescent secondary antibody was diluted with 1% BSA in the ratio of 1:200. The 5 μg ml−1 DAPI was used to dye the cell nucleus. BMMSCs were observed under fluorescence microscope.
2.4. Biological evaluation of HA/PEEK scaffolds in vivo
2.4.1. Subcutaneous implantation in SD rats
Eight week-old male SD rats were randomly assigned into three groups designed as PEEK (with low crystallinity), 20%HA/PEEK (with low crystallinity) and titanium alloy. The PEEK and 20%HA/PEEK scaffolds were designed and fabricated as annulus (outer diameter 6 mm, inner diameter 4 mm, height 2 mm). And titanium alloy scaffolds were shaped as the same size. The materials were transplanted into the subcutaneous areas of SD rats. Samples were collected after 4 weeks and 8 weeks and treated with 4% paraformaldehyde for 24 h. All specimens were embedded with paraffin.
2.4.2. Alveolar socket implantation in beagles
The 18-month-old beagles were used for alveolar socket transplantation experiment. The beagles were divided into three experimental groups as PEEK (with low crystallinity), 20%HA/PEEK (with low crystallinity) and titanium alloy. All scaffolds were shaped as frustum (upper diameter 4 mm, lower diameter 3.5 mm, height 6 mm) to match the teeth defect. The premolars of beagles were extracted and the alveolar sockets were trimmed. Scaffolds were transplanted into the trimmed alveolar sockets and the wounds were closed. Samples were collected after 8 weeks and treated with 4% paraformaldehyde for 24 h. All specimens were embedded with paraffin.
2.4.3. Mandible and rib bone implantation in beagles
To further explore the application of the 20%HA/PEEK scaffold (with low crystallinity), we built a mandibular bone defect and rib bone defect models in beagles. A 33.7 mm × 8.5 mm × 5.7 mm defect was cut at the premolar area of one side of the mandibular bone, and the scaffold was fixed by mini-screws (figure 7(a)). A 30 mm long bone of the middle part of the 5th rib 40 mm away from the head of rib was taken away. The scaffold was designed as a 37 mm-long tube which the two broken ends of the 5th rib could be plugged into (figure 7(e)). Samples were collected after 12 weeks.
2.4.4. Histological staining
The 5 µm thick tissue sections were prepared for hematoxylin and eosin (HE) and toluidine blue O (TB) staining. All samples were treated in accordance with the manufacturer's recommended protocols.
2.5. Statistic analysis
All statistical analysis were performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Student's t-test was used to compare the differences between two groups and one-way analysis of variance was performed to assess the discrepancies between multiple groups. p < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of 3D-HA/PEEK scaffolds
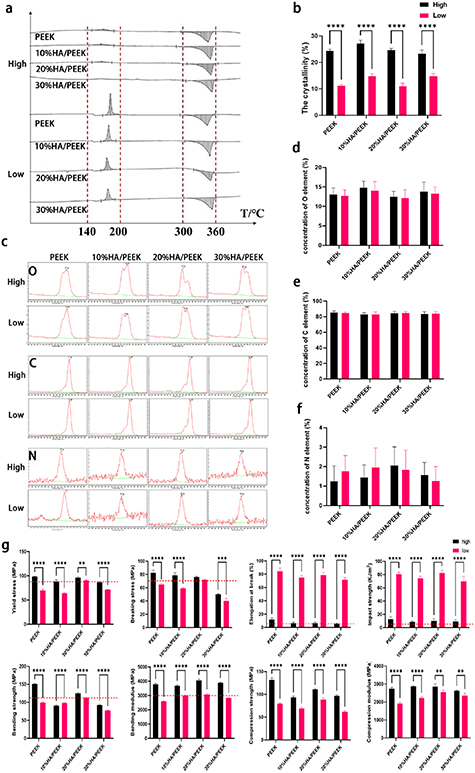
As well known, HA could give PEEK scaffolds a great improvement of bioactivity. But the optimal ratio is still controversial. In scanning electron microscope (SEM) images, we saw that the HA powders were basically sphericity (figure S3). Using two typical printing modes named as high/low crystallinity printing modes, the HA/PEEK scaffolds with different concentration of HA could demonstrate different crystallinity. TG-DSC proved that the two printing modes did endow PEEK, 10%HA/PEEK, 20%HA/PEEK, 30%HA/PEEK scaffolds with different crystallinity (figures 2(a) and (b)). Using XPS, we found that the mixture of HA and the change of crystallinity had little influence on the element composition of the PEEK scaffolds, with the C, O, N element in PEEK, 10%HA/PEEK, 20%HA/PEEK, 30%HA/PEEK scaffolds with both high and low crystallinity all at the same level (figures 2(c)–(f)).
Figure 2. Characterization of HA/PEEK scaffolds. (a) Thermogravimetry differential scanning calorimetry (TG-DSC) of PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds fabricated by two printing modes named as high/low, the recrystallization phenomenon and the final crystallinity marked by the red lines; (b) quantitative analysis of the crystallinity, **** p < 0.0001; (c) x-ray photoelectron spectroscopy (XPS) spectrum of O, C and N element of PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds fabricated by two printing modes named as high/low; (d)–(f) show quantitative analysis of O, C, N elements respectively. Data are expressed as means ± standard deviation; (g) quantitative analysis of yield stress, breaking stress, elongation at break, impact stress, bending strength, bending modulus, compression strength and compression modulus of PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds fabricated by two printing modes named as high/low. The red lines indicate the requirements of Chinese national standard specification. Data are expressed as means ± standard deviation, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Download figure:
Standard image High-resolution image3.2. Evaluation of mechanical properties
According to the Chinese standard specification for PEEK polymer for surgical implants application (YY/T 0660-2008), the requirements for the mechanical properties of PEEK composites includes yield stress >90 MPa, breaking stress >70 MPa, elongation at break >5%, bending strength >110 MPa, bending modulus >3 GPa, impact strength >4 KJ m−2, which are indicated by red dotted lines in the following statistical charts. There were obvious differences between the scaffolds with high/low crystallinity (figure 2(g)). The HA/PEEK scaffolds with high crystallinity were characterized by higher strength, hardness, rigidity, and poorer elasticity and toughness, which made them prone to brittle fracture under stress. However, those with low crystallinity would undergo elastic deformation to resist external force and avoid brittle fracture. Based on the appropriate mechanical properties showed in scaffolds with low crystallinity, the low crystallinity printing mode was used to further evaluate the influence of HA mixture ratio on the bioactivity of the scaffolds. The mixture ratio of HA would affect the mechanical properties of scaffolds as well, though the difference caused by the mixture ratio only showed in the bending strength.
3.3. Evaluation of cytotoxicity and cell proliferation
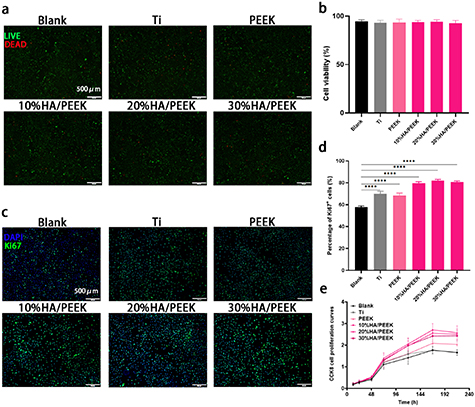
Based on the results of the mechanical evaluation, we found that scaffolds with low crystallinity were more suitable. Since titanium and its alloy is still the usual material for the bone reconstruction and dental implant, we used titanium alloys (Ti) as the positive control group. BMMSCs were seeded on the Ti, PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds and cultured for 24 h. We could see from the SEM that the cells adhered to the surface of different materials and unfolded themselves especially on the 20%HA/PEEK scaffold (figure S4). Live/dead staining showed that most of cells in all five groups were live (green), and few cells were dead (red) (figure 3(a)). The quantitative analysis also showed that there was no significant difference in cell survival rate between groups (figure 3(b)), which proved that HA/PEEK materials printed with low crystallinity have no obvious cytotoxicity, as well as Ti and pure PEEK scaffolds. CCK8 and Ki67 staining were performed using the extraction liquid of the five scaffolds. BMMSCs were cultured for 1, 3, 5, 7, 9 d. CCK8 proliferation curves showed that the addition of HA increased the proliferation of BMMSCs after 7 d (figure 3(e)), which was proved by the results of Ki67 staining as well (figures 3(c) and (d)).
Figure 3. Cell viability and proliferation analysis of HA/PEEK scaffolds with low crystallinity. (a) The live/dead staining of BMMSCs seeded on the scaffolds for 3 d; (b) quantitative analysis of cell viability; (c) fluorescence microscope images of Ki67 staining of BMMSCs cultured in the extraction liquid for 7 d; (d) quantitative analysis of percentage of Ki67+ cells, **** p < 0.0001; (e) CCK8 cell proliferation curves of BMMSCs cultured in the extraction liquid for 12 h, 24 h, 48 h, 72 h, 5 d, 7 d and 9 d.
Download figure:
Standard image High-resolution image3.4. Evaluation of cell migration
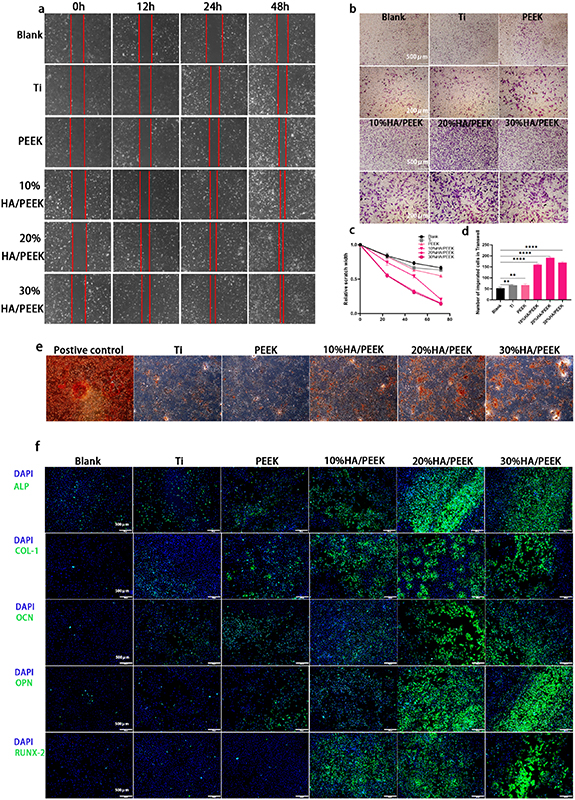
The results of cell scratch test showed that the cell scratch closure speed in HA/PEEK groups was faster, suggesting stronger cell migration ability (figure 4(a)). The statistical results showed that after 24 h, a significant reduction of the scratch width began to appear in 20%HA/PEEK and 30%HA/PEEK groups (figure 4(c)) (p < 0.05). We further used the Transwell plates to elucidate the attraction of HA/PEEK scaffolds to BMMSCs by putting the materials on the lower chambers and BMMSCs on the upper chambers. BMMSCs could move through the 8 μm pores of the filter membrane in all groups, while the number of cells was much larger in 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK groups (figure 4(d)). By adding HA to the PEEK scaffold, the composites showed a proved ability to attract cells and promote the cell migration, which was obvious when the HA mixture ratio was 20% and 30% (w/w).
Figure 4. Cell migration assessment and osteogenic evaluation of HA/PEEK scaffolds with low crystallinity in vitro. (a) Images of the cell scratches cultured in the extraction liquid for 0 h, 12 h, 24 h, 48 h; (b) crystal violet staining of BMMSCs on the lower side of membranes of Transwell chambers for 24 h; (c) quantitative analysis of the relative scratch width; (d) quantitative analysis of the number of migrated cells in Transwells. Data are expressed as means ± standard deviation, ** p < 0.01, **** p < 0.0001; (e) alizarin red staining of BMMSCs cultured with osteogenic induction fluid, Ti, PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds with low crystallinity for 14 d; (f) the immunofluorescence staining of ALP, COL-1, OCN, OPN, RUNX-2 of BMMSCs cultured with blank medium, Ti, PEEK, 10%HA/PEEK, 20%HA/PEEK and 30%HA/PEEK scaffolds with low crystallinity for 7 d.
Download figure:
Standard image High-resolution image3.5. Evaluation of osteogenic differentiation in vitro
As shown in the alizarin red staining, BMMSCs co-cultured for 14 d with 20%HA/PEEK and 30%HA/PEEK scaffolds with low crystallinity produced much more red calcified nodules compared with Ti, PEEK and 10%HA/PEEK groups, but less than positive control group which was induced by osteogenic induction fluid (figure 4(e)). The expression of the osteogenic marker genes was detected by immunofluorescence staining. All these genes including ALP, COL-1, OCN, OPN and RUNX-2 were high expressed because of the adding of HA, and were expressed at the same level in the Ti and PEEK group after culturing for 7 d (figure 4(f)). The 20%HA/PEEK and 30%HA/PEEK scaffolds with low crystallinity both demonstrated a significant improvement of the osteogenesis.
3.6. Evaluation of tissue integration of subcutaneous implantation in SD rats
To evaluate soft tissue integration, we implanted Ti, PEEK and 20%HA/PEEK scaffolds with low crystallinity into the subcutaneous areas of 8 week male SD rats (figure 5(a)). We found that the rats all kept in good health with stable bonding of scaffolds and no ulcers on the back skin after 4 weeks and 8 weeks. The tissue sections showed continuous soft tissue–material interfaces in all three groups after implanted for 4 weeks (figure 5(b)) and 8 weeks (figure 5(c)). And there seemed to be a trend for the soft tissue to grow into the inner lacunae of 20%HA/PEEK scaffold with low crystallinity which was reserved in the bioprinting process for better tissue integration (figure 5(b)).
Figure 5. Subcutaneous transplantation of HA/PEEK scaffolds in SD rats. (a) Images of the skin transplanted with Ti, PEEK (with low crystallinity) and 20%HA/PEEK (with low crystallinity) scaffolds after 8 weeks; (b) TB staining of the subcutaneous tissue sections of SD rats implanted with Ti, PEEK (with low crystallinity) and 20%HA/PEEK (with low crystallinity) scaffolds after 4 weeks; (c) TB staining of the subcutaneous tissue sections of SD rats implanted with Ti, 3D-PEEK (with low crystallinity) and 20%HA/PEEK (with low crystallinity) scaffolds after 8 weeks (S: scaffold; F: fibers).
Download figure:
Standard image High-resolution image3.7. Reconstruction of tooth loss in beagles
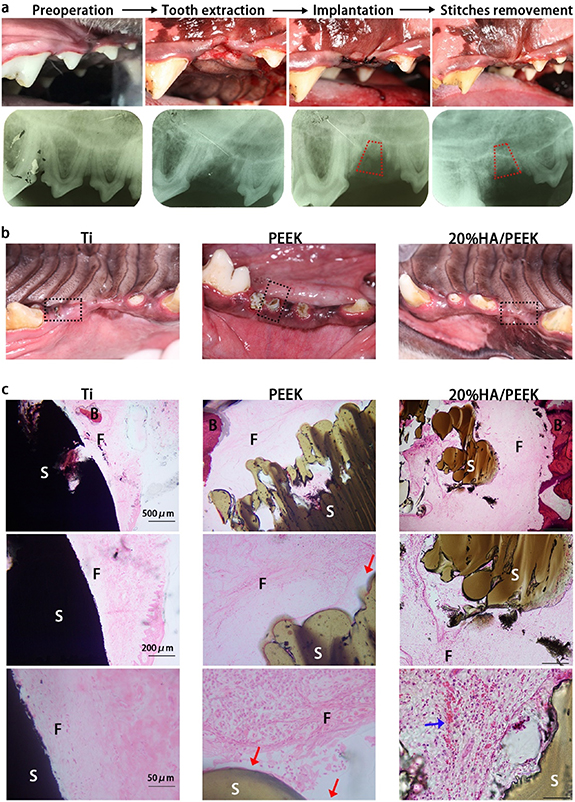
To imitate the tooth implantation to see the scaffolds' capacity as dental implants to reconstruct the tooth loss, we implanted the designed frustum scaffolds (figure S5) into trimmed alveolar sockets in beagles. After 4 weeks, the wounds of Ti scaffolds and 20%HA/PEEK scaffolds with low crystallinity all closed well, while PEEK scaffolds with low crystallinity (figure 6(b)) and one of the Ti scaffolds (figure S6) appeared to extrude out of the gingiva after 8 weeks. Two of three PEEK scaffolds with low crystallinity had an unusual mobility and one had already fall out after 8 weeks. The success rate was shown in table 1, with Ti scaffolds and 20%HA/PEEK scaffolds sharing a 100% success rate. The tissue sections demonstrated that both Ti and 20%HA/PEEK scaffolds could achieve a tight combination with the surrounding tissue, while PEEK scaffolds tended to have gaps between the tissue (figure 6(c)). There were fibers similar to periodontal ligament between Ti scaffolds and alveolar bone, but the orientation of fibers was parallel to the surface of Ti scaffolds, unlike the vertical structure as Sharpey's fiber (figure 6(c)). New-born blood vessels could be seen in the connective tissue formed between 20%HA/PEEK scaffolds and the alveolar bone. Unsurprisingly, compared with pure-PEEK scaffolds, adding HA in an appropriate proportion in PEEK scaffolds could activate the scaffolds, so that they could better combine with soft/hard tissues. Moreover, 3D printing created regular pores in the scaffolds allowing the tissues to grow into and obtain higher implant stability than titanium alloy scaffolds. The 20%HA/PEEK scaffolds seemed to be a better dental implant material than titanium alloy.
Figure 6. Alveolar socket implantation of HA/PEEK scaffolds in beagles. (a) Photographs and dental films of the alveolar socket implantation including reoperation, tooth extraction, implantation and stitches removement. The red trapezium showed the location of the scaffold. (b) Photographs of the alveolar bone implanted with Ti, PEEK (with low crystallinity) and 20%HA/PEEK (with low crystallinity) scaffolds after 4 weeks; (c) HE staining of the alveolar socket tissue sections of beagles implanted with Ti, PEEK (with low crystallinity) and 20%HA/PEEK (with low crystallinity) scaffolds after 8 weeks. The red arrows showed the gaps between the scaffold and surrounding tissue; the blue arrow showed the new-born blood vessels (S: scaffold; F: fibers; B: alveolar bones).
Download figure:
Standard image High-resolution imageTable 1. Prognosis of alveolar socket implantation in beagles. The prognosis of Ti, PEEK and 20%HA/PEEK scaffolds were assessed according to whether they had mobility or dislocated from the alveolar socket.
| Alveolar sockets implants | Total number | Prognosis | |
|---|---|---|---|
| Mobility | Dislocation | ||
| Ti | 3 | 2 | 1 |
| PEEK | 3 | 0 | 0 |
| 20%HA/PEEK | 3 | 0 | 0 |
3.8. Reconstruction of large-scale bone defects in beagles
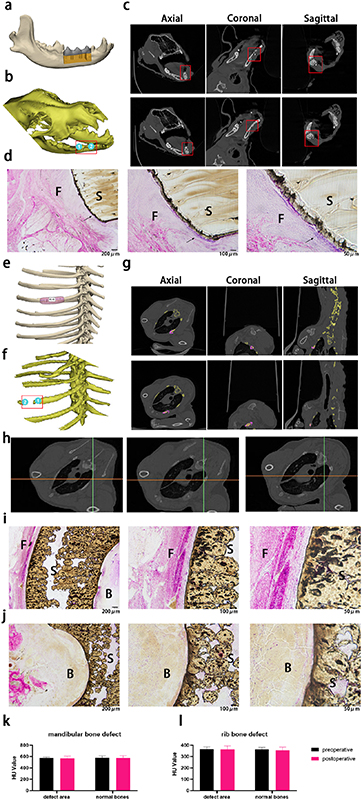
Considering that the fixture of rib bone fracture similar to dentofacial bone defect was largely affected by external force due to daily breathe movement and mastication, we chose two sites in beagles, mandible and rib, to build defect models and conduct the implantation. After 12 weeks of implantation in vivo, we photographed computed tomography (CT) and performed 3D model reconstruction of mandible (figure 7(b)) and rib (figure 7(f)) according to the images. The 3D model showed us the location of the defect. Two sites were selected in the anterior and posterior parts of the mandible defect area as examples. When observing the CT images from the sagittal, coronal and axial directions, it was found that the bone in the mandible defect area was continuous and not absorbed (figures 7(c) and (k)), demonstrating that 20%HA/PEEK scaffold with low crystallinity successfully endured the complex external forces without any harm to both surrounding tissue and the scaffold itself, largely due to the flexible mechanical characteristics. We also saw from the tissue sections that the scaffolds could combine to the surrounding tissue closely and showed little inflammation (figure 7(d)), which was inseparable from the excellent biological performance brought by the reasonable addition of HA.
Figure 7. Reconstruction of large-scale mandible and rib defect with 20%HA/PEEK scaffolds. (a) Design drawing of the mandible defect reconstruction 20%HA/PEEK scaffold with low crystallinity. (b) 3D-model of the beagle's head reconstructed by the CT images taken 12 weeks after transplantation. The red square included the location of the transplanted scaffold. Two observation sites were marked as and. (c) CT images of site 1 (the first line) and site 2 (the second line) from axial, coronal and sagittal direction (from left to right). (d) HE staining of mandibular bone tissue sections of beagles implanted with 20%HA/PEEK (with low crystallinity) scaffold after 12 weeks. The black arrow showed the tight connection between the scaffold and surrounding tissue. (e) Design drawing of the rib defect reconstruction 20%HA/PEEK scaffold with low crystallinity. (f) 3D-model of the beagle's chest reconstructed by the CT images taken 12 weeks after transplantation. The red square included the location of the transplanted scaffold. Two observation sites were marked as and. (g) CT images of site 1 (the first line) and site 2 (the second line) from axial, coronal and sagittal direction (from left to right). Bone tissue was marked as yellow and the scaffold as purple. (h) CT images of the lung tissue taken from the axial direction. (i) HE staining of the outer side of rib bone tissue sections of beagles implanted with 20%HA/PEEK (with low crystallinity) scaffold after 12 weeks. (j) HE staining of the inner side of rib bone tissue sections of beagles implanted with 20%HA/PEEK (with low crystallinity) scaffold after 12 weeks. Value of hounsfield unit on CT of the mandibular defect (k) and rib defect (l) (S: scaffold; F: fibers; B: alveolar bones).
Download figure:
Standard image High-resolution imageThe need to participate in breathing activities requires the ribs a physiological mobility caused by external force. To realize the function, the ribs are flexible and not easy to fold, which also conforms to the mechanical properties of 20%HA/PEEK scaffolds with low crystallinity. The two broken ends of the rib defect were selected as the two observation sites, and the CT image showed that the scaffold was closely combined with the bone tissue (figure 7(g)) and the density maintained the same as the normal bones (figure 7 (l)). We could also observe normal lung tissue from CT images, which meant that the scaffold will not affect adjacent organs (figure 7(h)). From tissue sections, we observed that the inner and outer surfaces of the tune-shaped scaffold were connected with the outer fibrous connective tissue (figure 7(I)) and the inner bone tissue (figure 7(j)) respectively. These results illustrated that 20%HA/PEEK scaffolds could be an ideal bone tissue engineering material and had a great potential to be used in the reconstruction of other hard tissue defect.
4. Discussion
The injury of hard tissue, especially those of high mineralized tissues like bones and teeth, often requires an exogenous material for reconstruction. An ideal type of this bone or tooth repair material is expected to display good mechanical properties which allow it endurable under the complex forces during mastication, and proper bioactivities for regeneration. The elastic modulus of PEEK is close to that of cortical bone, which is conducive to solving the problem of stress shielding caused by the inapt elastic modulus of titanium [26, 27]. In addition, PEEK can be used as a raw material for 3D printing, which greatly broadens its application in the medical field, especially in regenerative medicine. However, the bio-inertia of PEEK makes it hard to produce effective combination with the surrounding tissue, let alone to promote osteogenesis. Two major methods have come forward to activate PEEK material. One is to blend other biomaterials into it, which would do damage to its mechanical properties [12, 14]. The other option is to construct an activated surface on PEEK without affecting its inherent properties, but the surface modifying is unstable [11, 28, 29]. We have not yet found previous studies succeeding to achieve the balance between the mechanical properties and biological activation of PEEK scaffolds.
In this study, we successfully constructed a composite of HA and PEEK by changing the internal crystallinity of the material, taking into account both mechanical and biological properties. The micro crystal structure of polymer materials affects the macro physical and chemical properties. The heat treatment conditions during 3D-bioprinting can change the crystallinity. In the process of high-temperature printing, the printed samples need to gradually recover from the high-temperature state to room temperature, and the internal molecular chain structure is gradually arranged to form a crystal structure in the process of slow temperature decline. If the external temperature drops suddenly, the arrangement process of molecular chains will be limited, making it difficult to form a neat and regular crystal structure and resulting in the decrease of crystallinity. The cooling rate of the polymer changes with the external temperature followed by the change of the crystallinity, which is easily affected by the characteristics of heterogeneous materials. Therefore, it is difficult to accurately control the thermal conditions in the preparation process. FDM as a common 3D-bioprinting technology, can create complex thermal conditions with the accurate control of cooling rate thus to control the thermal properties of the polymer materials during the melting and extruding processes. Geng et al [30] used the air-force cooling in FDM and succeeded to improve the mechanical properties of the 3D printed polyphenylene sulfide, which also gave us the idea to apply this principle to PEEK. And in this study, we designed two printing modes corresponding to low/high crystallinity based on FDM, which was proved by TG-DSC (figure 2(a)).
The addition of HA endows PEEK scaffolds with good biological properties and enables the polymer scaffolds to better integrate with natural tissues and even promote regeneration, but at the same time this composite structure brings mechanical properties different from pure PEEK. As we can see from figure 2(g), the mixture of HA did change the mechanical properties compared with pure PEEK material, mainly in the reduction of bending stress and compressive stress and little change of corresponding modulus, but the relationship between this change and the mixing ratio is not clear. However, changes in mechanical properties brought about by HA mixing could be completely masked by those by changes in crystallinity. The 20%HA/PEEK fabricated by high and low crystallinity printing mode both met the national standard for PEEK for surgical implants application, but the impact resistance and elastic deformation ability of HA/PEEK prepared by printing with low crystallinity were more suitable as bone/dental restorative materials, considering that the material especially those to repair teeth loss or facial bone defect would be subjected to various directions and magnitudes of force transmitted to normal bone tissue when performing its function [3]. Following in vitro studies confirmed that different mixture ratio of HA was associated with the proliferation, adherence and differentiation of stem cells represented by BMMSCs (figures 3 and 4). The osteogenesis effect brought by mixing HA into PEEK is very significant, and the crystallinity inside the mixed material is adjusted by FDM bio-printing, which can obtain stable mechanical characteristics of strength that meets the requirements of clinical use and toughness and elasticity suitable for bone conduction.
To optimize the clinical application strategy based on the mechanical properties of different materials, it is required that the materials have better elasticity, so as to resist external force through their own deformation without brittle fracture. Therefore, in this study, 20% HA/PEEK composites with low crystallinity printing with better strength and hardness, elasticity, toughness, impact resistance and fatigue resistance were selected as the experimental group for the evaluation in vivo. The ability of 20%HA/PEEK to bind to natural tissues was evaluated using a rat subcutaneous model. Whether it is the implant material for teeth loss or the restoration material for maxillofacial bone defects [31, 32], titanium alloys are very commonly used, thus titanium alloys were chosen as the positive control group. And we found that 20%HA/PEEK could achieve similar tissue integration to titanium alloys as dental implants, and better tissue ingrowth could be observed inside 20%HA/PEEK thanks in part to the porous structure produced by 3D printing. In order to further simulate the repair of clinical bone defects, we prepared an irreversible bone defect in the mandibular premolar region of beagles, and implanted a 3D printed 20%HA/PEEK scaffold strictly designed corresponding to the size of the defect, showing tight binding to surrounding bone tissue and no general health hazard. In addition to the field of stomatology, PEEK materials have been used in many medical researches [33–38], including many animal experiments and clinical application on various hard tissues such as tibia, femur, rib and so on [18, 39–42]. However, few animal experiments are conducted on the HA/PEEK scaffolds [28, 43]. As shown in figures 7, 20%HA/PEEK also showed excellent biological properties in the rib transplantation of beagles, which demonstrated that this scaffold could be used in the repair of different bone tissues. Both the effect of dental and bone reconstruction of 20%HA/PEEK scaffold with low crystallinity demonstrated it was versatile in the field of hard tissue engineering. However, the limitation of this study is that we were unable to analyze the force of PEEK implanted in animals due to technical reasons.
5. Conclusion
In this study, we designed a 20%HA/PEEK scaffold with low crystallinity by FDM for multiple applications in hard tissue engineering. The low crystallinity printing mode realized the regulation of the internal crystallinity of HA/PEEK composites and endow them with excellent mechanical properties. The 20%HA/PEEK scaffolds showed the best ability to promote cell adhesion, migration, proliferation and osteogenesis compared to pure PEEK, 10% HA/PEEK and 30% HA/PEEK. The 20%HA/PEEK scaffolds with low crystallinity further showed good biocompatibility in animal implantation experiments, and its osteointegration ability was comparable to that of titanium alloys and better than pure PEEK. And the scaffolds fabricated under this strategy did succeed to reconstruct different bone/tooth defects in beagles.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Funding
This study was supported by Nature Science Foundation of China (31971281), Innovative Talents Program of Sichuan Province (2022JDRC0043), Research and Develop Program, West China Hospital of Stomatology Sichuan University (RD-03-202106), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (20HXJS007), Miaozi Project in Science and Technology Innovation Program of Sichuan Province (2022081) and National Natural Science Foundation of China (82270958).
Authors' contributions
W G and X M guided and supervised the total progress of this study including the research design, experiment implementation, and manuscript editing. J C, J X and X H performed the experiments. J C and J X analyzed the data. All authors have read and approved this final manuscript.
Ethical statement
All animal procedures were in compliance with Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People's Republic of China. All experimental animal procedures were performed in strict accordance with the Guidelines for Care and Use of Laboratory Animals of Sichuan University. The animal experiments were approved by the Research Ethics Committee of West China Hospital of Stomatology, Sichuan University (Permit No. WCHSIRB-D-2021-048). All efforts were devoted to minimize animal suffering.
Supplementary data (7.2 MB DOCX)