Abstract
Bioactive borate glass (BG) has emerged as a promising alternative for bone regeneration due to its high osteoinductivity, osteoconductivity, compressive strength, and biocompatibility. However, the role of BG in large segmental bone repair is unclear and little is known about the underlying mechanism of BG's osteoinductivity. In this study, we demonstrated that BG possessed pro-osteogenic effects in an experimental model of critical-sized radius defects. Transplanting BG to radius defects resulted in better repair of bone defects as compared to widely used β-TCP. Histological and morphological analysis indicated that BG significantly enhanced new bone formation. Furthermore, the degradation rate of the BG was faster than that of β-TCP, which matched the higher bone regeneration rate. In addition, ions from BG enhanced cell viability, ALP activity, and osteogenic-related genes expression. Mechanistically, the critical genes Smad1/5 and Dlx5 in the BMP pathway and p-Smad1/5 proteins were significantly elevated after BG transplantation, and these effects could be blocked by the BMP/Smad specific inhibitor. Taken together, our findings suggest that BG could repair large segmental bone defects through activating the BMP/Smad pathway and osteogenic differentiation in BMSCs.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Repair of large segmental bone defects is challenging in orthopaedic surgery. Autologous and allogeneic bone grafting are the most widely used treatment strategies for large bone defects. However, disadvantages of autologous bone grafts include the limited graft material available for autografts, extra damage to the harvest site, and the frequent need for a second operation [1, 2]. Moreover, allogenic bone grafts are associated with significant failure rates, poor mechanical stability, and immunological rejection [3]. Bone tissue engineering has emerged as a promising approach for treatment of large bone defects through which bone regeneration can be achieved via a combination of a scaffold with cells or osteoinductive growth factors [4, 5]. However, the realistic use of seed cells is hampered by their limited capacity to proliferate and a rapid loss of differentiation potential during in vitro expansion. Similarly, the applications of growth factors are also limited by high dose requirements, lower half-life, protein instability, high costs, and undesired side effects [6, 7]. Therefore, there is an urgent need to find an osteoinductive scaffold which can repair large bone defects.
Bioactive material provides an alternative solution for the repair and regeneration of bone defects. An ideal bioactive material for bone graft substitute should be biocompatible, biodegradable, and osteoinductive with a suitable microstructure for cell proliferation and differentiation [8]. Hydroxyapatite (HA) and β-TCP porous osteoconductive materials, composed of the same ions as bone, have been widely used in bone regeneration. However, they resorb slowly or undergo little conversion into a bonelike material in vivo [9]. Our previous studies showed that bioactive borate glass (BG) is one of the promising scaffolds for bone regeneration, which has good osteoinductivity, osteoconductivity, compressive strength, and biocompatibility [10–14]. Additionally, BG degrades faster and converts more completely to hydroxyapatite in vivo [15]. However, the application of BG in repairing large segmental bone defects has not been evaluated.
In this study, a critical-sized radius defect model of a rabbit was used to evaluate the ability of BG to repair large segmental bone defects. To gain insight into the osteogenic potential of BG, the impact of BG on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), the major endogenous cell source for bone remodeling and regeneration [16], was assessed. In addition, the effects of BG dissolution products on the activity of the BMP/Smad signal pathway in BMSCs was also investigated. Our results show that BG demonstrates better bone regeneration effects as compared to β-TCP. Our study also shows that the BG dissolution products significantly improve osteogenic differentiation of BMSCs through activating the BMP/Smad signaling pathway. Furthermore, BG significantly improves osteogenic differentiation of BMSCs, leading to bone defect restoration and regeneration. To the best of our knowledge, this study, for the first time, demonstrates a promising therapeutic potential of BG in large segmental bone defects.
2. Materials and methods
2.1. Synthesis of bioactive BG implants
BG with the composition of 6Na2O–8K2O–8MgO–22CaO–54B2O3–2P2O5 (mol%) was used to fashion a scaffold. The powders and porous scaffolds of BG were prepared as described previously [10, 14, 17, 18]. Both BG (with an average pore size of 500 μm and 58% porosity) and β-TCP (Bio-lu Biomaterials Co., Ltd, Shanghai; with an average pore size of 500 μm and 75% porosity) cylindrical implants were the same size (5 mm in diameter and 15 mm in length) and were sterilized for use in animal study.
2.2. In vivo studies
2.2.1. Animal surgery
The study protocol was approved by the Animal Care Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. All experiments were carried out in accordance with the guideline of the local Animal Welfare Committee. During the study, 26 male New Zealand White Rabbits (NZWR) (2.5–3 kg) were kept in individual cages under standard conditions and were allowed to be fully active. The animal surgery was performed as previously described [19, 20]. Briefly, the rabbits were anesthetized by intravenous injection of penobarbital (30 mg kg−1). A 3 cm incision was made over the left distal radius. The soft tissue was dissected and the radius was exposed by blunt separation. A 15 mm segmental diaphyseal defect was created with an oscillating saw under irrigation with 0.9% saline. The radius bone defects were randomly filled with materials or with nothing as follows: (1) control without any implantation (n = 6); (2) β-TCP (n = 10); (3) BG (n = 10). The wounds were closed with 4-0 resorbable sutures. Carprofen was given to relieve pain. Water and food were provided ad libitum.
Fluorescence labeling was used to study new bone formation and mineralization within the bone defect using the protocol previously described [21]. Briefly, alizarin red S (30 mg kg−1; Sigma-Aldrich) and calcein green (10 mg kg−1; Sigma-Aldrich) were intraperitoneally injected at six and nine weeks post-surgery. After 12 weeks, the animals were sacrificed and fixed in 10% neutral formalin for 48 h.
2.2.2. Radiographic and micro-CT evaluation
Standardized anterior-posterior radiographs were taken at 4, 8 and 12 weeks after surgery using x-rays to qualitatively assess bone regeneration and defect bridging.
The specimens, which contained the 1.5 cm segmental defect and 0.5 cm of proximal and 0.5 cm of distal cortical bone adjacent to the defect, were fixed in 70% alcohol. A high-resolution micro-CT system was used to evaluate the healing of the defects. The specimens were scanned continuously at a resolution of 25 μm per voxel. After scanning, the images were reconstructed and 3D analyses were performed. The quantifications of bone mineral density (BMD) and bone volume (BV) were performed using the same standardized threshold.
2.2.3. Double fluorescent labeling and histological analysis
The specimens were dehydrated in a graded series of alcohol baths and embedded in polymethymetacrylate (PMMA). To guarantee the same region was compared with different groups, the position of radius was standardized. The samples were cut in longitudinal sections in a thickness of 250 μm using a saw microtome (Leica SP1600, Germany). The sections were glued onto a transparent plastic plate and polished to a final thickness of 60 μm. The sections were examined for fluorescent labeling under a confocal laser scanning microscope (CLSM) (Leica, Germany). The excitation/emission wavelengths used were 543/580–670 nm (alizarin red, red) and 488/500–550 nm (calcein, green). For further observation of osteoid and mineralized tissue, the sections were stained with van Gieson's picric-fuchsine.
2.3. In vitro studies
2.3.1. hBMSCs isolation
All experiment protocols were approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Written consents were obtained from all donors (age 40–68 years). The BMSCs were isolated and cultured as previously described [22, 23]. Briefly, bone marrow was aspirated and suspended in α-MEM (Gibco) supplemented with 10% Fetal Bovine Serum (FBS, Gibco). The culture media was changed every 2 d and the non-adherent cells were discarded. Remaining cells were trypsinized and passaged when they reached 80% confluence. Passages 4 and 5 were used for the following experiments.
2.3.2. Attachment and morphology of hBMSCs on borate glass scaffold
The BG and β-TCP cylindrical porous scaffolds (8 mm in diameter × 2 mm) were sonicated for 4 h, rinsed in distilled water, and autoclaved at 125 °C/0.14 MPa for 30 min. All scaffolds were pre-incubated in the medium for 24 h before cell seeding. Then hBMSCs were seeded on the scaffolds at an initial density of 1 × 105 cells/scaffold and the culture media was changed every 2 d. The cells were cultured for 3 d, after which the scaffolds were washed with PBS and then fixed with 2.5% glutaraldehyde for 2 h. The scaffolds were coated with gold and the morphological characteristics of the attached cells were observed by a scanning electron microscope (SEM, MX2600, CamScan). Cell seeded scaffolds were also stained with Live/Dead assay kit (LIVE/DEAD Viability/Cytoxicity Kit, Invitrogen, CA) and observed under a confocal fluorescence microscope (Leica DMI6000B, Germany).
2.3.3. Preparation of borate glass extracts
The dissolution extracts of BG were prepared by adding 2 g BG powders to 10 ml α-MEM culture medium supplemented with 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. Then the pH of BG extracts was adjusted to 7.2–7.4 using ammonia. After incubation at 37 °C for 24 h, the mixture was centrifuged and the supernatant was collected. To remove the particulates, the supernatant was filtered through a 0.22 μm filter (Millipore, USA). The resulting supernatant, supplemented with 10% FBS, was denoted as original extract. The original extract was diluted x times with α-MEM to obtain the final extract denoted as the 1/x extract which was used for the cellular experiments. All the extracts were supplemented with 10% FBS and analyzed for the concentrations of Ca, P and B by inductively-coupled, plasma optical emission spectroscopy (ICP-AES; Varian Co., USA) (table 1).
Table 1. Concentrations of Ca, P and B ions in the media.
| Media | Ca (mM) | P (mM) | B (mM) |
|---|---|---|---|
| α-MEM | 1.97 ± 0.06 | 1.27 ± 0.03 | 0 |
| Original extract | 4.45 ± 0.13 | 2.03 ± 0.08 | 7.84 ± 0.21 |
2.3.4. Proliferation of hBMSCs cultured in ion extract
A CCK-8 proliferation assay was performed to investigate cell viability. Briefly, 5 × 103 hBMSCs were seeded in 96-well plates and incubated for 24 h. Then the culture medium was replaced by 2 mL of fresh culture medium containing 1/2, 1/4, 1/8, 1/16, 1/32 diluted borate extracts, respectively. The α-MEM was used as a control. On day 1, 3 and 7, A CCK-8 assay was performed by adding 10 μl CCK-8 solution to each well and incubated at 37 °C for 3 h. Then 100 μl media was transferred and read at 450 nm with a microplate reader (iMark™; Bio-Rad, USA). All experiments were done in triplicate and repeated three independent times.
2.3.5. Alkaline phosphatase (ALP) activity assay
The activity of ALP was measured using an ALP Detection Kit (Beyotime, China). Briefly, hBMSCs were cultured in the presence of different concentrations of borate extracts for 7 and 14 d, respectively. Then the cells were lysed in 200 μl 0.2% Triton X-100. The solution was centrifuged at 14 000 rpm for 15 min at 4 °C. The supernatant was mixed with a working solution of ALP assay kit according to the manufacturer's instructions (Beyotime, China). The absorbance at 450 nm was read with a microplate reader (iMark™; Bio-Rad, USA). The ALP activity was normalized to total protein content as measured by the bicinchoninic acid protein assay kit (Pierce, USA) as previously described [24].
2.3.6. Real time polymerase chain reaction (RT-PCR)
To investigate the effect of borate extracts on osteogenic-genes or BMP/Smad-related genes expression, the total RNA of the hBMSCs cultured in media with 1/4 extract were isolated after 3 and 10 d, or 1 and 3 d, respectively. Then RT-PCR was performed. Briefly, total RNA was obtained with the TRIZOL reagent (Invitrogen, USA). Complementary DNA was synthesized by cDNA Synthesis kit (TakaRa, Japan) from 1 μg total RNA according to the manufacturer's instructions. RT-PCR was performed with a real-time PCR kit (SYBR Premix EX Taq, TaKaRa). GAPDH was used as a housekeeping gene to normalize gene expression. Primer sequences used for real-time PCR are shown in table 2.
Table 2. Primers used for real time polymerase chain reaction (RT-PCR) analysis.
| Genes | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| ALP | TGGAGCTTCAGAAGCTCAACACCA | ATCTCGTTGTCTGAGTACCAGTCC |
| Runx2 | CATGAGAGCCCTCACA | AGAGCGACACCCTAGAC |
| Ocn | CATGAGAGCCCTCACA | AGAGCGACACCCTAGAC |
| SMAD1 | CACAAACATGATGGCGCCT | CATAGTAGACAATAGAGCACCAGTGTTT |
| SMAD5 | GAAGCTTGCTGGTAATCTTAAGAATTTTC | GCTTGTATCCATAGGCTGGGAA |
| Dlx5 | CCAACCAGCCAGAGAAAGAA | GCAAGGCGAGGTACTGAGTC |
| GAPDH | ATCCCATCACCATCTTCC | GAGTCCTTCCACGATACCA |
2.3.7. Western blot analysis
hBMSCs cultured with and without 1/4 extract were analyzed by western blotting. The total cellular proteins were first extracted. The cells were lysed in ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 0.1% SDS) containing 1 mM phenylmethylsulphonyl fluoride (PMSF) (Sigma, USA) and phosphatase inhibitor cocktail (Sigma, USA). The cell lysates were cleared by centrifugation at 4 °C and 12 000 rpm for 15 min. The protein concentrations of the lysates were quantified using a BCA assay Kit. The cell proteins were separated by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. After incubation in a 5% BSA blocking solution for 1 h, the membranes were incubated overnight at 4 °C with anti-phospho-Smad1/5, anti-Smad1, and anti-β-actin antibodies (all from Cell Signaling Technology, USA). The signal inhibitor used for BMP/Smad was noggin (R&D Systems, USA). The noggin was dissolved in PBS containing 0.1% BSA at a concentration of 100 ng ml−1 for subsequent experiments. The membranes were then washed three times with PBS-Tween-20 and incubated with horseradish peroxidase (HPR)-conjugated secondary antibodies (Sigma, USA) at 37 °C. The immunoreactive bands were observed using the ECL chemiluminescence reagent (Millipore, USA)
2.3.8. ALP and alizarin red S (ARS) staining
ALP staining was performed as previously described [25]. Briefly, hBMSCs were cultured in 1/4 original extract for 10 d. Cells were fixed with 4% paraformaldehyde and incubated in a mixture of nitro-blue tetrazolium and 5-bromo-4chloro-3-indolylphosphate.
Alizarin Red S staining was performed to detect extracellular calcium content as previously described [26]. Briefly, the hBMSCs were seeded in 6-well plates and cultured in 1/4 original extract for 14 d. Cells were rinsed with PBS and fixed in 4% paraformaldehyde for 15 min, then stained with 1% (w/v) Alizarin Red S for 30 min at room temperature.
2.4. Statistical analysis
All data are shown as mean ± standard deviation (SD). Differences between groups were assessed by one-way analysis of variance (ANOVA) with GraphPad Prism software. P values <0.05 were considered statistically significant.
3. Results
3.1. BG enhances repair of large segmental bone defects in vivo
3.1.1. Gross view and histological staining
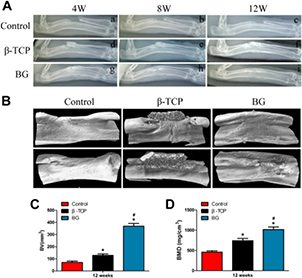
The gross view of the radius implanted with BG showed better bone repair at 12 weeks after surgery (figure 1(A)) compared to β-TCP transplantation. The morphology of the newly formed radius in BG group look is similar to those of the normal radius. In the β-TCP group, much of the implanted scaffold remained and a small amount of callus formed close to the scaffold after 12 weeks. In the control group, most of the defects were filled with fibrous tissue and no bone union was observed. The ends of the bone defects were sclerotic and the medullary cavities were blocked at 12 weeks post-implantation, suggesting that the bone defect was not healed.
Figure 1. Effects of BG treatment on bone regeneration. (A) Gross morphology of specimens and (B) van Gleson's picric-fuchsine staining of repaired radius treated with β-TCP or BG at 12 weeks after implantation. Red and black represent the newly formed bone (NB) and residual material, respectively.
Download figure:
Standard image High-resolution imageVan Gieson's picric-fuchsine staining was performed to evaluate the new bone formation and in vivo degradation of the scaffold. The new bone formation was much more evident in the BG implantation than in the β-TCP group after 12 weeks (figure 1(B)). Furthermore, very little BG was observed in the BG transplantation group, while a massive amount of β-TCP was detected in the β-TCP transplantation group. There was no obvious formation of bone tissue in the blank control group. Taken together, these data demonstrate that BG treatment has a better effect on bone repair as compared to β-TCP.
3.1.2. Analysis of bone formation by radiographic and micro-CT
To assess bone regeneration and bone union within the defects, x-rays were taken at 4, 8 and 12 weeks after surgery (figure 2(A)). In the control group, the segmental defect was not healed and non-union was apparent at all time points (figures 2(A(a)–(c))). In the β-TCP group, β-TCP did not degrade at 4 weeks post-surgery; it began to degrade at 8 weeks and a small amount of callus formed in the interspaces between the implants and bone tissue, and the β-TCP was partly degraded at 12 weeks (figures 2(A(d)–(f))). In contrast, the implanted material bonded with the proximal and distal host bone and partially degraded at 4 weeks in the BG group. Moreover, at 8 weeks massive amounts of callus formed and most of the implanted BG was degraded. At 12 weeks, the bone defects were fully bridged and the implanted BG completely degraded. The boundary between new bone and normal bone was indistinct. The medullary cavity reformed and achieved recanalization in the BG group (figures 2(A(g)–(i))).
Figure 2. The radiographs and micro-CT evaluation of a rabbit radius bone defect model repaired with different scaffolds at different time points after implantation. (A) Radiographs to show the defects received no graft (a)–(c), β-TCP (d)–(f), or BG (g)–(i), respectively, at different times after surgery. (B) Representative 3D reconstruction by micro-CT to demonstrate the repaired radius from the control, β-TCP and BG groups (from left to right) at 12 weeks after implantation. Morphometric analysis of the bone volume (C) and bone mineral density (D). (*P < 0.05 compared with the control group, #P < 0.05 compared with the β-TCP group.)
Download figure:
Standard image High-resolution imageThe images of new bone formation were then reconstructed by micro-CT and BV and BMD in the bone defect were calculated by using auxiliary software of micro-CT with a threshold of 800 defined as bone tissue. The results showed that the new bone in the BG group was greater than that in the β-TCP group or control group at 12 weeks after surgery (figure 2(B)). Morphometric analysis further revealed that BV and local BMDs in the BG group were significantly increased compared to the β-TCP group and control group, respectively (figures 2(C) and (D)).
3.1.3. BG enhances mineral deposition and new bone formation
Immunofluorescence staining of alizarin red and calcein further revealed that, at week 6 and 9, the area of mineralized deposition of new bone formation in the BG group was significantly increased compared to the β-TCP group or the control group (figure 3).
Figure 3. New bone formation and mineralization was determined histomorphometrically by fluorochrome-labeling analysis. Red: alizarin red; Green: calcein. (A) Row a, b, and c represent fluorochrome-labeling area for each group. Row a3, b3, and c3, represent merged images of the two fluorochromes for the same group, and row a4, b4, and c4 represent merged images from a plain microscope. (B) The analysis of the percentage of each fluorochrome area for different groups (*P < 0.05 compared with the control group, #P < 0.05 compared with the β-TCP group.).
Download figure:
Standard image High-resolution image3.2. Enhancing proliferation and osteogenic differentiation of BMSCs by BG in vitro
3.2.1. BG scaffolds promote attachment and survival of hBMSCs
The attachment and survival of hBMSCs on BG was shown in figure 4(A). hBMSCs could attach on the BG scaffold at day 1. Under the use of Live/Dead staining, it showed that hBMSCs on BG had a higher survival rate even after 7 d, indicating that the BG is non-cytotoxic.
Figure 4. Effect of BG on the attachment, survival, proliferation, and ALP activity of hBMSCs. (A) SEM showing hBMSCs attached on BG after 24 h cultured. (a) Low magnification and (b) high magnification. Live/dead cell staining showing hBMSCs cultured on BG at 1 d (c) and 7 d (d) after seeding. (B) Cell proliferation of hBMSCs cultured with different BG extracts for 24 h. (C) ALP activity of hBMSCs cultured with different BG extracts for 7 and 14 d (*P < 0.05 compared with the control group, #P < 0.05 compared with other groups.).
Download figure:
Standard image High-resolution image3.2.2. Effect of BG extracts on BMSCs proliferation
The concentrations of B, Ca, and P in the extracts are shown in table 1. We found that BG extracts contain much higher amounts of B, Ca, and P than α-MEM.
To investigate the effect of extracts on the proliferation of hBMSCs, cells were cultured with different extracts for 1, 3 and 7 d. As determined by the CCK-8 proliferation assay, no significant difference was detected between cell growth in either extract or in the control media at 1 and 3 d. After 7 d of culture, however, cell proliferation in the 1/4, 1/8, 1/16, 1/32 extracts significantly increased (figure 4(B)).
3.2.3. BG extracts promote ALP activity and osteogenic-related gene expression
To investigate the effect of BG extracts on the osteogenic differentiation of hBMSCs, ALP activity was examined at 7 and 14 d after being cultured in different extracts. As shown in figure 4(C), ALP activity on day 7 was significantly elevated in 1/4 extract whereas the ALP activity on day 14 was significantly elevated in all the extracts.
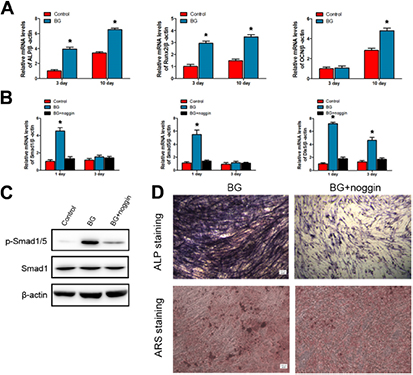
To further confirm the effect of BG extracts on osteogenic differentiation of hBMSCs, hBMSCs were cultured in 1/4 extract for 3 and 10 d, then the expression of osteoblastic markers in hBMSCs was examined by RT-PCR. As shown in figure 5(A), the expression of ALP and Runx2 were significantly enhanced by the extracts of BG over 10 d. The gene expression of OCN showed no significant difference among the three groups at day 3 whereas it was dramatically increased in cells cultured with the BG extracts at day 10.
Figure 5. Involvement of BMP/Smad signaling in the BG-induced osteogenic responses from hBMSCs. (A) Expression of osteogenic genes was assessed by qRT-PCR in hBMSCs cultured with 1/4 BG original extracts for 3 and 10 d. (B) BMP/Smad signaling genes (Smad1/5, and Dlx5) expression in hBMSCs cultured with 1/4 BG original extracts for 1 and 3 d with or without noggin. hBMSCs cultured in α-MEM were served as the control group. (C) Expression of p-Smad1/5 proteins in hBMSCs cultured with 1/4 BG original extracts for 12 h with or without noggin. hBMSCs cultured in α-MEM served as the control group. (D) ALP and ARS staining of hBMSCs cultured with 1/4 BG original extracts for 10 and 14 d with or without noggin (*P < 0.05 compared with other groups.) Scale bar: 100 μm.
Download figure:
Standard image High-resolution image3.2.4. Activity of BMP/Smad pathway in BMSCs stimulated by BG extracts
Data described above demonstrated that the 1/4 extract of BG was the most effective to induce hBMSCs osteogenic differentiation. Therefore, we next sought to investigate whether the BMP/Smad signaling pathway was activated during BMSC differentiation after treatment with 1/4 BG extract. The gene expression levels of key molecules (Smad1/5 and Dlx5 transcription factors) in the BMP/Smad signaling pathway were examined using RT-PCR [27]. As shown in figure 5(B), cells cultured in the control group expressed a low level of these genes, while cells showed significant higher expression of Smad1/5 and Dlx5 after incubation with the 1/4 BG extracts at day 1. Specifically, expression of Dlx5 in BG group was remarkably higher than that in the control group at day 3.
To clarify whether activation of BMP/Smad is required for the hBMSCs osteogenic differentiation induced by 1/4 BG extracts, the BMP/Smad signaling pathway was blocked with a specific inhibitor, noggin, for 24 h prior to 1/4 BG extract. Subsequently, qRT-PCR, Western blot, ALP, and ARS staining were preformed to assess osteogenic activity. Data from these experiments showed that the BG-induced 1/4 BG extract-induced up-regulation of Smad1/5 and Dlx5 mRNA expression and p-Smad1/5 proteins were suppressed by noggin (figures 5(B) and (C)). The ALP and ARS staining also revealed that 1/4 BG extract-induced osteogenic activity was remarkably inhibited by the noggin treatment (figure 5(D)). Taken together, these data suggest that BG-induced osteoblast differentiation is achieved through activating BMP/Smad signaling.
4. Discussion
BG is a new bioactive glass with many advantages as mentioned in the beginning. However, up to now, there are no reports regarding the application of BG in large segmental bone defects. In this study, the repair of critical-sized radius bone defects demonstrated that BG can heal large segmental bone defects. Further analysis in vitro demonstrated that the BG extracts could promote the proliferation and osteogenic differentiation of BMSCs, which might be involved in the activation of the BMP/Smad signal pathway. All the results showed that BG can be used as an excellent bone regeneration material to treat large segmental bone defects.
β-TCP is a well characterized osteoconductive biomaterial and is clinically used for bone repair and regeneration [28], but it lacks osteoinductive activity and has a low degradation rate in vivo. In the present study, x-rays showed that the newly formed bone completely bridged the defects in the BG group. The gross view revealed that the repaired radius closely resembled that of a normal radius. In agreement with the above finding, micro-CT analysis and fluorosence labeling confirmed there was much more newly formed bone in the BG group. The control group showed the bone defects being filled with fibrous tissue and little new bone formation at 12 weeks after implantation. As for β-TCP with only osteoconductive capacity, the in vivo results showed that little new bone formed at the end of the experiments. Moreover, an ideal bone substitute not only can induce new bone formation, but also degrade at a suitable rate. Therefore, it is desirable to develop biomaterials with a suitable degradation rate. From the x-ray images, gross view, and histomorphometric analysis, the BG almost totally degraded at 12 weeks after implantation. However, in the β-TCP group, the implanted scaffold degraded much slower than the BG, suggesting that the degradation rate of BG is better coordinated with the rate of bone regeneration. Previous studies have also demonstrated that BG converted completely to apatite in vivo [15, 29].
Although BG was found to promote bone regeneration in vivo, the molecular mechanism of this effect has not been elucidated yet. It is supposed that bone regeneration by bioactive glasses may occur through bioactive ions released by these materials during their degradation [30]. After treatment with different concentrations of boron ions, gene expression of osteoblastic markers and protein levels of BMPs were increased in MC3T3-E1 cells [31]. In the current study, we investigated the effect of the ionic products of BG on BMSCs proliferation and osteogenic differentiation. ALP is an earlier marker for osteogenic differentiation. Runx2 and OCN have also been regarded as osteogenic markers. In this study, the increased ALP activity and expression of osteogenic markers demonstrated the BMSCs were induced into osteogenic differentiation by BG extracts. Meanwhile, the expression of the critical genes, Smad1/5 and Dlx5, in the BMP/Smad signal pathway was also detected. Several signal pathways have been proven to be involved in bone regeneration, such as BMPs pathway [32, 33], Wnt pathway [34, 35], and fibroblast growth factor pathway [36, 37]etc. Among those signal pathways, the BMP/Smad signal pathway has been reported to participate in the process of biomaterials stimulated osteogenesis. Liu et al reported that the BMP signaling pathway is associated with cerium-promoted osteogenic differentiation of MSCs [38]. Liu et al demonstrated that biomimetic nanofibrous scaffolds promote bone regeneration by activating the BMP/Smad signaling pathway [39]. In this study, the addition of BMP/Smad inhibitor to the BG extracts stimulated BMSCs led to a suppressed Smad1/5 and Dlx5 mRNA expression, and decreased ALP and ARS staining intensity, which indicated that the BMP/Smad signal pathway might be fully or partially involved in the process of BG stimulated osteogenesis.
Another concern with BG is the concentration of boron released during the degradation of the scaffold. A low concentration of boron is beneficial for bone development and regeneration, while a high concentration of boron can result in side effects and toxicity [40]. Fu et al showed that an extract of BG supported the proliferation and osteogenic differentiation of BMSCs below a threshold value [41]. Previous study has reported that a boron content of <100 mg kg−1 mass of the rabbit would be a safe threshold [42]. Our previous study also showed that the BG progressively degraded during the experiment period and the boron concentration in the blood sample was far below the toxicity level. No evidence of boron toxicity [17] was detected from the blood examination and histological section. In conclusion, the results showed that the BG was biocompatible and capable of stimulating bone formation in vivo.
5. Conclusion
In summary, the study showed that BG could significantly promote large segmental bone defect repairs better than β-TCP and the degradation of BG was also faster than that of β-TCP. Moreover, in vitro results demonstrated that, when cultured with a certain concentration of BG extracts, the proliferation and osteogenic differentiation of the hBMSCs were enhanced. The underlying mechanisms are involved in the activation of BMP/Smad signal pathway in osteogenic differentiation of BMSCs. The BG has the potential to be a good bone regeneration material with excellent application prospects.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (grant Nos: 81371938 and 51372170). We particularly thank Dr J Zhou, Department of Pharmacology & Toxicology Medical College of Georgia, Georgia Regents University for the editorial assistance on the manuscript.