Abstract
The appropriate porosity and pore size of barrier membranes were associated with the transportation of biomolecules required for new bone formation and angiogenesis. In this study, we fabricated three-dimensional (3D)-printed resorbable polycaprolactone (PCL) membranes with different porosities (30%, 50%, and 70%) to evaluate the effective pore size for guided bone regeneration (GBR) membranes. To analyze mechanical properties and cytocompatibility, PCL membranes prepared using extrusion-based 3D printing technology were compared in dry and wet conditions and tested in vitro. The proliferation rates and pattern of fibroblasts and preosteoblasts on PCL membranes with different porosities were determined using a cell counting kit-8 assay and scanning electron microscopy. PCL membrane porosity did not affect cell proliferation, but osteogenic differentiation and mechanical properties were increased with lower porosity (30%) on day 14 (p < 0.001). Similar results were found in an in vivo calvarial defect model; new bone formation was significantly higher in PCL membranes with lower porosity (p < 0.001). These results indicate that 3D-printed PCL with 30% porosity (130 μm pore size) is an excellent pore size for GBR membranes.
Export citation and abstract BibTeX RIS
1. Introduction
The placement of endosseous dental implants for prosthetic support requires adequate bone volume at the desired locations. If bone volume is inadequate, several surgical techniques may be used to reconstruct the deficient ridge for implant placement [1–3]. Among them, guided bone regeneration (GBR) is the most commonly used method, and it has satisfactorily treated bone defects in clinical situations [4–7]. The basic principle of GBR includes blocking unwanted invasion of tissue into the bone defect area using the barrier membranes and inducing vascular ingrowth to form a chamber for stable growth of the autologous bone, and eventually, for bone regeneration without other tissue competition [4, 6, 8–11]. For successful GBR, the barrier membrane must have biocompatibility, mechanical properties for sufficient space maintenance, cell occlusiveness for blood clot protection, integration with the connective tissues, recipient site ossification, and clinical manageability [6, 9, 12]. Occlusivity is closely associated with membrane porosity [13]. The barrier membrane pores allow the migration and growth of the pluripotential and osteogenic cells (e.g. osteoblasts derived from the periosteum, adjacent bone, and/or bone marrow) and enhance the initial angiogenesis, which is important for new bone formation [6, 14–16].
Various barrier membrane materials have been used in GBR procedures [17]; these can be classified as non-resorbable and bioresorbable membranes. Non-resorbable membranes, such as extended polytetrafluoroethylene (e-PTFE) and titanium (Ti) mesh [18], are widely available in clinical settings [19, 20] and have limited immunologic reactions and excellent space maintenance abilities. Nevertheless, they require a second surgery for membrane removal and are at a higher risk of infection due to premature membrane exposure [17, 21]. In contrast, collagen, a representative bioresorbable membrane, has excellent biocompatibility and good integration with the connective tissues, which helps with early wound stabilization. Its hydrophilic properties contribute to excellent manageability [23], but its unfavorable mechanical properties and fast degradation and absorption significantly inhibit the formation of space for bone regeneration [24, 25]. To overcome the drawbacks of existing non-resorbable and bioresorbable membranes, a barrier membrane was recently prepared using synthetic bioresorbable materials [26–32].
Synthetic bioresorbable membranes have unique material compositions for controlling degradation rate, mechanical durability, and handling properties through a wide variety of manufacturing procedures. Three-dimensional (3D) printing technology, which is based on computer-aided design/computer-assisted manufacturing, is a method of preparing complicated 3D structures through layer-by-layer processes [27, 33]. The barrier membrane is especially important for preventing excessive penetration of fibrous tissue into the bone defect while allowing neovascularization and bone formation [6]. Differences in the intensity of bone regeneration depending on the pore size have been observed [34], and biomaterial scaffold porosity and pore size play a critical role in bone formation [35]. Porosity and pore size can be adjusted using the 3D printing method [26, 36, 37], and 3D scaffolds with a fully interconnected architecture can be prepared. Thus, the tissue engineering field is actively studying 3D technology biomaterials [28, 38, 39].
One aspect of 3D printing membrane development has been trying to prepare membranes of biodegradable polymer combined with bioceramic materials using the multi-head deposition system (MHDS), a type of 3D printing technology [29]. In addition, a bone morphogenetic protein was loaded onto the 3D-printed biodegradable polymer membrane to confirm its excellent bone regeneration ability [31]. Many studies on 3D-printed barrier membranes have been conducted [28–31, 38], but the effects of porosity and pore size of 3D-printed barrier membranes on new bone formation have rarely been studied.
Thus, it is necessary to investigate the effects of various porosities of 3D-printed barrier membranes on new bone formation in the GBR procedure. In this study, barrier membranes with 30% porosity (130 μm pore size), 50% porosity (300 μm pore size), and 70% porosity (700 μm pore size) were designed and fabricated using 3D printing technology, and GBR was performed in the rabbit calvarial defect models to compare the effectiveness of 3D-printed barrier membrane porosities for bone regeneration.
2. Materials and methods
2.1. Manufacturing process of polycaprolactone (PCL) barrier membranes using 3D printing technology
2.1.1. Preparation of PCL material
The 3D-printed barrier membrane was prepared using PCL (Resomer® C, Type IV 1.53–1.87 dl g−1; Evonik Industries AG, Essen, Germany) through the thermal melting process. PCL granules were heated and melted in a 10 ml steel syringe of the MHDS and kept at 80 °C for dispensing.
2.1.2. Fabrication of 3D-printed PCL membranes
PCL was dispensed from the steel nozzle of the dispensing head at 80 °C and 550 kPa. The 3D-printed PCL membrane was prepared in three layers through the layer-by-layer process. The membrane's pore structure was designed as a triangular type with 300 μm line, and different porosities and pore sizes were used for the experimental groups: 30% porosity (130 μm pore size), 50% porosity (300 μm pore size), and 70% porosity (700 μm pore size).
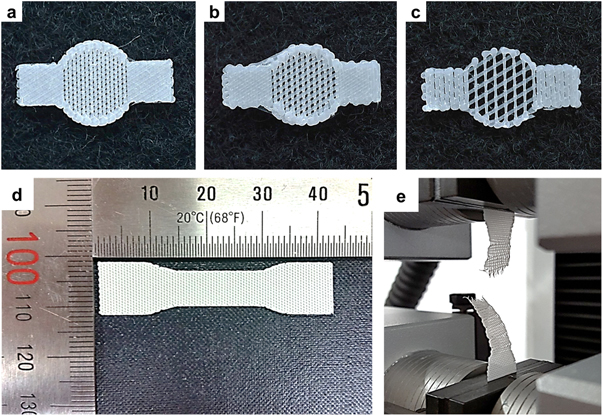
A 3D-printed PCL membrane with a circular shape and an 8 mm diameter was designed to treat 6 mm calvarial defects. In addition, two rectangular wings (4.0 × 4.5 mm) disposed at 180° around the membrane were designed to fix the membrane with screws (figures 1(a)–(c)).
Figure 1. Images of the 3D-printed PCL membranes. (a) 30% porosity (130 μm pore size); (b) 50% porosity (300 μm pore size); (c) 70% porosity (700 μm pore size) and the mechanical tensile test. (d) specimen dimensions (42.2 mm × 10.5 mm × 0.25 mm); (e) the tensile test was performed using a single-column tensile test machine.
Download figure:
Standard image High-resolution image2.1.3. Mechanical testing of 3D-printed PCL membranes
A membrane-type specimen mechanical tensile testing was fabricated using the 3D printing system. The experimental conditions were based on ISO527 (tensile test on plastics), and the specimen dimensions were determined according to type 4 of ISO527-3. The overall specimen dimensions were 42.2 × 10.5 × 0.25 mm (figures 1(d) and (e)). The tensile test was performed using a single-column tensile test machine (Model 3345, Instron Co., Norwood, MA, USA) under dry and wet (after soaking the membranes in medium for 18 h) conditions; the printed strut size was 300 μm. Load and displacement were monitored at a constant cross-head speed of 5 mm min–1.
2.2. In vitro experiments
2.2.1. Cell culture and proliferation analysis
Mouse fibroblasts (NIH3T3, ATCC #CRL-1658) were cultured in Dulbecco's modified Eagle medium plus 10% FBS and 1% penicillin and streptomycin. Preosteoblasts (MC3T3-E1, ATCC #CRL-2593) were grown in α-minimum essential medium (α-MEM) containing 10% FBS and 1% penicillin and streptomycin (all from Invitrogen, Carlsbad, CA, USA). Before cell seeding, PCL membranes (10 × 10 × 0.3 mm) were pre-wetted in culture medium for 2 h; cells were then seeded on the membranes at a density of 3 × 105 cells/200 μl. Cell-seeded membranes were incubated at 37 °C and 5% CO2, and proliferation rates on the scaffold were measured with the cell counting kit-8 (CCK-8, Dojindo, Rockville, MD, USA) at 1, 4, 7, and 14 d of incubation.
2.2.2. Osteogenic differentiation and alizarin red stain
For osteogenic differentiation, MC3T3-E1 cells were cultured for 1 d; then the media was replaced with osteogenic differentiation media consisting of α-MEM supplemented with 20% FBS, 10−8 M dexamethasone, 0.2 mM ascorbic acid, 10 mM β-glycerol phosphate (all from Sigma Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). After differentiation for 7 or 14 d, all membranes were stained with alizarin red S for analysis of the calcium deposition of cells on the membrane. To quantify staining, the alizarin red S stain was extracted by 10% cetylpyridinium chloride for 10 min at room temperature and the optical density was measured at 570 nm using a microplate reader (Epoch, BioTek, Winooski, VT, USA).
2.2.3. Scanning electron microscopy (SEM)
The morphology of the cells on membrane was observed using high-resolution SEM with acceleration voltage of 10 kV (Nova NanoSEM 450, FEI, Hillsboro, OR, USA).
2.3. In vivo study
2.3.1. Experimental animals
Eight 12 to 13 week old male New Zealand white rabbits (average weight 3.4 kg, range 3.3–3.5 kg) were used for this study. Rabbits were individually housed in a light- and temperature-controlled environment and provided with food and water ad libitum. The animal selection, animal management, and surgical procedures were performed in accordance with the Ethics Committee on Animal Experimentation at Chung Buk University (CA-15-13).
2.3.2. Experimental design
Four groups were used for the in vivo study: the control group (no membrane), and 3D-printed PCL membrane groups with 30%, 50%, and 70% porosity; bone grafting materials (Bio-C, Cowellmedi Co., Busan, South Korea) were used in all four groups. Four defects were formed on each experimental animal cranial bone for a total of 32 defects in eight animals. Each of the four defects in one rabbit was randomly assigned to one of the four groups. The number of animals used for the statistical analyses was eight per group. Experimental animals were sacrificed four weeks after surgery.
2.3.3. Surgical procedure
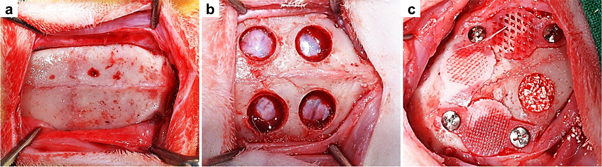
General anesthesia was induced by an intramuscular injection of 0.5 ml tiletamine plus zolazepam (125 mg ml−1; Zoletil; Bayer Korea Co., Seoul, South Korea) and 0.5 ml xylazine hydrochloride (10 mg kg−1 body weight; Rompun; Bayer Co., Seoul, South Korea). The cranium area was shaved from the nasal bone to the occipital protuberance and disinfected with povidone–iodine. Lidocaine (2%) containing epinephrine (1:100 000) was infiltrated into the cranial area to reduce local hemorrhaging. An approximately 25–30 mm midsagittal incision was made on the rabbit skull, and the periosteum was removed to expose the parietal bone. Two 6 mm diameter calvarial defects were formed beside the sagittal suture on each right and left cranial bone without damaging the dura with a dental-trephine bur while saline solution was being supplied. The bone grafting material was quantified (about 0.03 g) and grafted into each defect. No membrane (control group) and the 3D-printed membranes with 30%, 50%, and 70% porosity were randomly placed onto the calvarial defects, and each was fixed with a titanium pin (Dentium Co., Seoul, South Korea) to improve membrane stability. These membranes covered the defects in a manner similar to the GBR procedure. The pericranium was sutured using 4-0 vicryl, and the skin was closed in layers with 4-0 silk. Eight specimens were obtained from each group (figure 2).
Figure 2. Surgical procedures for the in vivo study. (a) The parietal bones were exposed by removing the periosteum; (b) four calvarial defects were formed with a trephine bur (6 mm diameter); (c) all of the defects were filled with the grafting material, and 3D-printed membranes with different porosities (30%, 50%, and 70%) or no membrane (control group) was randomly applied on the grafted area.
Download figure:
Standard image High-resolution image2.3.4. Post-operative care and sacrifice
After surgery, rabbits received 1 mg kg−1 gentamicin (Kookje Co., Seoul, South Korea) and 0.5 ml kg−1 pyrin (Green Cross Veterinary Products Co., Seoul, South Korea) intramuscularly three times daily for 3 d. Rabbits were individually caged and received food and water ad libitum. Animals were allowed to recover for 4 weeks postsurgery, after which they were sacrificed by CO2 inhalation. To collect the specimens, the skin was dissected and the defect sites were harvested along with the surrounding bone and 3D-printed membrane. Harvested specimens were fixed in neutral buffered formalin (Sigma Aldrich Co., St. Louis, MO, USA) for 2 weeks and prepared for micro-computed tomographic (μCT) analysis and histomorphometric analysis.
2.3.5. μCT analysis
After fixation, 3D μCT images were made to analyze the new bone volume (NBV) of the defect area. The specimens were wrapped with Parafilm M® (Bemis Co., Neenah, WI, USA) to prevent drying during the scanning process. All samples were scanned with 130 kV energy, 60 μA intensity, and a 7.10 μm pixel resolution using a bromine filter (0.25 mm) (Skyscan-1173, version 1.6, Bruker-CT Co., Kontich, Belgium). Then the Nrecon reconstruction program (version 1.6.10.1, Bruker-CT Co., Kontich, Belgium) was performed. The applied scan and reconstruction parameters were identical for all specimens. The overall augmented contour was evaluated in the 3D reconstructed view. Boundaries were set to standardize the region of the augmented volume to be analyzed. NBV (mm3) was calculated as the area occupied by NBV within the region of interest (figure 3).
Figure 3. Images of the region of interest. (a) Reconstructed image; (b) total bone (bone graft material + new bone) image; (c) bone graft material image.
Download figure:
Standard image High-resolution image2.3.6. Histomorphometric analysis
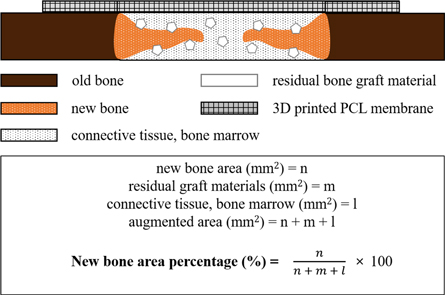
After the completion of the μCT analysis, specimens were cleaned with running water, and calcium was removed using a rapid decalcifying solution (Calci-Clear Rapid, National Diagnostics, Atlanta, GA, USA). After confirming calcium removal, the ethanol concentration was increased for dehydration. Upon the completion of dehydration, dealcoholization, paraffin infiltration, and paraffin embedding were conducted. The paraffinized specimen block was longitudinally sectioned at each defect center using a microtome, then mounted to a slide. The thickness of the final slide was 4 μm. Hematoxylin–eosin (H&E) staining was used to observe the newly regenerated bone, then the specimen was mounted to prepare the final slides. The most central sections from each block were selected for histologic and histometric evaluation. Selected slides were imaged using an optical microscope (BX51, Olympus Co., Tokyo, Japan) connected to a computer, a CCD camera (SPOT Insight, Scientific Co. Inc., Campbell, CA, USA), and an adaptor (U-CMA3, Olympus Co., Tokyo, Japan). An i-Solution (ver. 8.1, IMT i-Solution, Inc., Coquitlam, BC, Canada) was used to analyze the captured images. The general specimen images were observed at ×12.5 magnifications, and ×100 and ×400 magnifications were used for histometric analysis. For the histometric analysis, an identical professionally trained and blinded investigator measured the new bone area (NBA; %) as the area occupied by new bone/the area of defect ×100 (figure 4).
Figure 4. Schematic drawing of the histometric analysis.
Download figure:
Standard image High-resolution image2.4. Statistical analysis
All experimental data are expressed as means, standard deviations, and medians. All statistical analyses were conducted using software R (version 3.1.3). The experimental group (control, 30%, 50%, or 70% group) was set as an independent factor, and the rabbit number was set as a random factor. To compare the radiographic and histomorphometric parameters between groups, non-parametric analysis introduced by Brunner and Langer was used for the group difference tests a non-parametric mixed model was used [40, 41]. The statistical significance level was 5% and post hoc analyses were performed by pairwise comparison.
3. Results
3.1. Mechanical testing of PCL membranes
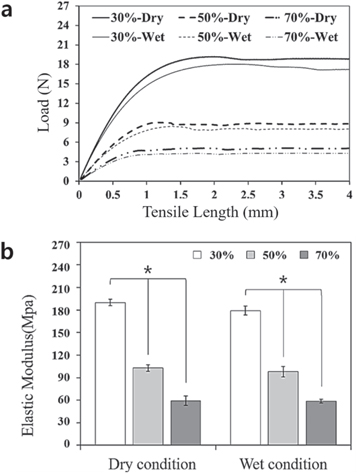
To evaluate the mechanical properties of 3D-printed PCL membranes with different porosities (30%, 50%, and 70%), the tensile test was performed in dry and wet conditions to confirm differences in mechanical behavior. The tensile test results of membranes with different porosities are shown in figure 5 and table 1. For the load-displacement curve (figure 5(a)), although there were no significant differences in the maximum load of each membrane in dry and wet conditions, the maximum load clearly differed between the membrane groups with different porosities. The maximum load of the membrane with 30% porosity in dry and wet conditions was approximately 2- and 4-times higher than that of the membranes with 50% and with 70% porosity, respectively. As shown in figure 5(b), the calculated elastic modulus of the membrane with 30% porosity was two and four times higher than that of the other membrane groups at dry and wet conditions. In addition, the elastic modulus of the membranes decreased as porosity increased in both dry and wet conditions (p < 0.001). However, the elastic modulus was not significantly different between dry and wet conditions.
Figure 5. Tensile test results (load-displacement curve, (a) and elastic modulus (b) of 3D-printed PCL membranes with three different porosities (30%, 50%, and 70%) in dry and wet conditions.
Download figure:
Standard image High-resolution imageTable 1. Maximum tensile load and elastic modulus of 3D-printed PCL membranes with three different porosities (30%, 50%, and 70%) in dry and wet conditions (means ± SDs; n = 3).
| Group | Maximum tensile load (N) | Elastic modulus (MPa) | ||
|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |
| 30% | 20.0 ± 0.7 | 18.8 ± 0.5 | 190.1 ± 4.3 | 179.2 ± 5.9 |
| 50% | 8.8 ± 0.4 | 8.2 ± 0.5 | 102.8 ± 4.4 | 98.0 ± 7.0 |
| 70% | 5.2 ± 0.5 | 5.0 ± 0.5 | 59.4 ± 6.3 | 58.6 ± 2.6 |
3.2. In vitro results
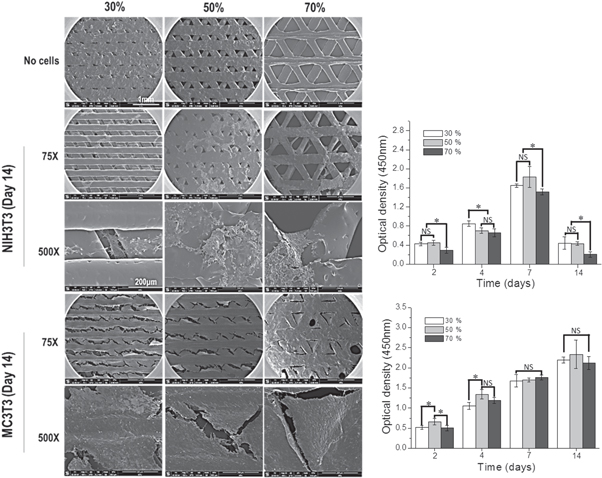
In order to verify the effects of PCL membrane porosities (30%, 50%, and 70%) on cells, we used fibroblasts (NIH3T3) and preosteoblasts (MC3T3-E1), which play an important role in alveolar bone formation. To analyze cell morphology on membranes, cell-seeded membranes were fixed with 2.5% glutaraldehyde and observed by SEM. As shown in figure 6, NIH3T3 cells were detached and did not cover the membrane surface on day 14 of culture on the membrane, regardless of porosity. On the other hand, MC3T3-E1 covered the whole membrane at 30% and 50% porosity and covered 90% of the 70% porosity membrane. These results were confirmed by a CCK-8 assay, which verified proliferation. Fibroblasts proliferated through day 7, after which the cells detached from the membrane. By day 14, the optical density was decreased to that on day 2; on the other hand, MC3T3-E1 cells continued to proliferate over time, and covered the membrane surface on day 14, as seen by SEM. However, these results did not show a significant difference depending on porosity, which indicates that the PCL porosity did not affect cell attachment or growth.
Figure 6. Cell morphology and proliferation assay of NIH3T3 and MC3T3 cells on PCL membranes with three different porosities (30%, 50%, and 70%).
Download figure:
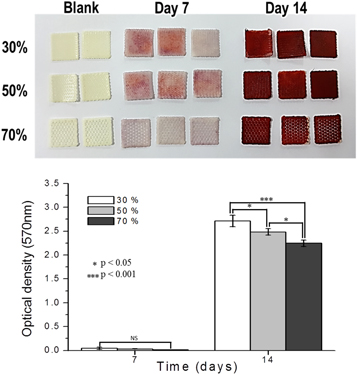
Standard image High-resolution imageNext, osteogenic differentiation was evaluated on the three PCL membranes with different porosities (30%, 50%, and 70%). MC3T3 cells were seeded onto each membrane. They were then maintained in culture media for 3 d, after which the media was replaced with osteogenic differentiation media. After 7 and 14 d, alizarin red staining was performed to measure calcium deposition; the stained alizarin red was extracted using 10% cetylpyridinium chloride and quantified (figure 7). The results of the quantitative assay showed that differences were marginal at 7 d, but osteogenic effects were observed at 14 d in the following order: 30% > 50% > 70% porosity, with a significant difference (p < 0.001) between 30% and 70% porosity. Thus, the in vitro data demonstrates very little difference in cell attachment or growth depending on porosities in cell attachments or growth, but osteogenic effects increased with lower porosity.
Figure 7. Alizarin red stain for the MC3T3 cells on the PCL membranes with three different porosities (30%, 50%, and 70%).
Download figure:
Standard image High-resolution image3.3. In vivo results
3.3.1. Clinical findings
All animals survived the surgical procedure, and all 32 defects were healed. During the healing period, no membrane exposure or loss was observed, and no abnormal findings such as inflammation or separation in the surgical site were observed.
3.3.2. μCT tomographic findings
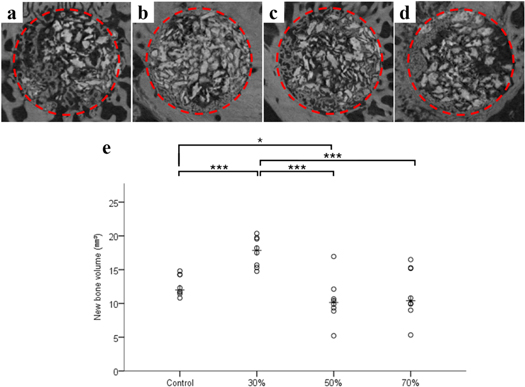
The volumetric measurements are summarized in figure 8 and table 2. The differences in NBV (mm3) were significant among the groups (p < 0.001). The 30% group showed a significantly high level of new bone formation among the experimental groups (p < 0.001). The control group showed a significantly high level of new bone formation compared with that of the 50% group (p = 0.019) (figure 8(e)).
Figure 8. Micro-computed tomographic images of each group. (a) Control group; (b) 30% group; (c) 50% group; (d) 70% group and scatter plot and median of new bone volume (mm3, (e)).
Download figure:
Standard image High-resolution imageTable 2. Volumetric analysis within the area of interest (n = 8; mm3).
| Group | Mean ± SD | Median |
|---|---|---|
| Control | 12.66 ± 1.55 | 12.00a,b |
| 30% | 17.64 ± 2.18 | 17.86b,c,d |
| 50% | 10.43 ± 3.31 | 10.15a,c |
| 70% | 11.50 ± 3.82 | 10.40a |
| p value | <0.001 | |
aSignificant difference compared to 30% group (p < 0.05). bSignificant difference compared to 50% group (p < 0.05). cSignificant difference compared to control group (p < 0.05). dSignificant difference compared to 70% group (p < 0.05).
3.3.3. Histologic findings
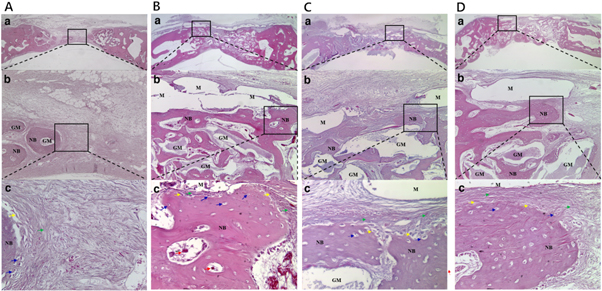
In the control group (figure 9(A)), a small amount of new bone formation was derived from the existing bone and around the bone grafting materials, but most of the new bone tissues were immature. The grafting material was unevenly positioned, and a large amount of fibrous tissues was observed in the whole defect area.
Figure 9. Control group (A), 30% group (B), 50% group (C), and 70% group (D). H&E staining of histological sections of defect sites 4 weeks after surgery. A small amount of new bone formation and a large amount of fibrous tissues was observed in control group. A significant amount of new bone formation and a small amount of fibrous tissue was observed in 30% group. New bone formation and fibrous connective tissues were observed in 50% group. New bone formation and fibrous connective tissue were observed in 70% group. The membrane was absorbed during deparaffinization processing. The rectangles (a and b) indicate new bone formation. NB: new bone, GM: grafting material, M: membrane, green arrow: mesenchymal cell, red arrow: blood vessel, yellow arrow: osteoblast, blue arrow: osteocyte within a lacuna (original magnification: ×12.5 [a], ×100 [b], ×400 [c]).
Download figure:
Standard image High-resolution imageIn the 30% group (figure 9(B)), more new bone formation was observed from the existing bone around the bone grafting materials and the lower area of the membrane. Newly formed blood vessels were observed in the new bone formation area. The immature bone tissues showed a plate structure in the lower area of the defect bordering the dura, but they did not show complete continuity due to the presence of fibrous bone. The bone grafting material was spread evenly. A small amount of fibrous tissue was observed below the pore in the membrane center. Tissues free from inflammation were clearly observed around the membrane. In all the specimens, the membrane was absorbed during the deparaffinization process.
In the 50% group (figure 9(C)), new bone formation was observed from the existing bone and around the bone grafting materials, but most of these new bone tissues were immature. The bone grafting material was spread evenly. Fibrous tissues were observed in the lower area of the membrane pore. Tissues free from inflammation were clearly observed around the membrane.
In the 70% group (figure 9(D)), new bone formation was observed from the existing bone around the bone grafting materials and the lower area of the membrane, but most of these new bone tissues were immature. The bone grafting material was unevenly positioned. A large amount of fibrous tissue was observed in the lower area of the membrane pore. Tissues free from inflammation were clearly observed around the membrane.
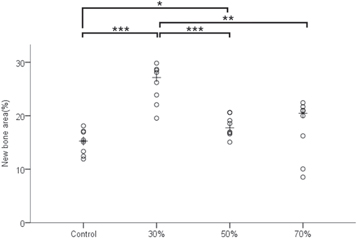
3.3.4. Histometric findings
The histometric measurements are summarized in figure 10 and table 3. Differences in NBA (%) were significant between the groups (p < 0.001). The 30% group had a significantly higher level of new bone formation compared with that of the other experimental groups (p < 0.05). The 50% group had a significantly higher level of new bone formation than the control group (p < 0.05).
Figure 10. Scatter plot and median of new bone area (%). '*' indicates statistical significance (p < 0.05). '**' indicates statistical significance (p < 0.01). '***' indicates statistical significance (p < 0.001).
Download figure:
Standard image High-resolution imageTable 3. Histometric analysis within the area of interest (n = 8; %).
| Group | Mean ± SD | Median |
|---|---|---|
| Control | 15.04 ± 2.30 | 15.27a,b |
| 30% | 25.85 ± 3.67 | 27.16b,c,d |
| 50% | 18.03 ± 2.01 | 17.75a,c |
| 70% | 17.60 ± 5.47 | 20.47a |
| p value | <0.001 | |
aSignificant difference compared to 30% group (p < 0.05). bSignificant difference compared to 50% group (p < 0.05). cSignificant difference compared to control group (p < 0.05). dSignificant difference compared to 70% group (p < 0.05).
4. Discussion
Many factors that contribute to a successful GBR outcome, including occlusion and stability, size of the barrier perforations, peripheral sealing between the barrier and the host bone, adequate blood supply, and access to bone-forming cells [6]. The barrier membrane pore size is associated with the transportation of biomolecules required for new bone formation and angiogenesis [6]. In addition, the porous and the fully interconnected structure could reduce problems with membrane separation and exposure due to tightly integrated membranes and tissues after soft tissue penetration [29].
The few reports in the literature show no effect of porosity on the amount of apposited bone [42]. However, porosity is expected to enhance osteogenesis, and numerous studies have verified this hypothesis [13, 16, 35]. Hutmacher et al reported that highly porous matrices formed a space not only for graft revascularization [16], but also for facilitating progenitor cell migration, proliferation, and differentiation to induce bone regeneration. According to Rispoli et al [13], a larger pore size allows faster-growing cells to overpopulate the defect site and inhibit the infiltration and activity of bone-forming cells. This decreases the resulting surface area of the material, which could limit initial cell adhesion onto the membrane. On the other hand, cell migration is limited if the pores are too small, resulting in avascular tissues [13]. Few studies on membrane pore size for effective bone regeneration have involved the appropriate porosity and pore size of 3D-printed barrier membranes. Therefore, in this study, the effects of 3D-printed barrier membrane with different porosities on new bone formation were investigated between experimental groups involving 30% (130 μm pore size), 50% (300 μm pore size), 70% (700 μm pore size), and control (no membrane) groups.
Various methods, including solvent casting/particulate leaching, phase separation, emulsion freeze-drying, gas forming, and fiber bonding have been used in conventional manufacturing of porous structures [30]. However, these methods have limitations in controlling membrane pores, pore size, and customized 3D microstructure, and require the use of cytotoxic organic solvents [20]. In contrast, the novel 3D printing technology method, can reproduce desirable pore types and a consistent pore size while maintaining a fully interconnected structure without using organic solvents. Among 3D printing technologies, the fused deposition modeling system prepares products by heating and melting polymer to form thin threads through pneumatic pressure and accumulates them to form multiple layers [43, 44]. The MHDS (an extrusion-based 3D printing system) is an advanced type of fused deposition modeling system; it is composed of four heads that can simultaneously process four membranes. Thus, this system can mass-produce membranes. In this study, barrier membranes with fully interconnected triangular pores were fabricated through MHDS.
PCL, the biodegradable synthetic polymer used in this study, has been approved by the United States Food and Drug Administration. PCL's properties include availability, non-toxicity, biocompatibility, and high elasticity [44]. A GBR membrane should be flexible enough to cover irregular bone defects [29], and PCL membranes tend to elongate without early fracture under a tensile load, so they are flexible enough to cover irregular defect sites [29]. Further, it takes three to four years for PCL-based polymer to be completely degraded [45, 46]; thus, the space for bone regeneration can be sufficiently maintained when working with scaffold. In addition, since the melting point is as low as 59 °C–64 °C, fully interconnected architectures can be prepared using MHDS.
In a previous study [29], 3D-printed synthetic bioresorbable membrane fabricated by MDHS with a blend of PCL, poly (lactic-co-glycolic acid) (PLGA), and β-tricalcium phosphate (β-TCP) promoted new bone formation in vitro and in vivo. When the PCL/PLGA/β-TCP membrane was compared to Ti-mesh, the new bone formation around the implants and osseointegration were similar to those of the Ti-mesh [30], and as recombinant human bone morphogenetic protein 2 was loaded on the blended PCL/PLGA/β-TCP membrane, significantly more new bone was formed [31]. In this study, only PCL was used to exclude the influence of material that could affect new bone formation, and the effects of different porosities of 3D-printed barrier membranes on new bone formation were evaluated.
Buser et al suggested barrier membrane mechanical strength is a key factor in successful GBR [9]. When we conducted tensile tests, the maximum load of the 30% group was two to four times greater than those of the 50% and 70% groups in both dry and wet conditions, confirming its excellent ability to maintain space. In this study, the excellent mechanical properties of the 30% group contributed to achieving stable bone tissue regeneration. While collagen membranes show excellent biocompatibility and are the most widely used, their weak tensile strength in wet conditions limit for wide clinical application [45]. The mechanical properties of collagen membranes are very different depending on the conditions. Moistening the membranes considerably alters their mechanical properties [47], and tensile strength drops sharply in wet conditions [30]. However, the 3D-printed synthetic bioresorbable membrane in this experiment had stable tensile test results in both dry and wet conditions [30], which may be due to the highly hydrophobic properties of the PCL material.
In a previous study examining soft tissue responses using polyurethane sponges [48], the smallest pore was rapidly filled by a dense, collagenous connective tissue which ultimately showed vacuolization; on the contrary, sponges with the largest pore size were filled with a loose-meshed alveolar tissue. According to Chvapil et al [49], material porosity is a decisive factor for tissue permeation, and the pore size should exceed 100 μm to ensure rapid penetration of highly vascular connective tissues, as small pores that were inadequate for capillary penetration tended to be filled with avascular tissues. Shim et al suggested the effective scaffold pore size for bone tissue engineering was 200–400 μm [32]. Therefore, in this study, we designed PCL membranes with three different porosities: 30% (130 μm pore size), 50% (300 μm pore size), and 70% (700 μm pore size). As a result, the 30% group (130 μm pore size) had excellent new bone formation as well as osteogenic differentiation in the in vitro assay. This may be due to pore size larger than 100 μm, which has been suggested as the smallest size [35] for migration and proliferation of pluripotential and osteogenic cells and enhancing initial angiogenesis [6, 14–16], as well as the ability to maintain space enhanced with a better tensile strength than other experimental groups.
According to Lee et al [50], if the cortical bone is removed and blood supply is sufficient, it is important to prevent the leakage of bone-marrow-derived blood and factors required for initial bone regeneration from the defect site. As it more effectively prevented leakage of bone formation factors from the defect site better than other experimental groups, the 3D-printed barrier membrane with 30% porosity (130 μm pore size) eventually contributed to excellent new bone formation.
In the histologic findings of this study, the control and 70% groups showed a small amount of randomly scattered grafting materials. In the control group, grafting materials were scattered due to the absence of a barrier membrane. According to a grafting material manufacturer, the particle size of the grafting material ranged from 410 to 1000 μm. In the 70% group, some of the grafting material particles leaked from the defect area and scattered. In addition, in the histometric findings of this study, the 70% group had more variable results than did other groups. This may be due to reduced stability of the barrier membrane with increased porosity. The 30% group had more stable new bone formation than did the other experimental groups because they had two to four times higher space-maintaining ability than did the 50% and 70% groups in dry and wet conditions, based on the tensile test results.
In this study, the control group showed significantly higher μCT findings than the 50% group despite the absence of the membrane (p = 0.041). This may be due to osteoconduction and new bone formation, which were affected by the periosteum in the initiation of osteogenesis and angiogenesis of donor bone graft healing [51], although they were unfavorable in terms of space maintenance compared with the other experimental groups.
Among the membranes used in the GBR procedure, non-resorbable membranes are widely used in specific indications requiring a space for bone regeneration and stable maintenance due to their excellent space-maintaining ability, despite the requirement for a second surgery to remove the membrane and the risk of infection from premature membrane exposure [6]. In particular, Ti-mesh is widely available in clinical settings [17, 18] because of its high strength and rigidity, low density, low weight, and resistance to corrosion [22, 52]. Commercially available Ti-mesh commonly has millimeter-level macroporosity [18]. Macroporous characteristics of Ti-mesh can be sharp where the material is cut or bent, which may provide an easy pathway for microbial contamination into the healing site [22], so they should be removed. In addition, it is difficult to produce Ti-mesh with various pore size and structure. Membrane porosity, pore size, and structure can be easily adjusted using 3D printing technology, as shown in this study. The 3D-printed barrier membrane with 30% porosity showed excellent mechanical properties and could sufficiently maintain the space while during new bone maturation due to PCL's slower degradation rate. Moreover, no additional surgery was required to remove the membrane due to complete degradation. Accordingly, replacing the conventional Ti-mesh with 3D-printed barrier membranes may provide an excellent tissue engineering solution.
In this study, the experimental group with 30% porosity 3D-printed barrier membranes had a significantly better ability to form new bone in all aspects than did the other groups. However, the limited sample size and the inadequacy of an experimental group that did not use grafting materials may be limitations of this study. In further studies, a larger sample size, experimental groups with and without grafting materials, and observations over time may be necessary.
5. Conclusions
In this study, synthetic, bioresorbable, 3D-printed barrier membranes with different porosities were prepared using the MHDS 3D printing system. When evaluating GBR in calvarial defect rabbit models, the 3D-printed barrier membranes with 30% porosity (130 μm pore size) showed the best ability to form new bone among the experimental groups in μCT and histometric findings. In addition, the maximum load of the 3D-printed barrier membrane with 30% porosity was approximately two to four times higher than the 50% and 70% groups in both dry and wet conditions. Despite the limitations of this study, these results may be a reference for determining porosity and pore size when preparing 3D-printed barrier membranes for GBR.
Acknowledgments
This research was supported by a grant from the Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Korean Ministry of Health & Welfare (grant number: HI14C3309) and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A03015562).
Conflicts of interest
The authors declare no conflict of interest.