Abstract
Wound or injury is a breakdown in the skin's protective function as well as damage to the normal tissues. Wound healing is a dynamic and complex phenomenon of replacing injured skin or body tissues. In ancient times the Calendula officinalis and Hibiscus rosa-sinensis flowers were extensively used by the tribal communities as herbal medicine for various complications including wound healing. But loading and delivery of such herbal medicines are challenging because it maintains their molecular structure against temperature, moisture, and other ambient factors. This study has fabricated xanthan gum (XG) hydrogel through a facile process and encapsulated C. officinalis and H. rosa-sinensis flower extract. The resulting hydrogel was characterized by different physical methods like x-ray diffractometer, UV–vis spectroscopy, Fourier transform infrared spectroscopy, SEM, dynamic light scattering, electronkinetic potential in colloidal systems (ZETA) potential, thermogravimetric differential thermal analysis (TGA-DTA), etc. The polyherbal extract was phytochemically screened and observed that flavonoids, alkaloids, terpenoids, tannins, saponins, anthraquinones, glycosides, amino acids, and a few percentages of reducing sugar were present in the polyherbal extract. Polyherbal extract encapsulated XG hydrogel (X@C–H) significantly enhanced the proliferation of fibroblast and keratinocyte cell lines in comparison to the bare excipient treated cells as determined by 3-(4, 5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazolium bromide assay. Also, the proliferation of these cells was confirmed by BrdU assay and enhanced expression of pAkt. In an in-vivo study, wound healing activity of BALB/c mice was carried out and we observed that X@C–H hydrogel showed significant result compared to the other groups (untreated, X, X@C, X@H). Henceforth, we conclude that this synthesized biocompatible hydrogel could emerge as a promising carrier of more than one herbal excipients.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The wound is divided into acute (large skin injuries, burn, etc) and chronic (diabetes foot ulcers) wounds, and chronic wound represents a significant health problem worldwide (Han and Ceilley 2017). Wound healing is a complex step-wise dynamic process that recovered the damaged tissue through four different phases: hemostasis, inflammation, proliferation, and remodeling (Amsden 2015, Alibolandi et al 2017). Over the past few years, different types of wound healing agents including some plant products have been found which are helpful for promoting and regeneration of wounded tissue (El-aie et al 2015). Biomaterials are the most important factors for stimulating and promoting wound healing. The damaged tissue is a favorable habitat for microorganisms like Pseudomonas aeruginosa, Klebsiella pneumonia, Acinetobacter baumannii, Staphylococcus aureus, and Enterococcus faecalis, it reduces the wound healing potential. Polymeric biomaterials can be used to protect the wounded portion from pathogens, hydrate the wound, and enhanced the wound healing activity. That is why plant extract, antibacterial agents, or extracellular components incorporated in polymeric biomaterials are induced granulation tissue formation in the wounded area (Das and Baker 2016, Murray et al 2019). Biomedical research field become an emerging and promising sector for drug delivery systems, through the development of biopolymeric materials from natural sources. Hydrogel is one type of three-dimensional hydrophilic network composed of polymeric chains, it is linked by various physical or chemical bonds like covalent bonds, hydrogen bonds, and van der Waals interactions. Recently hydrogel was used for wound dressing materials because of their excessive water or exudates absorbing capacity, facilitating gas exchange, non-adherence, maintaining a moist environment, and capability of releasing bioactive compounds (Jaiswal et al 2019). The hydrogel is an essential homopolymer or utilizes various synthetic monomers for its synthesis such as poly (acrylate acid), poly (ethylene glycol) (PEG), poly (vinyl alcohol) (PVA), etc (Hoare and Kohane 2008). Natural hydrogels have been synthesized from natural sources such as collagen, fibrin, gelatin, agarose, alginate, xanthan, pectin, guar gum, gum arabic, starch, heparin, alginate, chitin, chitosan, etc (Sharma et al 2015). Xanthan gum (XG) is a macromolecular polysaccharide; it is isolated from Xanthomonas campestris. XG is composed of a β-1, 4-glycosidic bond, and a side chain trisaccharide which contains glucuronic acid, citric acid, and mannose. XG is a biocompatible, biodegradable, cost-effective natural polysaccharide; it is widely used as a thickener, rheological modifier, stabilizer, emulsifier in food, cosmetic, pharmaceutical, textile, and oil recovery industries (Hu et al 2019). It also shows a special type of chemical structure, excellent pour ability, thickening, and rheological properties, and is highly stable to heat, acid, and alkali conditions. Hydroxyl groups and carboxyl groups are cross-linked with citric acid and generate ester bonds, forming a chemical hydrogel with a porous structure that renders its high absorbance capacity.
Different types of natural herbal products such as Aloe vera, Punica granatum Linn, Curcuma zedoaria, Azadirachta indica, etc are already tested for wound healing activity. But molecular pathways associated with a different phase of wound healing and modulation of the factors involved in the signaling by plant products are partially explored. For example, improved vascularization, granulation tissue formation, re-epithelization, and collagen deposition were observed by the extract of Ampelopsis japonica (Lee et al 2015). PI3K-dependent proliferation and migration of fibroblast were observed by the flower extracts of Calendula officinalis (Dinda et al 2015). Additionally, in in-vivo experiments, it stimulates granulation tissue formation by altering the expression of α-smooth muscle actin as well as connective tissue growth factor in excisional wounds of BALB/c mice (Dinda et al 2016). Thus, different plant products act differentially on the healing process encompassing the promotion of some specific stage. Thus, the use of a polyherbal mixture comprising more than one plant product is necessary. For example, the root of Astragalus propinquus and Rehmannia glutinosa reduce oxidative stress and induce angiogenesis in diabetic mice (Tam et al 2014). In addition to angiogenesis, improving tissue granulation, induces re-epithelialization and collagen synthesis by the herbal mixture of Alchemilla vulgaris and Mimosa tenuiflora (Choi et al 2018). The herbal extract of Vitex negundo L. (VN), Tridax procumbens L. (TP), and Emblica officinalis Gaertn. (EO) promote wound healing by proliferation and migration of fibroblast and keratinocytes at the affected sites (Talekar et al 2017). Thus, combinations derived from plant products are needed to explore further for proper management of wounds without any side effects. Moreover, the proper delivery of such agents needs to be explored scientifically.
In such an effort, we have used two herbal flower excipients C. officinalis and Hibiscus rosa-sinensis. These two are utilized medicinally either in the form of ointments, tinctures, infusions, creams, or liquid extracts. The extract compasses different types of components like polysaccharides, flavonoids, triterpene alcohols, phenol acids, tannins, glycosides, sterols, carotenoids, saponosides, etc. The wound healing activity of Calendula has been documented previously (Khairnar et al 2013). A total of 250 species of Hibiscus are widely distributed in tropical and subtropical regions of different countries and have various medicinal properties including antitumor, antihypertensive, and antioxidant properties (Purushothaman et al 2016, Shedoeva et al 2019). The leaves and flowers promote hair growth and aid in the healing of ulcers (Bhaskar and Nithya 2012). The herbal extracts contain major flavonoid constituents like rutin and quercetin which are suggested to have significant proliferative activities (Almeida et al 2015, Doersch and Newell-Rogers 2017). Additionally, for controlled and localized delivery we have synthesized herbal excipient encapsulated XG hydrogel patch and evaluated its physical as well as biological activities. We observed that the synthesized hydrogel polymer also helped with the sustained release of polyherbal excipients from the patch. We also found polyherbal excipient encapsulated biopolymeric hydrogel patch stimulate the proliferation, migration, and epithelization of human lung fibroblast (WI-38) and human keratinocyte (HaCaT) cell lines compared to free herbal excipient treated cells. In an in-vivo experiment, we topically applied X, X@C, X@H, and X@C–H hydrogel in an excision wound of BALB/c mice, and observed that X@C–H is more effective in wound healing compared to a bare excipient or single excipient loaded hydrogel. Thus this biocompatible XG hydrogel is a potential vehicle for the delivery of herbal excipients for wound healing. Studies using hydrogel for the delivery of wound healing agents are limited. One step further we have loaded polyherbal excipients for successful delivery in wounds and tested their potential in vivo experiments.
Our study highlighted the loading of the much-needed polyherbal extract on XG hydrogel, and through in vitro and in vivo studies we demonstrated the efficacy of such synthesized materials in wound healing. Reports are available about the loading of a single herbal product in hydrogel and only in vivo studies were conducted (Rubio-Elizalde et al 2019, Elegbede et al 2020, Wang et al 2020, Najafpour et al 2022, Rathod et al 2022). Additionally, the thermally stable, smaller size, and sustained release of hydrogel make it an ideal delivery agent for wound healing, particularly polyherbal excipients.
2. Materials and methods
XG (approx. MW 300 kDa), MTT (3-(4, 5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazolium bromide), phosphate buffer saline (PBS) and sodium dodecyl sulfate, were purchased from SRL, India. PEG 8000 (HI Media, India) and Absolute ethyl acetate obtained from Merck. Dulbecco's modified eagle's medium (DMEM) F12, DMEM, fetal bovine serum (FBS), and antibiotics were purchased from Thermo Fisher Scientific (Waltham, MA, USA). BrdU (5-Bromo −2ʹ-deoxyuridine) was purchased from Sigma-Aldrich, (St. Louis, MO, USA) and Vectashield mounting medium containing4ʹ6-diamidino-2-phenylindole (DAPI) was obtained from Vector Laboratories, Inc. (CA, USA). Primary antibodies pAkt Ser-473 (1:1000, rabbit polyclonal, Santacruz biotechnology, USA), GAPDH (1:2000, mouse monoclonal, cell signaling, USA), Akt (1:1000, rabbit monoclonal, cell signaling, USA). Anti-rabbit, anti-mouse HRP-linked, and mouse anti-BrdU antibodies were purchased from Abcam, UK. The acrylamide solution was purchased from Bio-Rad (California). Deionized water was used throughout the experiment with a conductivity of less than 18 mω.
2.1. Plant material collection and authentication
The C. officinalis (family Asteraceae) flowers were collected from Burdwan, India, and H. rosa-sinensis (family Malvaceae, red color flower) flowers were collected from Jadavpur university campus, Kolkata, India. Both plant materials were authenticated by the Agri horticultural society of India, Kolkata.
2.2. The extraction process of C. officinalis and H. rosa-sinensis flowers
The fresh petal of C. officinalis flowers (60 g) was collected and shade dried. The dry flowers were macerated in 50% ethanol (500 ml) for 5 d at room temperature and filtered through Whatman filter paper no 1 (Merck Millipore). The filtrate was lyophilized and stored at −20 °C (Dinda et al 2016).
H. rosa-sinensis, flowers were shade-dried at room temperature and crushed by a mechanical grinder. The 500 g of fine flower powder was suspended in 1500 ml of 32% ethanol for 24 h at room temperature. The mixture was filtered by filter paper (Whatman no: 1). The filtrate was lyophilized and stored at −20 °C of the final ethanol-free clear residue was used for the study (Shivananda et al 2007, Shen et al 2017).
2.3. Preliminary phytochemical screening
The phytochemical screening test was used to detect the phytochemicals (flavonoids, terpenoids, reducing sugar, alkaloids, tannins, saponins, anthraquinones, and amino acids) in polyherbal extract (Khan et al 2021).
2.4. Flavonoids detection test
For the flavonoid test, we used 1 ml polyherbal extract and a few drops of dilute sodium hydroxide, after mixing it formed yellowish color. After that added a few drops of dilute acid and it becomes a colorless sample indicating the existence of flavonoid components in the polyherbal extract.
2.5. Terpenoids detection test
For the terpenoid test an equal volume of chloroform and concentrated hydrogen sulfuric acid was added within the 2 ml of polyherbal extract. The formation of reddish-brown color and mentioned the presence of a terpenoids compound in the polyherbal extract.
2.6. Reducing sugar detection test
The reducing sugar was detected by Benedict's reagents. Here we used 1 ml polyherbal extract and mixed it with 2 ml Benedict's reagent, and boiled it for 3–5 min in a water bath. The brick red color sample indicated the presence of reducing sugar in the polyherbal extract.
2.7. Alkaloids detection test
For the alkaloids detection test, 2 ml of Wagner's reagent was added to 2 ml of polyherbal ethanolic extract, and the formation of brownish precipitation, confirmed the presence of alkaloids in the polyherbal extract.
2.8. Tannins detection test
The presence of tannins in the polyherbal extract was confirmed by the formation of brownish-green color with the addition of 0.1% ferric chloride to 2 ml of polyherbal extract.
2.9. Saponins detection test
For the saponins detection test, 2 ml polyherbal extract was added with the same volume of Benedict's reagent. The presence of saponins was confirmed by bluish-black color precipitation.
2.10. Anthraquinones detection test
The presence of anthraquinones in the polyherbal extract was confirmed by the 1 ml crude extract and 10% hydrogen chloride was boiled in a water bath and allowed to cool. Then an equal volume of chloroform and a few drops of 10% ammonia were added subsequently and heated. The formation of a rose-pink color showed the presence of anthraquinones.
2.11. Amino acids detection test
For the amino acid detection test, 1 ml polyherbal extract was added with a few drops of Ninhydrin reagent. The formation of purple color indicates the presence of amino acids in the polyherbal extract.
2.12. Glycosides detection test
The presence of glycosides in the polyherbal extract was confirmed by the 1 ml polyherbal extract added with 1 ml concentrated sulfuric acid. The formation of reddish color precipitation and indicates the presence of glycosides in the polyherbal extract.
2.13. Synthesis of hydrogel
2.13.1. XG hydrogel synthesis
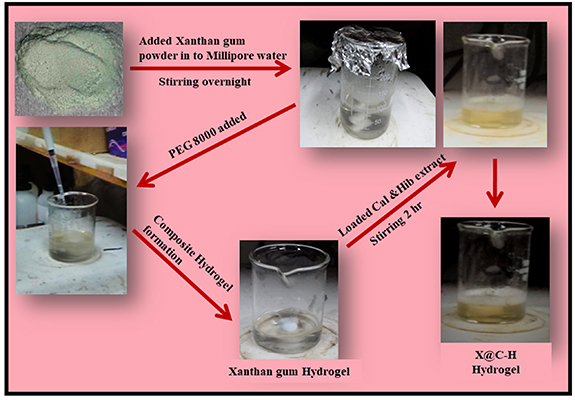
Initially, XG powder (SRL, India) (5 mg ml−1) was dissolved in millipore water under vigorous stirring for 12 h followed by the addition of the cross-linking agent PEG-8000 (Hi Media, India). The solution turned into a semi-transparent gel after the complete dissolution of the gum and was named X.
2.13.2. Herbal excipient loaded hydrogel patch formation
The drug solution was added dropwise into the dissolved polymeric solution and continued stirring for 1 h. Finally, the polyhedral extract loaded XG hydrogel (X@C–H) was collected and dried by lyophilization technique.
2.13.3. Characterization of the hydrogel patch
The x-ray diffractometer (XRD) patterns of the hydrogel patch samples were recorded by XRD model D8, Bruker AXS, Wisconsin, USA, using Cu-kα target employing wavelength of 1.5418 å and operating at 35 kV with a scan speed of 1 s/step. In the case of field emission scanning electron microscopy (FESEM) and Fourier transform infrared spectroscopy (FTIR) measurements, the dry hydrogel powder was prepared by employing the lyophilization technique. In the case of FESEM, the dry powder was then cast on carbon grids and sputtered coated by using gold plasma. The FESEM was employed for the morphological study of hydrogel patches using Inspect f50 (Fei, Netherlands). The FTIR study was done using FTIR-8400s, Shimadzu, in the wave number range from 400 cm−1 to 4000 cm−1. The samples were prepared by mixing dried hydrogel powder with KBr. In this case, the samples to KBr ratio was maintained at 1:50. The mixed powder was then pressed by using a hydraulic press system. A pure KBr pallet was also prepared for baseline correction during the measurement. The TGA and DTA study was done using the DTG-60H DTA-TGA instrument. UV–Vis (Bio-Tech) was used for measured the absorbance intensity. The average particle size distribution and diameter of hydrogel were measured by dynamic light scattering (DLS). In the case of DLS, all the samples were initially immersed in distilled water maintaining a concentration of 5 mg ml−1. Then the samples were further exposed to ultrasound for 30 min. Such vigorous ultrasound readily prepared a hydrogel solution of the respective sample. The charges of the hydrogel were also measured by the Zetasizer (NanoZS90, Malvern instruments Ltd, UK).
2.13.4. Swelling studies
The swelling characteristics of XG hydrogel (5 mg ml−1) were measured in a buffer solution of various pH (pH 5.4 and 7.4) at 37 °C using a standard protocol (Seeli and Prabaharan 2017). The dry hydrogel (S0) was weight precisely and submerged in a petri dish containing buffer solutions and incubated for 24 h. The weight of the swollen hydrogel (St ) at different times was measured after removing excess water with tissue paper. The swelling ratio of the hydrogel at time t was determined using the following formula,
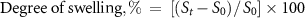
where St and S0 are the weights of the hydrogel at time t and 0 h respectively.
2.13.5. Drug release study
In vitro study of drug release, was performed from a polyherbal encapsulated XG hydrogel patch at four different pH (7, 7.5, 8, and 8.5) for a period of 10 h. The percentage of encapsulated excipient of the hydrogel patch was calculated by absorbance maxima of herbal excipient solution at 540 nm using a spectrophotometer (epoch microplate spectrophotometer, USA). The DLC (drug loading capacity) and DEE (drug encapsulation efficiency) were calculated by the following equations (Alle et al 2020),

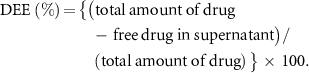
2.13.6. Fibroblast cell culture
Human lung fibroblast cell lines WI-38 and human keratinocyte cell line HaCaT were cultured in DMEM and DMEM F12 medium (Thermo Fisher Scientific (Waltham, MA, USA)) respectively with 5% FBS (Thermo Fisher Scientific), penicillin/streptomycin (100 units/ml), amphotericin-B (anti-fungal) and incubated at 37 °C, and 5% CO2 (Heraeus, Thermo Scientific, MA, USA).
2.13.7. Cell proliferation assay
Fibroblast cells WI-38 and HaCaT were seeded at a density of 2.5 × 104 cells per well in 24 well plates and exposed to XG hydrogel loaded with excipients at different concentrations (0–400 μg ml−1) for 24 h. The MTT assay method was performed using a standard protocol (Al Masum et al 2020).
2.13.8. Immunocytochemistry and BrdU incorporation assay
WI-38 and HaCaT cells were grown in respective mediums with 10% FBS in a coverslip placed in a 35 mm culture plate. The cells were treated with 200 μg ml−1 drug for WI-38 and 400 μg ml−1 for the HaCaT cell line and incubated for 24 h, and then 10 μm BrdU was added and incubated for 1 h for immunocytochemical analysis. Coverslips were washed in PBS, fixation with 4% paraformaldehyde for 30 min and permeabilized by 0.2% Triton X-100 at 4 °C for 10 min. The cells were treated with 2 N HCl for 30 min at room temperature. The 5% FBS was used for blocking and stayed for 1 h at room temperature. The cells were incubated with mouse monoclonal Anti-BrdU antibody (BD 44) at 1:100 dilutions in wash buffer and incubated at 4 °C for overnight. After washing, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (SC-2078) at 1:200 dilutions at room temperature in dark conditions for 1 h. The slides were then washed and mounted with a mounting medium containing DAPI. The cells were examined under a fluorescence microscope (Leica, Wetzlar, Germany). Each analysis was performed at least three times (Dinda et al 2015).
2.13.9. Scratch assay
The lung fibroblast cell line (WI-38) was seeded in a 35 mm plate and incubated for 24 h at 37 °C. Then, cells were treated with 200 μg ml−1 of the drug (X, X@C, X@H, X@C–H) and incubated for 24 h at 37 °C with 5% CO2. The same field of the wounded zone was photographed after 24 h time points. Scratch assay was done as per the method reported earlier (Dinda et al 2016).
2.13.10. Western blot analysis
After incubation with drugs, cell lysate was prepared followed by western blot analysis using a standard protocol (Dinda et al 2015). Here we have used primary antibody Akt (1:1000, rabbit monoclonal, Cell signaling, USA), pAkt (Ser 473) (1:1000, rabbit polyclonal, Santacruz biotechnology, USA), and GAPDH (1:2000, mouse monoclonal, Cell signaling, USA). GAPDH was used as the loading control.
2.13.11. Animal experiments
We used about 25–30 g male BALB/c mice with a minimum of 6–7 weeks old, for the experiments. The mice were fed a restricted vitamin-rich pellet diet and libitum-added water. Animals were maintained according to the guidelines of the National Institute of Nutrition, Hyderabad, India.
2.13.12. Experimental design of in-vivo experiments
The mice were divided into five groups each containing five animals Group 1: excision wound created mice (untreated), Group 2: excision wound created and applied with X hydrogel patch, Group 3: excision wound created and applied with X@C hydrogel patch, Group 4: excision wound created and applied with X@H hydrogel patch, Group 5: excision wound created and applied with X@C–H hydrogel patch.
All the mice of groups 2, 3, 4, and 5 were applied with the same dose rate for 12 d at an interval of 3 d. The dorsal hair of mice was cleaned and shaved with 70% ethanol. One full-thickness excisional wound on the side of the dorsal midline was created by a 5 mm diameter of a punch biopsy. The image of the wound region was taken on 1, 3, 6, 9, and 12 d.
2.13.13. Hematoxylin and eosin staining
Hematoxylin dye is used for staining basophilic structures, nucleic acid-containing molecules like chromatin, ribosomes, and cytoplasmic areas rich in ribonucleic acid (RNA), which are given a blue-purple contrast. Basic components such as muscle, red blood cell (RBC)s, collagen, and cytoplasm are counterstained by acidic eosin in different shades of red, pink, and orange color. All tissues were sectioned into 4 mm sizes and paraffinized. The sections were washed three times with xylene for deparaffinized and rehydrated with ethanol. The section was followed by staining with hematoxylin for 5 min and washed with tap water. Next, the slides dip in 0.5% acid alcohol and are washed three times in distilled water. The blueing agent was used for 1 min and the slides were washed three times in distilled water. The counterstain alcoholic eosin was used for 1 min (Dinda et al 2016). The slides were then dehydrated through a graded ethanol series, cleared in xylene, and mounted with coverslips. The hematoxylin and eosin-stained slides were observed by a bright-field optical microscope (Leica dm 2500, Germany).
3. Results
3.1. Phytochemical screening
The phytochemical screening was used for the observation of chemical constituents of polyherbal flower extract (C. officinalis and H. rosa-sinensis). These chemical constituents play an important role in wound healing. Table 1 summarized the result of the phytochemical screening test. The flavonoids, alkaloids, terpenoids, tannins, saponins, anthraquinones, glycosides, and amino acids are present in the polyherbal extract (supplementary figure S4). In the reducing sugar experimental test, we observed that the polyherbal extract formed yellowish-red color but the Benedict reagent test mentioned that reducing sugar formed a brick-red color. Here we have used dextrose sugar as a positive control of reducing sugar test and found that it formed a brick-red color. That is why this result indicates, the reducing sugar percentage is low of our synthesized polyherbal extract. (supplementary figures S4(B) and (C)). This polyherbal extract can be utilized for wound healing activity because it is made up of a medicinal compound and is highly therapeutic which plays a crucial role against wound infection and induce wound tissue regeneration.
Table 1. Phytochemical screening of polyherbal ethanolic extract.
| Phytochemicals | Result |
|---|---|
| Flavonoids | + |
| Alkaloids | + |
| Reducing sugars | + (few %) |
| Terpenoids | + |
| Tannins | + |
| Saponins | + |
| Anthraquinones | + |
| Amino acids | + |
| Glycosides | + |
3.2. Synthesized biopolymeric hydrogel patch
The synthesis process of the hydrogel patch is very facile, it is shown in figure 1.
Figure 1. Schematic representation of xanthan gum hydrogel and herbal extract encapsulated hydrogel preparation process.
Download figure:
Standard image High-resolution image3.3. Physical characteristics of the synthesized hydrogel samples
The XRD patterns of pure XG powder did not find diffraction maxima but cross-linking agent PEG 8000 was showing diffraction maxima of 19.1° and 23.2° (supplementary figure S2(b)). The samples X, X@C, and X@H was showing diffraction peaks at 19.1° and 23.2° whereas, diffraction peaks centered at 19.1°, 23.2° and 31° have been found in the case of the doped hydrogel sample (figure 2(a)). The absence of any sharp diffraction line in XRD indicates an amorphous pattern of the pure XG powder (Mohsin et al 2018) and PEG 8000 shows two sharp peaks at 19° and 23.33°, it is semi-crystalline type (Özdemir Dinç and Güner 2017). Therefore, X also carries two diffraction lines at 19° and 23.33° which is similar to PEG 8000 so it is formed a hydrogel. The herbal excipient doped samples X@C, X@H, and X@C–H show diffraction lines at 19.1° and 23.2° revealing the semi-crystalline nature (Sung et al 2010).
Figure 2. Physical characterization of xanthan gum hydrogel patch, (a) XRD of X, X@C, X@H, and X@C–H; (b) FTIR spectroscopy of X, X@C, and X@H and X@C–H; (c) UV–VIS spectroscopy of X, X@C, X@H, C@H, and X@C–H; (d) and (e) FESEM images of X and X@C–H respectively; (f) DLS of X, X@C, X@H, X@C–H; (g) water absorbance capacity of hydrogel measured by swelling ratio study at variant pH; (h) TGA, and (i) DTA of X, X@C, X@H, X@C–H.
Download figure:
Standard image High-resolution imageIn FTIR study is shown in figure 2(b) and supplementary figure S1(a). The XG is added to the PEG 8000 solution, and it is formed X hydrogel. The FTIR spectrum of the hydrogel shows special characteristics, that confirms the interaction between the two polymers and forms hydrogel. The 3600–3000 cm−1 region mentioned –OH group stretching vibration which is the first featured. In the case of PEG, the –OH group signal appears at 3478 cm−1, XG at 3256 cm−1, and in X at 3312 cm−1. As the content of X in the gels increases, the 3256–3478 cm−1 ranges of peak detecting the –OH group of stretching vibration, indicating the interaction between XG and PEG molecular chains. The second is featured in the region of 2500–3000 cm−1 which is the C–H group stretching vibration. The C–H group signal appears in PEG at 2885 cm−1, XG at 2913 cm−1, and X at 2893 cm−1. The hydrogel signals indicate a higher concentration of XG. A characteristic absorption signal occurs for pure PEG at 537, 862, 1116 and 1476 cm−1 for C–O–C, CH2, C–O, and CH2 respectively (Ofokansi et al 2016) while for pure XG at 1017, 1394 and 1644 cm−1 for COO–, COO– and C2H3O stretching respectively (Faria et al 2011, Zhang et al 2019). In the case of the bare hydrogel are shown other absorbance peaks at 1110 cm−1 (C–O stretching), 1070 cm−1 (C–O stretching), and 1608 cm−1 (C=C stretching), these signals are indicating its purity (figure 2(b)). The bands corresponding to the doped hydrogel sample are at 1043, 1533, and 3010 cm−1 depicting the proper incorporation of the poly-herbal excipients (figure 2(b)). Additionally, in FTIR the 1043 cm−1 peak corresponds to the polysaccharide C–O valence vibration (Mak et al 2013), and the other two major vibrations at 1533 and 3010 cm−1 are due to C–OH and –OH vibrations respectively (Al-Mussawi and Al-Hussani 2019). This –OH vibration possibly comes from the absorbed water of the hydrogel sample. Poly-herbal excipients doped hydrogel shows two extra absorbance bands centered around 1643 and 2130 cm−1 depicting the formation of C–OH and C–H bonds respectively (Mak et al 2013, Azizi et al 2018).
The absorbance of the hydrogel samples was measured after the complete dissolution of the mixture in water. The absorbance spectra were recorded in a UV–Vis spectrophotometer. Figure 2(c) does not show any prominent absorption maximum for the undoped hydrogel sample, whereas, the poly-herbal extract doped hydrogel offers a broad absorption maximum centered at around 352 nm, and it corresponds to n–π* transition of the herbal excipients indicating successful doping. This relates to the proper incorporation of the dopants as seen in XRD and FTIR data.
In FESEM, herbal excipient encapsulated hydrogel showed a more exfoliated structure of the gel sample than the bare vehicle (figures 2(d) and (e)). The doped hydrogel also shows a porous structure and less agglomeration. Our synthesized doped sample shows exfoliated microstructure suggesting increased porosity of the sample and reducing the agglomeration and thus providing better attachment probability to the substrate like in contact with a wound. The hydrodynamic diameter of pure hydrogel is 20.144 d.nm as measured by DLS whereas the polyherbal extract conjugated hydrogel (X@C–H) corresponding value is 1596 d.nm with poly-dispersity index (PDI) 0.64 (figure 2(f)). Additionally, the surface charge of the pure XG hydrogel is 0.136 mv, which is positive and pretty low to be stable enough (supplementary figure S3). Loading of poly-herbal excipient makes this hydrogel negative (−27.7 mV) and is quite stable (supplementary figure S3). It is quite interesting to observe the alteration of microstructure after the doping of poly-herbal excipients into the hydrogel patch. Our synthesized doped sample shows exfoliated microstructure suggesting increased porosity of the sample and reducing the agglomeration and thus providing better attachment probability to the substrate like in contact with a wound. The doped sample depicts an enhanced negative surface charge with a lower PDI (0.64) indicating the extract-encapsulated hydrogel forms a homogenous solution, which is suitable for any biological application. In reality, negative surfaces of the poly-herbal excipient strongly attract the hydrogel towards their surface, which further exfoliates the structure of the doped hydrogel and opens the clustered surface for better binding with the substrate.
3.4. The absorption capacity of hydrogel patch measured by the swelling study
The drug release capacity of hydrogel was evaluated by its swelling properties. After 24 h it has been found that the weight of bare XG was increased (figure 2(g)) and swollen hydrogel patch maintains its shape better at pH 7.5 than pH 5.4. The high drug loading capacity is due to the large surface area of the hydrogel patch.
3.5. Stability assay of the hydrogel patch samples
The thermal stability has also been measured using the DTA-TGA technique showing the stability of the hydrogel patch up to 200 °C. The thermal stability of the pure hydrogel patch has been tested (figures 2(h) and (i)) to validate their storage and uses. The temperature-dependent mass loss and differential thermograms were analyzed by the DTA-TGA instrument maintaining a heating rate of 10 °C min−1 in nitrogen atmosphere (flow rate 50 cc min−1). It is observed that the hydrogel undergoes dehydration at 60 °C–70 °C but no mass loss has been observed in this region. Apart from this, another major thermal transition occurs at 190 °C indicating high stability of the hydrogel sample. In reality, such hydrogel patches have a very fragile structure and need proper ambient conditions to sustain. TGA measurement enables us to find the softening temperature and phase formation temperature, which not only signifies its stability but also aids in designing its storage conditions.
The synthesized sample was exposed to various pH ranges (5.3, 7.5, and 9.6) in order to check the structural and chemical stability. It is found that there is no alteration in their absorbance spectra at different pH (supplementary figure S2(b)) and no change in absorbance spectra of the samples with time was observed (supplementary figure S2(a)). Simultaneously dissolving synthesized samples in different buffer solutions (H2O, PBS, and TBS) does not influence their stability (supplementary figure S2(c)). Additionally, pH and time-dependent stabilities of the sample have been found, which reveals the application potential of the hydrogel for biological purposes.
3.6. Herbal excipient encapsulated hydrogel patch showed pH-responsive drug release
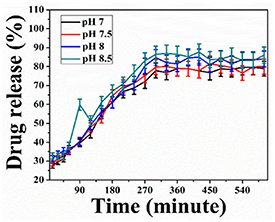
The release profile of herbal excipient (drug) encapsulated hydrogel patch at different pH was estimated and shown in figure 3. The DLC and DEE percentages of the XG hydrogel patch were found to be 71% and 85%, respectively. Approximately 55%, 58%, 70%, and 80% of the drug is released from X@C–H hydrogel at pH 7, 7.5, and pH 8, 8.5 (wounded environment) respectively, as shown in figure 3. However, the present outcomes confirmed that the pH-dependent release is very much more sensitive to in alkaline pH of 8 or 8.5 than the normal pH of 7 or 7.5.
Figure 3. Drug release studies at pH 7, 7.5, 8, and 8.5 of X@C–H hydrogel.
Download figure:
Standard image High-resolution image3.7. Synthesized hydrogel promotes cell proliferation
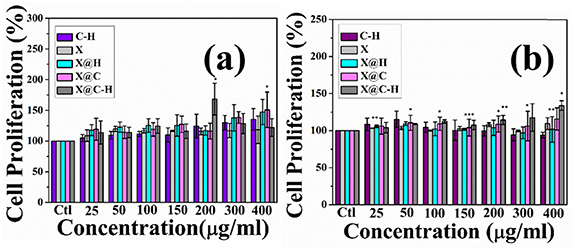
MTT assay was performed to explore the proliferation of WI-38 and HaCaT cell lines. The cells were seeded in 24 well plates and treated with our synthesized C–H, X, X@C, X@H, and X@C–H at different concentrations (0–400 μg ml−1) for 24 h. The maximum proliferation of WI-38 and HaCaT cells at 200 μg ml−1 and 400 μg ml−1 respectively and are shown in figures 4(a) and (b). We have found bare hydrogel (X) has no cytotoxic effects and X@C–H exhibited significant proliferation in a dose-dependent manner.
Figure 4. Assessment of cell cytotoxicity and cell proliferation of C@H, X, X@C, X@H on human fibroblast and keratinocyte cell lines (a) WI-38 and (b) HaCaT cells by MTT assays which shows cells number were increasing in a dose-dependent manner. The MTT results were expressed as mean ±SD of three independent experiments and where * (P < 0.05), ** (P < 0.01), and *** (P < 0.001).
Download figure:
Standard image High-resolution image3.8. Proliferative effect assessment of the excipient payloaded hydrogel by BrdU assay
We further studied the proliferation effect of polyherbal encapsulated hydrogel patch on WI-38 and HaCaT cells by counting BrdU-positive cells. After labeling with BrdU, cells were observed under a fluorescence microscope and counted for BrdU-positive cells. As shown in figure 5, X@C–H has more BrdU-positive cells (150% of cells are BrdU positive for both the cell lines) compared to X@C and X@H treated cells. Thus, we found more BrdU-positive cells in the fibroblast cell line compared to the keratinocyte cell after the treatment of X@C–H hydrogel. In addition, this polyherbal hydrogel patch significantly promoted the migration of the lung fibroblast cell line.
Figure 5. Effect of X, X@C, X@H, and X@C–H treated cell proliferation were determined by BrdU staining assay treatment with/without extract in WI-38 and HaCaT cell lines for 24 h and labeled with 10 μM BrdU before immunocytochemical analysis. The representative images of BrdU staining were shown in figures (a) and (b) respectively. The quantitative estimation of BrdU positive WI-38 and HaCaT cells was shown in the respective figures (c) and (d), where * (P < 0.05) and ** (P < 0.01).
Download figure:
Standard image High-resolution image3.9. Cell migration estimated by scratch assay
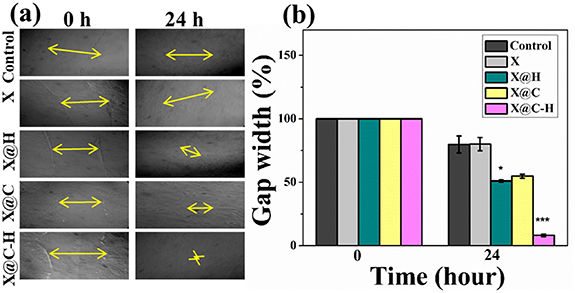
To assess the in vitro wound healing effect of X, X@C, X@H, and X@C–H hydrogel on WI-38 cell lines, the migration pattern was observed around the closure of a marked area scratched in confluent monolayer cells. The measure of gap width at the initial time (0 h) and final time (24 h) was calculated and plotted in figures 6(a) and (b). This result suggested an increased migrating ability of fibroblasts in response to the polyherbal extract encapsulated hydrogel patch treatment.
Figure 6. (a) The scratch area was formed in WI-38 monolayer cells and images have taken by a phase contrast microscope (10×) at 0 h and 24 h; (b) X, X@C, X@H, and X@C–H treated cell migration was measured using ImageJ Software and % gap width is calculated with different time point, where * (P < 0.05) and *** (P < 0.001).
Download figure:
Standard image High-resolution image3.10. Expression of the proliferation responsive protein analysis by Western blotting
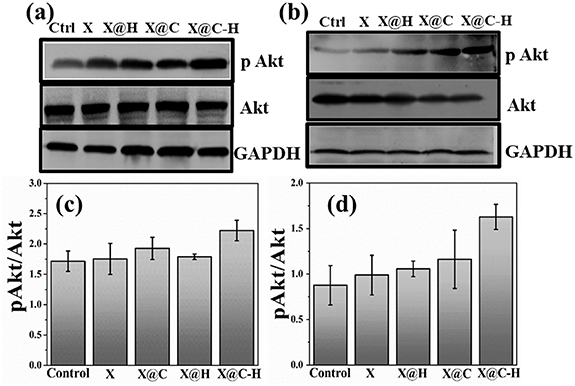
We next explored the expression of Akt and pAkt protein in X, X@C, X@H and X@C–H treated human lung fibroblast cell line WI-38 and keratinocyte cell line HaCaT. We have observed that, in both the cell lines, expression of pAkt enhanced in the presence of X@C–H than X, X@C or X@H treated cells (figure 7). Here also observed phosphorylation of Akt, when cells were treated with a hydrogel containing herbal excipients, indicating efficient release of excipients.
Figure 7. Western blot analysis of Akt (MW 60 kDa) and pAkt (MW 60 kDa) protein in response to X; X@H; X@C and X@C–H. This suggests the upregulation of pAkt protein expression upon delivery of polyherbal excipient encapsulated hydrogel patch in fibroblast and keratinocyte cell lines shown in (a) for WI-38 and (b) for HaCaT; (c) WI-38 and (d) HaCaT represent the band intensity of the proteins and was quantified using ImageJ software.
Download figure:
Standard image High-resolution image3.11. In vivo study
3.11.1. Excision wound created in male BALB/c mice model and measurement of wound size
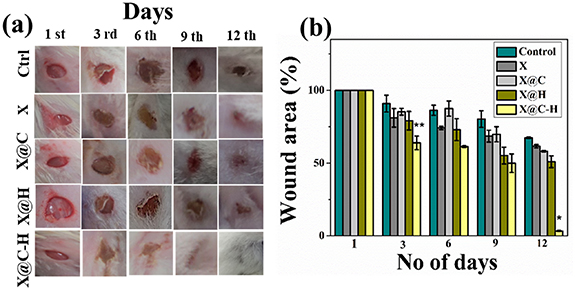
We found that X@C–H treated mice also healed within 9 d compared to other treated X, X@C, and X@H mice (figure 8(a)). After 12 d, the wounded area was measured following this process, wound sizes at any given time point after wounding were expressed as a percentage of the initial (day 1) wound area and the rate of wound closure was calculated using ImageJ software (NIH, USA). The rate of wound healing was shown in figure 8(b), on day 12 post-wounding the X@C–H treated groups showed complete wound closure compared to other groups of mice. The results indicated that the application of bare hydrogel does not induce any toxic effect on the mice neither it promotes the re-epithelialization of the epidermis. However, treatment with X@C–H hydrogel led to extensive re-epithelialization within 9 d and hair follicle appears on dermal sheath cells. Also, there was no sign of scars and hairlessness compared to the others groups.
Figure 8. Pictorial representation of in vivo experiments (a) excisional wounds of untreated and treated mice were monitored on 1, 3, 6, 9, and 12 d and the corresponding images were taken with a digital camera (Sony Cyber-shot DSC-WX80); (b) the wound areas were calculated using ImageJ software and were expressed as % of initial wound area against a number of days post wounding. Results were expressed as mean ± SD, where * (P < 0.05) and ** (P < 0.01) in comparison to control.
Download figure:
Standard image High-resolution image3.11.2. Histological study
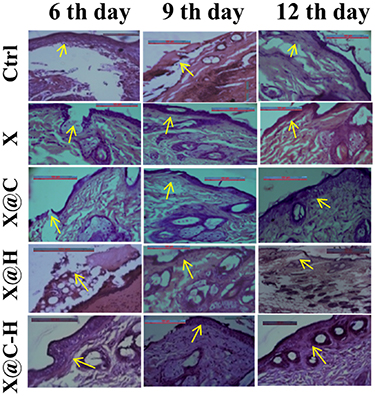
A histological study purposes eosin, as well as hematoxylin, was used to envisage the alterations in tissue morphology with the progression of wound healing upon application of various forms of hydrogel like X, X@C, X@H, X@C–H for different time intervals. The slides were observed under a bright-field optical microscope and found the fibroblast numbers are slightly increased in the dermis near a wounded portion with the genesis of new blood vessels (neoangiogenesis). While in control, the wound site was filled with necrotic debris and fibrin. Within 6 d, in the case of X@C–H applied animals, the granulation tissue had developed fully and the regeneration of the epidermis took place. On the other hand, it took 12 d for the formation of granulation tissue after the application of X@C and X@H hydrogels. While in control and bare hydrogel applied animals slowly developed the granulation layer. On day 12, the re-epithelialization of the wounded portion was completed in X@C–H applied animals compared to control, X, X@C, and X@H where re-epithelialization was not completed (figure 9). Hence, we can conclude that the X@C–H hydrogel showed more pronounced wound healing activities.
Figure 9. Histological analysis of wounded areas tissue from mice at different post-wounding days (6, 9, and 12) in treated, untreated animals. Original magnification 40X, scale bar-100 μm.
Download figure:
Standard image High-resolution image4. Discussion
Wound healing is a very slow, complex, and overlapping dynamic process that repairs the injured tissue through hemostasis, inflammation, proliferation, and remodeling phases (Amsden 2015, Alibolandi et al 2017).
In recent times different types of wound care products have been suggested that are more effective in promoting and regeneration of wounded tissue (Mohsin et al 2018). Among them, hydrogels are beneficial for control release of therapeutic agents of different types (Özdemir Dinç and Güner 2017). Here we have synthesized a biocompatible hydrogel patch from the abundantly available XG which acts as a promising vehicle for the delivery of polyherbal excipients using a facile method. For herbal excipients, we have used C. officinalis flower which was known to treat burn and ulcer wounds, and H. rosa-sinensis flower, known for wound healing and antibacterial property (Talekar et al 2017). Our prepared polyherbal ethanolic flower extract showed the presence of phytochemical constituents through the phytochemical screening process. The flavonoids, alkaloids, terpenoids, tannins, saponins, anthraquinones, glycosides, and amino acids are present in the polyherbal extract (supplementary figure S4). The reducing sugar screening result showed yellowish red color, indicating the reducing sugar percentage of the polyherbal extract is low. The result of reducing sugar of polyherbal extract is compared to a known reducing agent like dextrose (supplementary figure S4). This polyherbal extract can be used for wound healing activity because it contains highly therapeutic and medicinal compounds which play a crucial role against wound infection and promote wound tissue regeneration. This poly-herbal plant extracts along with their encapsulation in a cheap hydrogel and is not reported previously.
The absence of any sharp diffraction line in XRD indicates an amorphous pattern of the pure XG powder (Mohsin et al 2018) and PEG 8000 shows two sharp peaks at 19° and 23.33°, it is semi-crystalline type. Therefore, X also carries two diffraction lines at 19° and 23.33° which is similar to PEG 8000 so it is formed a hydrogel. The herbal excipient doped samples X@C, X@H, and X@C–H show diffraction lines at 19.1° and 23.2° revealing the semi-crystalline nature. Additionally, in FTIR the 1043 cm−1 peak corresponds to the polysaccharide C–O valence vibration, and the other two major vibrations at 1533 and 3010 cm−1 are due to C–OH and –OH vibrations respectively. This –OH vibration possibly comes from the absorbed water of the hydrogel sample. Poly-herbal excipients doped hydrogel shows two extra absorbance bands centered around 1643 and 2130 cm−1 depicting the formation of C–OH and C–H bonds respectively. Moreover, the appearance of an absorption band in the UV–Vis spectrum around 352 nm is due to the incorporation of the poly-herbal excipients and it corresponds to n–π* transition of the herbal excipients indicating successful doping. It is quite interesting to observe the alteration of microstructure after the doping of poly-herbal excipients into the hydrogel patch. The size values of pure hydrogel and doped hydrogel are suitable for hydrogel structure. Zang et al synthesized another type of hydrogel by using PVA and XG. Our synthesized doped sample shows exfoliated microstructure suggesting increased porosity of the sample and reducing the agglomeration and thus providing better attachment probability to the substrate like in contact with a wound. The doped sample depicts an enhanced negative surface charge with a lower PDI (0.64) indicating the extract encapsulated hydrogel forms a homogenous solution, which is suitable for any biological application. Additionally, pH and time dependent stabilities of the sample have been found, which reveals the application potential of the hydrogel for biological purposes. The thermal stability has also been measured using the DTA-TGA technique showing the stability of the hydrogel patch up to 190 °C. Such high temperature stability would definitely make this sample usable for biomedical applications.
In reality, negative surfaces of the poly-herbal excipient strongly attract the hydrogel towards their surface, which further exfoliates the structure of the doped hydrogel and opens the clustered surface for better binding with the substrate. The swelling ratio study is the most important property for the determination of its drug release capacity. The high drug loading capacity is due to the large surface area of the hydrogel patch.
We have found bare hydrogel (X) has no cytotoxic effects and X@C–H exhibited significant proliferation in a dose-dependent manner. The result could be due to the presence of major flavonoid constituents like rutin, quercetin, and gallic acid in Calendula and Hibiscus flower extract (Dinda et al 2016, Purushothaman et al 2016) which are reported to be significant proliferative activities on fibroblast cells. Thus, we found more BrdU-positive cells in the fibroblast cell line compared to keratinocyte cells after the treatment of X@C–H hydrogel. In addition, this polyherbal hydrogel patch significantly promoted the migration of the lung fibroblast cell line. Phosphorylation of Akt was observed when cells were treated with C. officinalis extract in our previous study (Shivananda Nayak et al 2007, Dinda et al 2016), here also observed phosphorylation of Akt when cells were treated with polyherbal extract encapsulated hydrogel and indicating efficient release of excipients. The in vivo results indicated that the application of bare hydrogel does not induce any toxic effect on the mice neither it promotes the re-epithelialization of the epidermis. However, treatment with X@C–H hydrogel led to extensive re-epithelialization within 9 d and hair follicle appears on dermal sheath cells. Also, there was no sign of scars and hairlessness compared to the others groups. It has been reported that ethanol extract from calendula and hibiscus flower attenuates inflammation, enhances fibroblast proliferation, and collagen deposition, and upregulates vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β1 expression in excisional wounds (Talekar et al 2017). However, we have developed a hydrogel-loaded polyherbal excipient and successfully deliver it to wounds for promoting healing.
5. Conclusion
This study hypothesized that the synthesized cost-effective biocompatible XG hydrogel has the potential to emerge as an ideal carrier for polyherbal excipients for the purpose of different types of wound amelioration in the near future.
Acknowledgments
The authors would like to thank the University Grants Commission (UGC), Govt. of India for the Junior Research fellowship [UGC Ref. No.: 735/ (CSIR-UGC NET JUNE 2017)] and the technical help from the CRNN, University of Calcutta, India.
Data availability statement
The data cannot be made publicly available upon publication because they are not available in a format that is sufficiently accessible or reusable by other researchers. The data that support the findings of this study are available upon reasonable request from the authors. Data will be available from 27 July 2022.
Funding
This work was supported by the DST Govt. of West Bengal (BT/ST/P/S&T/2G-13/2017), DBT Govt. of India (BT/PR25380/NER/95/1168/2017) and Rashtriya Uchchatar Shiksha Abhiyan (RUSA) 2.0 (Ref. No. R-11/400/2019, Date: 19 April 2019).
Ethics declarations
All applicable guidelines for the care and use of animals were followed. The experimental protocols were approved by the Bose Institute, P 1/12, CIT scheme VIIM, Kolkata-700 054, India animal ethics committee.
Conflict of interest
The authors have no conflict of interest to declare.