Abstract
Three-dimensional (3D) bioprinting is a disruptive technology for creating organotypic constructs for high-throughput screening and regenerative medicine. One major challenge is the lack of suitable bioinks. Short synthetic self-assembling peptides are ideal candidates. Several classes of peptides self-assemble into nanofibrous hydrogels resembling the native extracellular matrix. This is a conducive microenvironment for maintaining cell survival and physiological function. Many peptides also demonstrate stimuli-responsive gelation and tuneable mechanical properties, which facilitates extrusion before dispensing and maintains the shape fidelity of the printed construct in aqueous media. The inherent biocompatibility and biodegradability bodes well for in vivo applications as implantable tissues and drug delivery matrices, while their short length and ease of functionalization facilitates synthesis and customization. By applying self-assembling peptide inks to bioprinting, the dynamic complexity of biological tissue can be recreated, thereby advancing current biomedical applications of peptide hydrogel scaffolds.
Export citation and abstract BibTeX RIS
Three-dimensional (3D) bioprinting is a disruptive technology for creating organotypic constructs for high-throughput screening and regenerative medicine [5, 6]. The ability to rapidly, precisely and reproducibly deposit cells and bioactive moieties in a small area enables the design of complex 3D constructs comprising multiple cell types and microenvironments. The bioprinted constructs better mimic native tissues in the body, and are thus believed to better recapitulate in vivo physiology for drug screening and disease modelling [1–3]. Existing cell culture systems commonly used for cell studies and drug testing are typically two-dimensional (2D) monocultures consisting of only one cell type or co-cultures with simple bi-layer or micropatterned layouts. Due to altered cell–cell and cell–substrate interactions, 2D cultures often induce changes in the morphology and gene expression of primary and stem cells. Consequently, tissue-specific responses cannot be accurately predicted. Moreover, larger constructs can be printed as biofunctional organ transplants for replacing diseased or damaged tissue in patients, thereby relieving the shortage of donor organs.
Despite its promise, the development of bioprinting technologies is constrained by the lack of suitable bioinks to serve as the structural scaffold to maintain the shape of the printed construct, localise the cells, and provide a microenvironment that is conducive to maintaining cell survival and physiological function. Recent advancements in bioprinting modalities have focused on developing sophisticated instrumentation (such as cellular inkjet and extrusion-based bioprinters) and optimising techniques (such as lithography) for printing live cells and biomaterial scaffolds with high resolution and reproducibility [4]. However, many bioinks that can be deposited accurately without bleeding have high viscosities which are difficult to dispense accurately [7]. To circumvent this problem, stimuli-responsive [8, 9] or shear-thinning [10] materials which form hydrogels can serve as candidate matrices. An alternative strategy is the co-deposition of crosslinking or coacervation agents [11]. Instantaneous solidification of the bioink (and its payload of cells and biomolecules) will preserve the desired spatial localisation of cells and bioactive moieties. As such, another popular strategy is the utilisation of building blocks that require UV or photo cross-linking. However, care must be taken to avoid chemical crosslinkers and reaction side products (such as free radicals) which are cytotoxic and mutagenic. Biocompatibility of candidate bioinks is integral to the long term culture of cells. If the construct is slated as an engineered tissue for implantation, additional care must be taken to ensure that the bioinks used are non-immunogenic, non-mutagenic and non-hemolytic, to prevent adverse physiological side effects. Moreover, chemically well-defined bioinks which can be further modified are particularly prized as spatial and temporal cues to govern cell behaviour and cell fate can be grafted onto specific locations of the 3D scaffold during the bioprinting process.
Synthetic self-assembling peptides are ideal candidates as inks for bioprinting and biofabrication. Such biomaterials have been applied as in vitro cell culture substrates, implantable scaffolds, regenerative therapies and drug delivery matrices [12, 13]. Their use as bioinks, however, is unexplored. Short peptides which self-assemble into nanofibrous hydrogels in physiologically relevant conditions are of particular interest as bioinks. The 3D network of nanofibres resembles the native ECM which provides a hospitable microenvironment that promotes the survival of pluripotent stem cells and maintains the native cellular physiology of differentiated primary cells. As the networks entrap water during the self-assembly process, these hydrogels will prevent cellular dehydration during the printing process. There are several different classes of short peptides which form nanofibrous hydrogels, as defined by their secondary structure (figure 1). Early work on β-sheet peptides utilised motifs derived from Mother Nature. Well-known examples include RADA16 (whose precursor AEAK16-II was discovered in the Zuotin yeast protein) [14], silk-like and elastin-like polypeptides [15]. Periodic repeats of hydrophilic and hydrophobic amino acids induce peptide folding into β-sheet secondary structures which subsequently stack (via intermolecular hydrophobic interactions and electrostatic interactions/hydrogen bonding) to form nanofibres. Since then, scientists have moved towards designing de novo β-sheet motifs such as ABA multi-domain short peptides [16] and β-sheet nanotapes [17]. Another class of synthetic designed self-assembling peptides utilises β-hairpin (two β-strands linked by a kink) structures [18, 19] which pack in an orderly fashion along their hydrophobic faces to form bilayers. These bilayers further aggregate to form cross-linked fibrils. The coiled-coil motif, as exemplified by α-helical peptides, can also serve as a building block for self-assembly [20]. Collagen-mimetic peptides also fall under this category since collagen is α-helical by nature [21–23]. More recently, ultrashort amphiphilic peptides which form β-fibrils via α-helical intermediates have been demonstrated to self-assemble into nanofibrous hydrogels [24, 25].
Figure 1. Short self-assembling peptides that form nanofibrous hydrogel scaffolds. (a) β-sheet-forming short peptides typically consist of periodic repeats of ionic hydrophobic and hydrophilic amino acids, as exemplified by RADA16. This motif induces peptide folding into β-sheet secondary structures with hydrophobic and hydrophilic surfaces. During self-assembly of RADA16, the macromolecular fibers are stabilized by overlapping hydrophobic interactions between the alanine residues (A) and intermolecular ionic interactions between arginine (R) and aspartic acid (D) residues (Reproduced with permission from [12]. Copyright 2012 Elsevier.). For ABA multi-domain peptides, the B-domain forms β-sheet secondary structures while the flanking A-domain contains charged peptides which favour intermolecular repulsion. When pH changes neutralise the flanking lysine (K) residues, hydrophobic interactions in the B-domain predominate, leading to peptide self-assembly (Reproduced with permission from [16]. Copyright 2009 American Chemical Society.). Fibrillising peptides adopt a β-sheet nanotape conformation in the presence of specific stimuli (pH and salt changes). Pairs of nanotapes stack to form ribbons due to π–π interactions between aromatic (Q and F) residues. Ribbons subsequently aggregate to form fibrils and fibers (Reproduced with permission from [17, 28]. Copyright 2003 American Chemical Society.). (b) Peptides with the β-hairpin motif adopt the conformation in the presence of specific stimuli (such as light, pH, ionic and temperature changes) (Reproduced with permission from [49]. Copyright 2004 Elsevier.). The β-hairpins pack along their hydrophobic faces, forming bilayers which subsequently aggregate into fibrils. (c) Coiled-coil heptad α-helical peptides with complementary charges form staggered parallel heterodimer fibrils. This pairing is stabilised by hydrophobic and ionic interactions, as well as pairing between asparagine (N) residues (Reproduced with permission from [20, 35]. Copyright 2009 Nature Publishing Group.). (d) A class of ultrashort amphiphilic peptides are unique in that they α-helical intermediates despite having fewer than 7 amino acids. This secondary structure is attributed to antiparallel pairing of peptide monomers. Subsequent stacking and aggregation gives rise to fibrils which in turn condense to form nanofibers (Reproduced with permission from [24].). (a) β-sheet motifs. (b). β-hairpin motif. (c) α-helical motif. (d) Amphiphilic motif that form β-fibrils via α-helical intermediates.
Download figure:
Standard image High-resolution imageMany self-assembling peptides demonstrate stimuli-responsive gelation and tuneable mechanical properties, which facilitates extrusion before dispensing and maintains the shape fidelity of the printed construct in aqueous media. The innate propensity of short peptides to organise themselves into reproducible macromolecular structures is dictated by specific intermolecular interactions in the presence of specific environmental conditions [26, 27]. As such, peptide self-assembly can be tuned by modulating intrinsic factors (such as amino acid sequence, number of repeating units of the assembling motif, and peptide concentration) and external stimuli (such as temperature, pH and salt concentration). Stimuli-responsive properties can be exploited to facilitate dispensing by lowering the viscosity or preventing nozzle clogging and maintenance of shape fidelity upon deposition onto the substrate via rapid solidification. In general, self-assembly can be accelerated by adding energy to the system—by keeping the reservoir solution at low temperatures, hydrogelation can usually be prevented. Upon deposition onto a heated surface, self-assembly rapidly occurs and hydrogelation is observed. Most notably, the self-assembly of thermo-responsive peptides such as elastin-like polypeptides is triggered when the temperature is raised above their critical gelation temperature, which is dictated by their amino acid sequence [15]. As many self-assembling peptide systems are stabilised by electrostatic interactions, the pH of the solution also impacts self-assembly. By titrating the pH of β-sheet nanotapes with acidic solutions, they can be encouraged to fibrillise [28]. Similarly, β-hairpin peptides are also pH-sensitive [29]. Salt concentration is another major factor as ions in the solution stabilise charged residues. For example, the hydrogelation of self-complementary β-sheet peptides such as RADA16 is inhibited by high salt concentrations and enhanced at low salt concentrations. In contrast, the folding and organisation of β-hairpin peptides is enhanced by salt. Peptides can be designed such that they will form fibrils upon mixing with specific ions. Metal-triggered fibril assembly can be achieved by conjugating metal-binding ligands at the termini of collagen-mimetic peptides [30, 31]. Some peptide systems which only self-assemble when two complementary monomers are interact. This can be exploited by co-depositing two peptide solutions onto the same spot for instantaneous gelation. Pairs of complementary α-helical coiled-coil peptides form parallel heterodimer fibrils via electrostatic interactions [20]. Likewise, β-sheet peptides consisting of alternating hydrophobic and cationic (or anionic) amino acids do not self-assemble due to electrostatic repulsions. However, assembly is initiated when oppositely charged peptides are mixed [32]. In short, a variety of strategies are available to prevent pre-mature gelation of the peptide solution in the reservoir and ensure rapid gelation post-deposition onto the substrate for good shape fidelity. They range from controlling the temperature and pH to co-deposition of ionic triggers or complementary sequences. These techniques are similar to those applied towards stimuli-responsive polymeric bioinks.
One major advantage of using short self-assembling peptides as printable scaffolds is their inherent biocompatibility and biodegradability. Many self-assembling peptides are inspired by motifs found in nature. Moreover, they are by-and-large prepared from amino acids, one of nature's ubiquitous building blocks. Peptide biodegradability can be further modulated by the presence or incorporation of enzymatic cleavage sites [33]. Similarly, spatial and temporal cues can be incorporated through conjugation and encapsulation to regulate cell growth, fate and behaviour [34, 35]. Due to the strong intermolecular interactions that drive self-assembly, the resulting peptide hydrogels are very stable, which bodes well for their use in long term in vitro cultures and in vivo implants for tissue regeneration. Hydrogels prepared using β-sheet peptides such RADA16 are stable across a broad range of temperatures, wide pH range, as well as high concentrations of denaturing agents, urea and guanidium hydrochloride. Sonication transiently disrupts the fibrous network but the hydrogel re-forms within two hours. The stability of the supramolecular structure facilitates the incorporation of biochemical cues for modifying cell behaviour. The facile encapsulation of drugs and growth factors enables the slow, controlled release of these biomolecules to adjacent cells [36–38]. More importantly, chemical conjugation of cell-adhesion or differentiation motifs does not compromise the mechanical integrity of the bulk structure [39, 40]. β-hairpin peptide hydrogels can be similarly modified [41, 42]. The peptide scaffold can also be modified post-assembly via click chemistry. This strategy has been adopted for α-helical peptides [43] and ultrashort amphiphilic peptides [44, 45]. By providing topographical and biochemical cues which mimic the extracellular milieu, these hydrogel scaffolds are capable of supporting cell growth for in vitro and in vivo biomedical applications [12, 13].
Peptides shorter than 20 amino acids are easily prepared on a commercial scale by solid phase synthesis, which bodes well for commercialisation and wide-spread adoption of the bioink technology. Since these building blocks are extremely well-defined chemically, regulatory approval may be expedited by the ability to characterise each batch of product well. Longer polypeptides such as ELPs and SELPs are manufactured by recombinant technology, which also yields monodispersed products. In contrast, commonly used bioinks such as collagen, gelatin, hyaluronic acid, chitosan, alginate and decellularised extracellular matrix, are often derived from animal or plant sources. As such, their chemical composition is poorly defined which impedes regulatory approval due to potential immunogenicity and batch-to-batch variations. There is thus a niche for synthetic 3D cell substrates which resemble ECM and are amenable to chemical modifications to incorporate biologically active ligands for controlling cell behaviour.
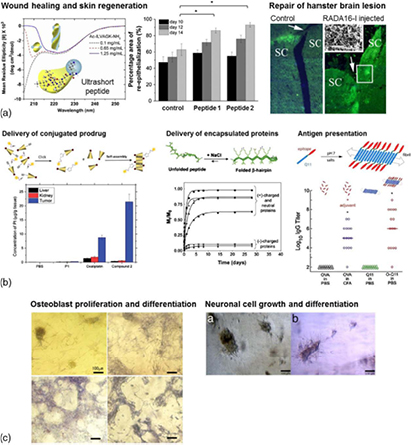
Self-assembling peptide scaffolds have a long and lauded history of being applied as implantable scaffolds for regenerative medicine and matrices for the delivery of encapsulated or conjugated bioactive therapeutics (figure 2). The β-sheet peptide RADA16 has been demonstrated to repair brain damage—injection into rodent models of optic tract lesions resulted in migration of neurons and formation of synapses within two days [12]. Similarly, when injected into mice myocardium, the recruitment of endothelial and myocyte precursors was observed [46]. Ultrashort amphiphilc peptides demonstrate accelerated skin regeneration when used as dressings for burn wounds [47]. They have also been applied as injectable localised drug delivery matrices for cancer therapeutics—the chemically conjugated prodrug was concentrated in the tumour tissue, thereby reducing systemic side effects [45]. β-hairpin peptide hydrogels have been used for sustained release of encapsulated proteins, wherein the release kinetics is influenced by the charge and molecular weight of the biomolecules [41]. In the development vaccines, β-sheet fibrillising peptides, such as Q11, have been modified for optimal antigen presentation to stimulate high antibody titres [48]. Self-assembling peptide hydrogels also function as in vitro 2D and 3D cell culture substrates, enabling the culture and expansion of specialised cells such as differentiated primary cells and pluripotent stem cells. This paves the way for the development of cellular models to understand cell–cell and cell–substrate interactions, as well as to elucidate the effect of specific biomolecules on cell behaviour for high-throughput screening assays.
Figure 2. Biomedical applications of short self-assembling peptides. (a) Regenerative applications include the use of β-sheet peptide RADA16 to repair damaged tissues. Injection of RADA16 into rodent models of optic tract lesions resulted in migration of neurons and formation of synapses (Reproduced with permission from [12]. Copyright 2012 Elsevier.). Ultrashort amphiphilc peptides can be applied as wound dressings for partial thickness burns, wherein they accelerate skin regeneration (Reproduced with permission from [47]. Copyright 2014 Elsevier.). (b) Peptide hydrogels have also been applied as injectable matrices for localised drug delivery. Cancer prodrugs chemically conjugated to ultrashort amphiphilc peptides via click chemistry demonstrate better localisation in the tumour tissue and hence reduced systemic side effects (Reproduced with permission from [45]. Copyright 2013 Royal Society of Chemistry.). Protein drugs can be encapsulated for sustained release, as demonstrated by β-hairpin peptide hydrogels (Reproduced with permission from [41]. Copyright 2010 Elsevier.). β-sheet fibrillising peptides have been modified for optimal antigen presentation to stimulate high antibody titres for vaccine development (Reproduced with permission from [48]. Copyright 2010 Royal Society of Chemistry.). (c) Self-assembling peptide hydrogels also function as in vitro 2D and 3D cell culture substrates. β-sheet peptides such as RADA16 can be modified with bioactive motifs which promote the proliferation and differentiation of osteoblasts (Reproduced from [50].). α-helical peptides promote the growth and differentiation of in vitro culture rat neuronal cells (Reproduced with permission from [20]. Copyright 2009 Nature Publishing Group.). (a) Regenerative medicine. (b) Delivery of bioactive therapeutics. (c) Synthetic cell culture substrate.
Download figure:
Standard image High-resolution imageBioprinting will enable the creation of more sophisticated organotypic constructs. The ability to precisely print multi-cellular and multi-domain tissue constructs in a 3D fashion with high resolution will be a significant advancement over the simple monocultures or co-cultured constructs currently used. In regenerative medicine, the patterning of vascular networks for efficient transport of nutrients and waste and support cells for production of growth factors and cytokines will enhance the integration of the implant with native tissue, encourage survival of implanted cells and modulate the immune response to prevent rejection. Scientists can also begin to develop better implants with greater understanding of the interplay between different cell types, culture substrate and biomolecules through the study of 3D biological constructs prepared by bioprinting. Such organotypic constructs will also better demonstrate the physiological effects of various biomolecules and chemicals, when used for screening assays. By being able to better predict the uptake, metabolism, toxicity, efficacy and biodistribution profile, the numbers of animals or patients required for in vivo testing can be reduced. Bioprinting can also be utilised to produce better drug delivery matrices wherein the release profile can be tailored by varying the dimensions of the micro-domains containing the biomolecule of interest. Different bioactive therapeutics can also be independently delivered by the same matrix implant, and better sustained release formulations will be developed. In short, bioprinting of short self-assembling peptide scaffolds will transform the fields of regenerative medicine, tissue engineering and drug delivery.
Peptide self-assembly is an elegant and expedient 'bottom-up' approach towards designing 3D biological scaffolds. Taking advantage of their biomimicry, stimuli-responsive properties, biocompatibility, biodegradability, ease of synthesis and functionalisation, they are ideal candidates for bioinks. By applying them to bioprinting, the dynamic complexity of biological tissue can be re-created, thereby advancing current biomedical applications of peptide hydrogel scaffolds.
Acknowledgments
This work was supported by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore).