Abstract
Objective. Optogenetic stimulation of the auditory nerve offers the ability to overcome the limitations of cochlear implants through spatially precise stimulation, but cannot achieve the temporal precision nor temporal fidelity required for good hearing outcomes. Auditory midbrain recordings have indicated a combined (hybrid) stimulation approach may permit improvements in the temporal precision without sacrificing spatial precision by facilitating electrical activation thresholds. However, previous research has been conducted in undeafened or acutely deafened animal models, and the impact of chronic deafness remains unclear. Our study aims to compare the temporal precision of auditory nerve responses to optogenetic, electrical, and combined stimulation in acutely and chronically deafened animals. Methods. We directly compare the temporal fidelity (measured as percentage of elicited responses) and precision (i.e. stability of response size and timing) of electrical, optogenetic, and hybrid stimulation (varying sub-threshold or supra-threshold optogenetic power levels combined with electrical stimuli) through compound action potential and single-unit recordings of the auditory nerve in transgenic mice expressing the opsin ChR2-H134R in auditory neurons. Recordings were conducted immediately or 2–3 weeks following aminoglycoside deafening when there was evidence of auditory nerve degeneration. Main results. Results showed that responses to electrical stimulation had significantly greater temporal precision than optogenetic stimulation (p < 0.001 for measures of response size and timing). This temporal precision could be maintained with hybrid stimulation, but only when the optogenetic stimulation power used was below or near activation threshold and worsened with increasing optical power. Chronically deafened mice showed poorer facilitation of electrical activation thresholds with concurrent optogenetic stimulation than acutely deafened mice. Additionally, responses in chronically deafened mice showed poorer temporal fidelity, but improved temporal precision to optogenetic and hybrid stimulation compared to acutely deafened mice. Significance. These findings show that the improvement to temporal fidelity and temporal precision provided by a hybrid stimulation paradigm can also be achieved in chronically deafened animals, albeit at higher levels of concurrent optogenetic stimulation levels.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Hearing loss affects over 20% of the global population and this figure is forecast to reach 25% by 2050. Hearing loss is commonly caused by loss of cochlear hair cells. Lost hair cells are not replaced by the body, leading to permanent hearing loss. Currently, the only available option for restoring hearing function following severe or complete hearing loss is the cochlear implant, which uses electrical charge to bypass the lost hair cells and directly stimulate the auditory nerve.
Although cochlear implants restore some hearing function to people suffering disabling hearing loss, many users still struggle to understand speech in noisy environments [1–3], appreciate music [4–7], and recognise speakers [8, 9]. A major contributor to these outcomes is the spread of charge in the highly conductive cochlear fluids resulting in broad activation of auditory neurons (ANs). Although in vivo studies of techniques such as current shaping and steering have achieved narrower regions of activation [10, 11], these results have not translated to significantly improved hearing outcomes in cochlear implant recipients [12, 13]. As such, there is a need for novel stimulation methods to achieve improved outcomes for users.
Optogenetics offers an alternative means of stimulating neurons with the potential to overcome the limitations of electrical stimulation. Optogenetics refers to the modification of neurons to express light sensitive actuators known as opsins, enabling neural modulation by visible light. Dozens of natural and engineered opsins have been discovered, offering a broad range of activation wavelengths, channel kinetics, and ion channel conductance properties suitable for a wide array of applications. It has been demonstrated that modification of ANs in the cochlea with excitatory opsins and subsequent optical stimulation reduces the region of activation compared to electrical stimulation [14, 15].
High stimulation rates are needed to preserve the temporal information of sound that encodes pitch and sound quality [16]. Many cochlear implants today use stimulation rates of 400–1000 pulses per second (pps), although there may be some benefit at even higher rates of stimulation at which acoustic-like neuron responses have been observed [17] and improvements in auditory perception have been reported [18]. However, when ANs were modified with Chronos-ES/TS, an opsin with one of the fastest channel kinetics to date, high spike probability (>80%) was reported using optical stimulation repetition rate below 100 pps, while only a few exceptional neurons were able to maintain good spike probabilities at repetition rates up to 500 pps [19].
Novel strategies are needed that can preserve the high stimulation rates of contemporary cochlear implants and confine the region of activation from each stimulating channel, permitting a greater number of independently activated regions. Hybrid stimulation that combines both electrical and optogenetic stimulation in the cochlea has been shown to achieve narrower regions of activation than electrical-only stimulation and higher maximum stimulation rates than optogenetic-only stimulation [20]. However, this research was conducted by recording from the auditory midbrain, a structure that is four synapses away from the cochlea and, as such, is an indirect measure of the effects on the auditory nerve. Furthermore, many studies of optogenetic stimulation in the cochlea do not account for the effects of hair cell-mediated responses or the degenerative aspects of hearing loss. To date, several studies have investigated the impact of chronic deafness on the responses of ANs to electrical stimulation [21–24], including speech perception outcomes in cochlear implant recipients [25]. For example, electrical stimulation of the auditory nerve following chronic deafness results in reduced jitter, reduced latency, and poorer entrainment compared to undeafened animals. However, it remains unclear how the degeneration of the auditory nerve resulting from hearing loss may impact responses to optogenetic stimulation.
Here, we conducted compound action potential and single unit recordings of the auditory nerve response to electrical-only, optical-only stimuli, and hybrid stimuli in optogenetically modified mice. Mice were acutely deafened shortly before or 2–3 weeks prior to recordings as models of short-term and chronic deafness, respectively, and so allowed investigation of the effects of nerve health on evoked responses. Our findings indicate that optogenetic and electrical stimuli can interact at the level of individual ANs to generate unique hybrid responses. Hybrid responses demonstrated increasingly optical-like characteristics as the power of the optogenetic stimulus increased. However, chronic deafness negatively impacted the temporal characteristics of optogenetic and hybrid responses, indicating optogenetic stimulation is impacted by nerve health.
2. Results
This study was performed in mice that expressed ChR2-H134R-EYFP fusion protein via the parvalbumin promoter. ChR2-H134R-EYFP was expressed throughout the cochlea in ANs and inner hair cells (IHCs), with weak expression in outer hair cells (figure 1(A)). The ChR2-H134R-EYFP protein was localised to the membrane of ANs and was observed in cell bodies as well as the peripheral and central fibres.
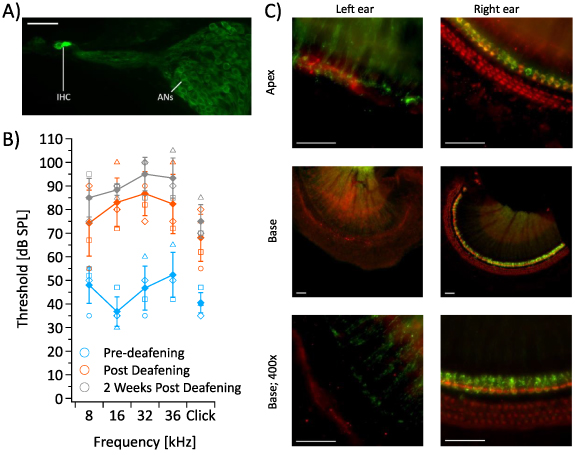
Figure 1. (A) ChR2-H134R-EYFP is strongly expressed in inner hair cells (IHC) and auditory neurons (ANs). Scale bar 50 µm. (B) Auditory brainstem response thresholds of mice before (n = 4) and after exposure of the left cochlea to neomycin sulphate (n = 3). Error bars represent standard error mean, individual mice are represented by unique symbols. (C) Whole mount preparations of the organ of Corti from the basal and apical regions of the cochlea from a chronically deafened mouse. A myosin VIIa antibody was used to stain hair cells (red). Significant hair cell ablation is observed in the treated (left) ear, with minimal to no hair cell loss seen in the untreated (right) ear. Scale bar 50 µm. Green = ChR2-H134R-EYFP.
Download figure:
Standard image High-resolution image2.1. Hair cell inactivation
Since it is possible for hair cells to be activated by electrical- [26], optogenetic- [27] or optoacoustic-stimulation [28], we developed a technique to thoroughly inactivate inner and outer hair cells and so mitigate the effect of hair cell-mediated responses on auditory nerve recordings. In our studies, mice were unilaterally deafened by perfusing neomycin sulphate through the left cochlea over 20–30 min. Auditory brainstem response (ABR) thresholds were recorded before and after deafening. A significant shift in thresholds was observed within one hour of neomycin sulphate exposure (p < 0.05 for all tones; RM-ANOVA, n = 4). Three mice were recovered and their hearing reassessed 2 weeks later; hearing thresholds remained significantly elevated in these animals (figure 1(B); p < 0.05 for all tones; RM-ANOVA, n = 3), and histology showed complete ablation of inner and outer hair cells in the left cochlea of all mice (figure 1(C)). Sporadic hair cell loss was also observed in the outer hair cell region of the right ear, although it appeared to follow no obvious pattern throughout the cochlea.
2.2. Stimulation set up
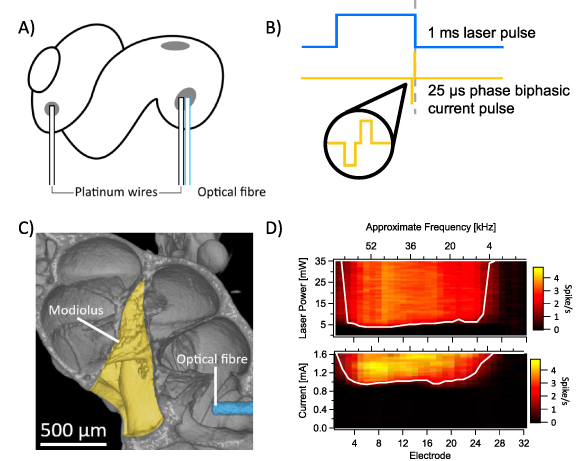
Hybrid stimulation relies on the interaction of simultaneous electrical and optogenetic stimulation of the auditory nerve. In our stimulation set up, optical stimulation (452 nm, 1 ms pulses) was provided by a 105 µm core optical fibre inserted into the round window of the cochlea and oriented towards the modiolus to maximise the spread of activated neurons, and so increase the probability of evoking hybrid responses. Charge balanced biphasic electrical stimulation was provided by two 100 µm platinum wires inserted into the round window in the base of the cochlea and a fenestration in the apical turn of the cochlea, respectively (figures 2(A) and (B)). Microcomputed tomography (micro-CT) scans confirmed the optical fibre was successfully directed towards the modiolus, projecting light on the auditory nerve where it exited the cochlea (figure 2(C)). To confirm the activation area, we recorded spiking activity in the contralateral inferior colliculus using a 32-channel recording array in response to 1 ms light pulses or the biphasic electrical pulses. The inferior colliculus is a midbrain structure of the auditory pathway that preserves the tonotopic arrangement of the cochlea and, as such, reflects the spread of activation in the cochlea. Indeed, multi-unit responses to optogenetic stimulation indicated broad activation across most frequencies of the adult mouse cochlea (activation range at 3 dB above threshold: 7.8–64.5 kHz for electrical, 26.4–59 kHz for optogenetic), in line with the positioning of the optical fibre, and high overlap between the two stimulation modalities (figure 2(D)).
Figure 2. (A) The stimulation set up consisted of a single 105 µm optical fibre inserted into the round window of the cochlea and directed towards the modiolus, a 100 µm platinum wire inserted into the round window, and a second 100 µm platinum wire inserted into a fenestration made in the apical wall of the cochlea. (B) Example stimuli waveforms, consisting of a 1 ms laser pulse (452 nm) and/or a 25 µs biphasic current pulse delayed by 942 µs (i.e. ending at the same time as the laser pulse). (C) Micro-CT scans confirmed positioning of the optical fibre (blue) in the round window, directing light towards the modiolus (yellow) where the auditory nerve exits the cochlea. (D) Multiunit recordings from the inferior colliculus for optogenetic stimulation (top) and electrical stimulation (bottom) show the region of activation within the cochlea by the two stimuli individually. White line marks the spatial tuning curve (i.e. threshold for each electrode). Electrodes 1–32 refer to shank electrodes, with approximate frequency estimated by best fit of best frequencies.
Download figure:
Standard image High-resolution image2.3. Facilitation of electrical activation
After confirming that our deafening and stimulating techniques were effective, we investigated how optogenetic stimulation facilitated activation of the auditory nerve by electrical stimulation at a rate of 4 pps. Compound action potentials in response to optogenetic, electrical, and hybrid stimuli were recorded, and activation thresholds compared across the different stimulation modalities. Activation thresholds were defined as the minimum power level presented that produced a visually identifiable response.
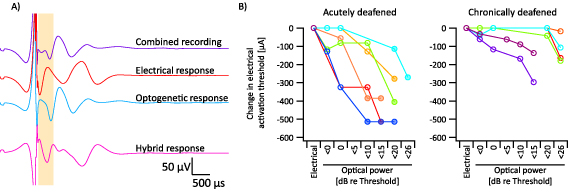
Despite our best efforts to maximally overlap the optogenetic and electrical stimuli, they did not overlap perfectly—i.e. some portions of the auditory nerve were only stimulated by optogenetic or electrical stimuli. As a result, there were three distinct responses to hybrid stimulation: responses of neurons activated by electrical stimulation only, those activated by optogenetic stimulation only, and those activated by interaction of electrical and optogenetic stimulation (i.e. hybrid responses). To isolate the hybrid responses, the responses at the equivalent electrical and optogenetic levels were subtracted from combined recordings. Finally, the difference between activation threshold current for electrical stimulation and hybrid stimulation were measured to evaluate the interaction of optogenetic and electrical stimulation (figure 3(A)).
Figure 3. (A) Representative example of hybrid response extraction. In brief, responses at the equivalent electrical and optogenetic level were removed from combined stimulation recording, and the residual response labelled as the hybrid response. (B) Facilitation of electrical activation thresholds by concurrent optogenetic stimulation in acutely deafened mice (left, n = 6) and chronically deafened (right, n = 5) mice. Filled circles indicate recordings where the level observed as the activation threshold was the minimum stimulation level recorded above zero. Different colours represent individual mice.
Download figure:
Standard image High-resolution imageHybrid stimulation was presented at several levels of sub- and supra-threshold optogenetic stimulation each combined with a range of electrical current levels. As the light power was increased, the activation thresholds for electrical stimulation decreased. In some cases, responses were observed at the lowest level of electrical stimulation presented above zero, and so the shift in activation thresholds was likely larger than presented here (figure 3(B)). The magnitude of this shift was significantly greater for acutely deafened animals compared to chronically deafened animals at all light levels (figure 3(B)). For example, at 15–20 dB suprathreshold optical stimulation, the electrical activation threshold was reduced by 328 ± 56 µA in acutely deafened mice compared to 7 ± 7 µA for chronically deafened mice (p < 0.05; t-test; n = 4 acute deaf, n = 4 chronic deaf). Indeed, the overall degree of facilitation was significantly different between the two groups, as measured by comparing the slopes of the change in electrical activation thresholds (p < 0.005; t-test, n = 6 acute deaf, n = 6 chronic deaf).
We did not observe any significant differences between the optogenetic nor electrical activation thresholds of the acutely deafened and chronically deafened mice.
2.4. Temporal precision at 100 pps
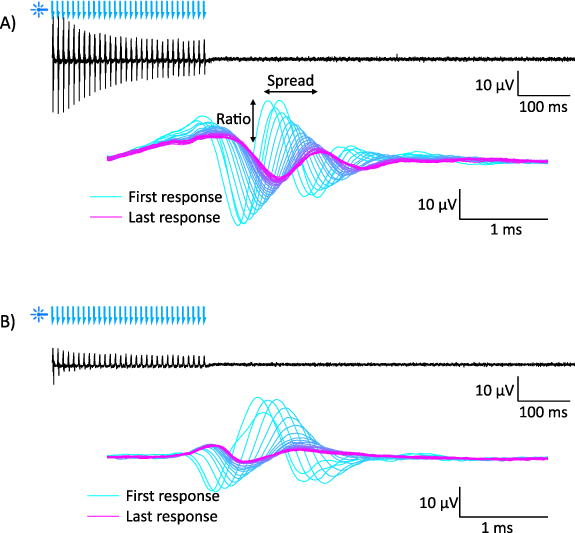
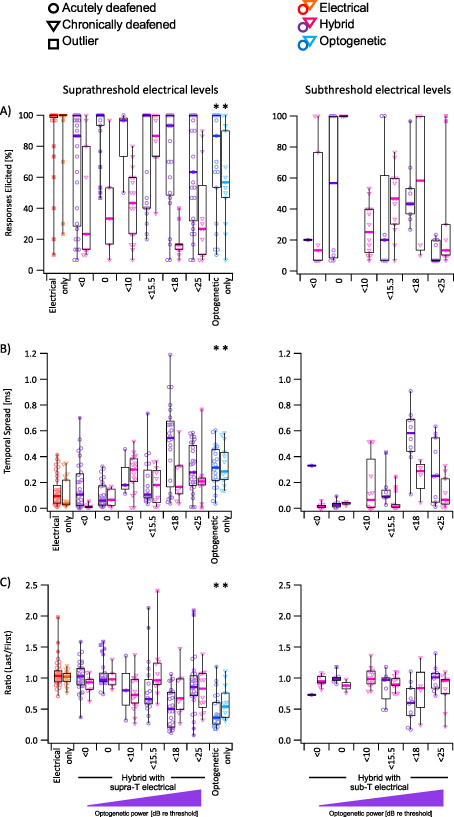
To understand if facilitation occurs at higher stimulation rates and, furthermore, to understand how the temporal characteristics of the auditory nerve response compares across the different stimulation modalities, we repeated our experiments using 100 pps burst stimulation over 300 ms, presented at a rate of 1 Hz (figure 4). As before, hybrid responses were identified as the residual of the combined stimulation recordings, and the electrical- and optical-only recordings. Three measures were taken from the responses: the percentage of responses elicited across the burst, the temporal spread (i.e. the time difference between the earliest and latest response of the burst), and the ratio (i.e. the relative change in peak-to-peak response amplitude from the first response to the last response). Recordings were conducted across a range of supra- and sub-threshold levels per animal and measures were taken from recordings that demonstrated more than two responses across the duration of the burst stimulus.
Figure 4. Representative examples of an optogenetic compound action potential recording at 100 pps in (A) an acutely deafened animal and (B) a chronically deafened animal. Black trace shows the recording over time (blue arrows = laser pulse), and blue-magenta traces show all responses from the black trace overlaid relative to stimulus.
Download figure:
Standard image High-resolution imageFor both acutely deafened and chronically deafened animals, electrical-only stimulation evoked a high median percentage of responses (acute median ± interquartile range = 100 ± 2%, chronic: 100 ± 0%). This was also true for optogenetic-only stimulation in acutely deafened animals (86.7 ± 47%), but not for optogenetic-only stimulation in chronically deafened animals, which was significantly lower at 56.7% ± 43% (p = 0.001, Wilcoxon–Mann–Whitney test, n = 17 for electrical, n = 13 for optogenetic). The median percentage of residual hybrid responses elicited using suprathreshold electrical stimulation was consistently high in acutely deaf mice (figure 5(A) purple bars). Medians between 87% and 100% were observed at all levels, except for the highest optogenetic powers used, where the median dropped to 63%. In contrast, the median percentage of residual hybrid responses elicited using suprathreshold electrical stimulation was consistently low in chronically deaf mice, peaking at 86.7% for <15.5 dB suprathreshold optogenetic stimulus.
Figure 5. (A) Percentage of stimuli which elicited a response for acutely deafened (circles) and chronically deafened (triangles) mice. Filled markers indicate outliers, filled square markers indicate far outliers (Tukey's method). Graphs on the left show responses to supra-threshold electrical stimulation (red/orange), hybrid stimulation using increasing optical power combined with supra-threshold electrical stimuli (purple/magenta), and supra-threshold optogenetic stimuli (dark blue/light blue). Graphs on the right show responses to hybrid stimulation using sub-threshold electrical stimuli. (B) Temporal spread of responses for acutely deafened (circles) and chronically deafened (triangles) mice for supra-threshold (left) or sub-threshold stimuli (right). (C) Ratio of the size of the last response and the first response for acutely deafened (circles) and chronically deafened (triangles) mice for supra-threshold (left) or sub-threshold stimuli (right). Asterisk (*) indicates significance between the marked column and the matching electrical column. Electrical stimuli ranged from 52 µA below threshold to 97 µA above threshold. Optogenetic stimuli ranged from 13 dB below threshold to 25 dB above threshold.
Download figure:
Standard image High-resolution imageThe temporal spread of electrical-only responses was low for both acutely and chronically deafened mice (94 ± 14 µs and 35 ± 15 µs, respectively, median ± variance), whereas optogenetic-only responses were significantly higher for both groups (315 ± 27 µs and 285 ± 26 µs for acutely and chronically deafened, respectively; p < 0.001 vs electrical; Wilcoxon–Mann–Whitney test, n = 17 for electrical, n = 13 for optogenetic) (figure 5(B)). Despite the observed differences in percentage of responses elicited for optogenetic-only responses, the temporal spread was not significantly different between acutely and chronically deafened mice. The temporal spread of hybrid responses evoked using suprathreshold electrical stimulation (purple bars) increased with increasing optogenetic stimulus power. However, similar to the trend observed with the percentage of responses elicited, the temporal spread of hybrid responses dropped at optogenetic stimulation powers above 18 dB re threshold (figure 5(B)). This trend was less clear in the chronically deafened mice, but temporal spread was generally higher at suprathreshold levels of optogenetic stimulation compared to sub- or threshold levels of optogenetic stimulation.
The amplitude of the response to electrical stimulation was stable across the burst, with both acutely and chronically deafened groups achieving median ratios (i.e. ratio of the response sizes of the last and first responses of the burst) near 1 (1.04 ± 0.05 and 1.02 ± 0.01, respectively, median ± variance) (figure 5(C)). In contrast, the amplitude of responses to optogenetic stimulation diminished during the burst, resulting in a ratio of 0.36 ± 0.08 in the acutely deaf mice and 0.54 ± 0.07 in the chronically deafened mice. These ratios were significantly lower than the ratio for electrical stimulation within the same group (p < 0.001, Wilcoxon–Mann–Whitney test, n = 17 for electrical, n = 13 for optogenetic) but were not significantly different to each other (p = 0.09, Wilcoxon–Mann–Whitney test, n = 21 for acutely deafened mice, n = 13 for chronically deafened mice). The ratio of hybrid responses followed a similar trend to temporal spread, with increasing optogenetic power eliciting responses with decreasing ratios, except at very high optogenetic stimulation levels.
Hybrid responses in acutely deafened animals appeared to demonstrate both good temporal fidelity and precision when optogenetic powers were close to activation threshold, whereas chronically deafened animals showed the best outcomes at higher powers (<15.5 dB). This trend was consistent across subthreshold and suprathreshold electrical stimulation.
Although combined stimulation could elicit hybrid responses at subthreshold levels of electrical stimulation, the degree of facilitation at 100 pps was much lower than that observed at 4 pps (data not shown). Subthreshold hybrid responses were observed in all animals but could not be elicited at levels lower than 55 µA below threshold.
Hybrid responses at subthreshold electrical stimulation levels typically evoked fewer responses across the burst than their supra-threshold counterparts. In this context, these responses rarely exceeded the temporal fidelity of their supra-threshold counterparts. However, at optogenetic stimulation levels close to threshold, these subthreshold electrical hybrid responses demonstrate good temporal precision, similar to that of their suprathreshold counterparts (figure 5).
Following final electrophysiology experiments, chronically deafened cochleae were processed to confirm exposure to neomycin sulphate under isoflurane anaesthesia induced hair cell loss as effectively as under the injectable anaesthesia used for the data in figure 1. Histology confirmed complete hair cell ablation in five of the six mice, with only one cochlea showing 42 remaining IHCs in the basal hook-turn region (data not shown).
2.5. Temporal precision of single units
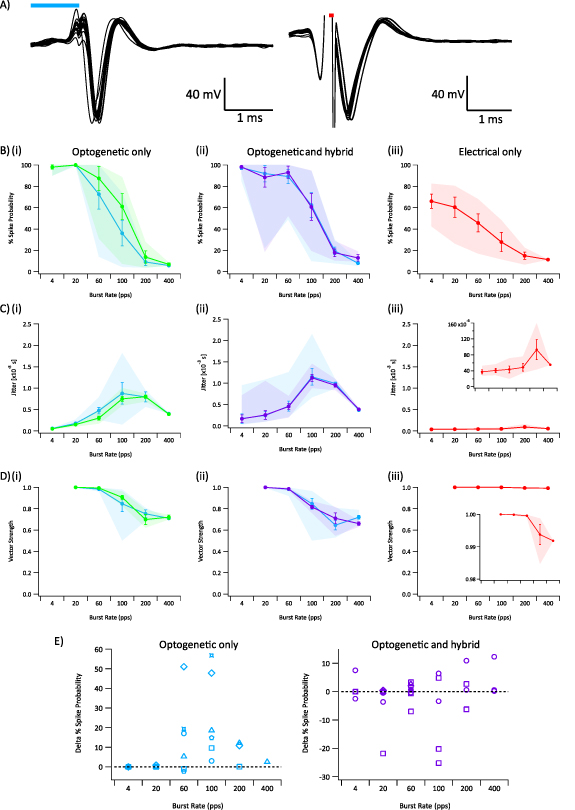
To gain deeper insight into the temporal properties of the three stimulation modalities, we conducted single unit recordings of the auditory nerve in acutely deafened mice. Glass micropipettes were inserted through the brain towards the auditory nerve where it enters the dorsal cochlear nucleus (DCN) and ANs identified by their response to optogenetic or electrical stimulation. Optogenetic-only (1 ms), electrical-only (58 µs or 230 µs), or hybrid stimuli were presented as a burst over 250 ms at rates of 4–400 pps at 1–2 Hz. Power levels presented were relative to threshold, defined as the power level that resulted in 80% spike probability at 4 pps.
Generally, spike probabilities in response to suprathreshold optogenetic stimulation decreased with increasing stimulation rate. Temporal precision also diminished, as evidenced by increased jitter and decreased vector strength, at high stimulation rates. Optogenetic responses demonstrated a sharp drop off in spike probability and increased temporal precision around 100 pps even at very suprathreshold power levels. Increasing the power of optogenetic stimulation by 2–3 dB improved the average spike probability at 100 Hz (p < 0.05; RM-ANOVA, n = 6) (figure 6(E)), but did not significantly improve temporal precision.
Figure 6. (A) Example auditory neuron responses to suprathreshold optogenetic (left) and electrical (right) stimuli. Blue and red bars indicate the optogenetic and electrical stimulus respectively. (B) Spike probability of single unit auditory neurons in response to (i) suprathreshold optogenetic stimuli (blue line = lower optogenetic stimulation, green line = higher optogenetic stimulation), (ii) hybrid and suprathreshold optogenetic stimuli (blue line = suprathreshold optogenetic-only, purple line = combined suprathreshold optogenetic and subthreshold electrical stimulation), (iii) threshold electrical stimulation. (C) Jitter of single unit auditory neurons, i.e. the variance of spike times. (D) Vector strength, i.e. synchronicity. (B)–(D) Shaded regions indicate minimum and maximum, line indicates average, error bars represent standard error mean. (E) Difference in spike probabilities between (left) low and high suprathreshold optogenetic stimulation, and (right) suprathreshold optogenetic stimulation and combined suprathreshold optogenetic and subthreshold electrical stimulation. Markers indicate same neuron. Optogenetic stimuli ranged 5–23 dB relative to threshold. Electrical stimuli ranged −5–0 dB relative to threshold.
Download figure:
Standard image High-resolution imageElectrical-only stimulation at threshold resulted in significantly greater temporal precision than optogenetic-only at stimulation rates between 20–200 pps (jitter: p < 0.005, vector strength: p < 0.05; two-tailed t-test, n = 5 for electrical, n = 6 for optogenetic). Even at the low electrical stimulation levels used here, the mean spike probability was similar to optogenetic stimulation at high stimulation rates. For example, the mean spike probability for threshold electrical stimulation at 200 pps was 14.9 ± 3.4%, compared to 13.7 ± 5.8% for suprathreshold optogenetic stimulation.
A subset of neurons were stimulated with both optogenetic-only and hybrid (using suprathreshold optogenetic and subthreshold electrical) stimulation, allowing direct comparison of measures. We observed an improvement in the spike probabilities in a subset of these neurons in response to hybrid stimulation compared to optogenetic-only stimulation (figure 6(E)), but not an improvement in temporal precision. The degree of improvement was dependent on the optogenetic power used, with the most improvement seen at lower optogenetic stimulation powers.
2.6. Histological evidence of nerve degeneration
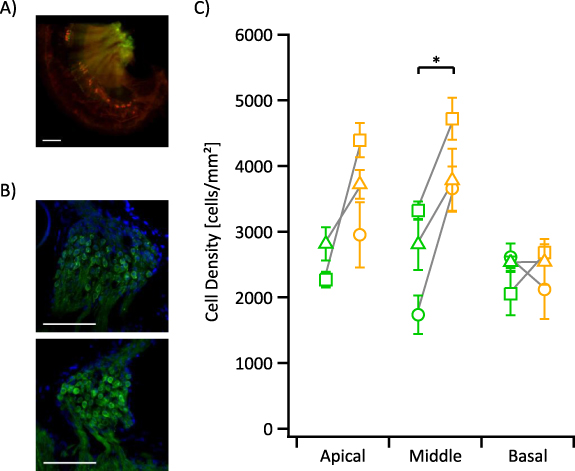
Following electrophysiology experiments, we confirmed deafening in the chronic deaf mice via whole mount preparations (cochlea pairs n = 2) or whole cochlea imaging (n = 3). Whole mount preparations and whole cochlea imaging showed no surviving inner or outer hair cells except for one animal, which had 42 surviving IHCs in the basal hook turn region (figure 7(A)).
Figure 7. (A) Whole mount dissections show the surviving basal inner hair cells (red) observed in a single animal. (B) Representative images of mid-modiolar cryo-sections show the cell bodies of auditory neurons (green) in Rosenthal's canal in the middle turn for the left deafened ear (top) and right control ear (bottom). (C) Average cell densities in the left deafened ears (green) and right control ears (yellow) of chronically deafened animals for each cochlear turn. Error bars indicate standard error mean. Scale bar 100 µm. Green = ChR2-H134R-EYFP. Red = myosin VIIa. Blue = DAPI; labelling of nuclei. Asterisk (*) indicates significance (p < 0.05; paired t-test).
Download figure:
Standard image High-resolution imageAN densities in left (deafened) and right (control) ears were evaluated in Rosenthal's canal of the apical, middle, and basal turns (averaged over 2–4 mid-modiolar sections per turn per animal; n = 3). There was significant degeneration of ANs in the middle turn Rosenthal's canal of deafened ears compared to the control ears (p < 0.05; paired t-test). Although degeneration was also evident in the apical turn of deafened ears, the reduction in AN density was not significant due to the small sample count, as the apical turn of one animal could not be assessed due to severe fibrosis. The remaining animals showed only minor fibrosis near the site of fenestration for the deafening procedure. No difference in AN density between deafened and control ears was observed in the basal turn (figure 7(C)).
3. Discussion
We characterised and compared responses of the auditory nerve in acutely deafened and chronically deafened mice to three modalities of stimulation: optogenetic, electrical, and hybrid stimulation, focussing on the temporal precision of the evoked responses.
3.1. Hair cell inactivation
This study is the first to examine optogenetic responses in a model of chronic deafness with near-complete inactivation of both inner and outer hair cells, ensuring no indirect activation of the auditory nerve via the hair cells. Previously published techniques, such as acoustic deafening and systemic aminoglycoside treatments (e.g. intraperitoneal kanamycin injections) achieve incomplete deafening, with many IHCs remaining at safe doses [29–32]. By directly perfusing the aminoglycoside neomycin sulphate directly through the left cochlea over 20–30 min, an extended time-period compared to our previous study [20], both inner and outer hair cells were effectively inactivated. Despite the unilateral ablation of hair cells in the left cochlea, ABR recordings indicated responses at high acoustic power (figure 1(B)). This is likely due to contamination from responses from the non-deafened ear, despite occlusion of the ear canal. Consequently, hearing thresholds presented here likely underestimate the severity of deafening. Deafening under isoflurane anaesthesia was less effective than deafening under combined ketamine/xylazine anaesthetic, consistent with a previous study that demonstrated the protective effects of isoflurane [33]. We only observed surviving hair cells in the basal region of one animal, and these hair cells showed gross morphological changes, suggesting their contribution to the recorded responses was negligible. Even allowing for the known protective effects of isoflurane anaesthesia, direct perfusion of neomycin was effective at mitigating hair cell mediated responses to stimuli, providing an effective model to test auditory nerve responses with little impact of hair cell mediated responses.
Additionally, this deafening technique was sufficient for the development of nerve degeneration in the 2–3 weeks following treatment. We observed substantial loss of auditory neuron cell bodies in Rosenthal's canal in the apical and middle turns, but not the basal turn. This aligns with the observation of surviving hair cells in the basal region of the cochlea, indicating that the sensory hair cells and neurons in this region are slower to degenerate following aminoglycoside exposures.
3.2. Facilitation
A major limiting factor of cochlear implant user outcomes is poor spatial precision of electrical stimulation. Previous studies have shown that in combining electrical and optogenetic stimulation, both the spread of activation and energy needs can be minimised [20, 34]. These studies investigated combined stimulation in the cochlea using in vitro techniques or in vivo recordings from the auditory midbrain. The results presented here are the first to investigate hybrid stimulation at the level of the auditory nerve in vivo. Our results confirm that optogenetic and electrical stimulation can combine at the level of the auditory nerve and evoke responses from individual neurons. Similar to results from the auditory midbrain and in vitro auditory nerve culture, we found the addition of optogenetic stimulation reduces the amount of electrical charge needed to activate the auditory nerve. Although we did not measure the spatial precision in this study (rather, we intentionally maximised the region of activation of our separate stimuli), a previous study using similar stimulation equipment established that combined stimulation with a more focused optical stimulus can improve the spatial precision of activation over electrical and optogenetic stimulation alone by reducing the electrical charge used to activate the auditory nerve [20].
We observed a clear difference between the degree of facilitation in acutely deafened and chronically deafened mice, with acutely deafened mice demonstrating a significantly greater increase in facilitation. This is most likely a consequence of the degenerating auditory nerve resulting from chronic deafness. Indeed, a study of the auditory nerve following a 10 d regime of intraperitoneal aminoglycoside deafening treatment in guinea pigs showed a significant decrease in auditory neuron density within 7 d of completing treatment [35].
How degeneration of the auditory nerve leads to poorer hybrid facilitation is not clear. One hypothesis is that there is reduced opsin expression and/or trafficking to the plasma membrane. Assuming that the mechanism of facilitation depends on the optogenetically-induced depolarising current, reduced plasma membrane expression would decrease the resulting optogenetic depolarisation, and thus the probability of facilitation. We expect this would also lead to an increase in the optogenetic activation threshold, which was not observed. Alternatively, degeneration of peripheral dendrites following chronic deafness has been shown to move the site of electrical activation centrally [21–24], where channel expression and myelination may be sufficiently different to impact the overlap of optogenetically and electrically induced depolarising currents. Studies investigating voltage gated ion channel distribution across auditory neurons show that ion channels are distributed evenly along the unmyelinated peripheral dendrites, but are localised at the nodes of Ranvier along the myelinated central axon [36, 37]. Although opsins show similar spread of expression across dendrites, they do not appear to show any localisation to the nodes of Ranvier in auditory neurons. This disparity in expression of opsins and voltage gated ion channels along the central axon may reduce the overlap between optogenetically induced and voltage gated ion channels, and thus reduce facilitation. A third hypothesis is that degeneration may impact the ability of the neuron to restore its resting potential following optogenetic stimulation. Natively evoked depolarising currents along the axon of a neuron rely on the controlled flow of sodium and potassium ions across the cell membrane. In contrast, the opsin used in this study, ChR2-H134R, is a non-selective cation channel, permitting the flow of calcium, sodium, potassium, and hydrogen ions across the cell membrane. This abnormal ion flow may take longer to restore in a degenerated neuron than in a healthy neuron.
Our results provide preliminary insight into the effects of degeneration on optogenetic responses of the auditory nerve. Although previous studies have demonstrated the changes in the auditory nerve response to electrical stimulation following chronic deafening [21–24], this study presents the first examples of optogenetic responses in such a model. Further research into the mechanism linking degeneration and optogenetic stimulation outcomes will be useful towards the clinical application of optogenetics and hybrid stimulation as novel stimulation strategies for next generation cochlear implants.
3.3. Spike probabilities
Traditionally, electrical stimulation has been the primary means of stimulation for neuroprostheses due to its safety and reliability in evoking responses from neural tissue, especially at high stimulation rates [38]. In contrast, optogenetic stimulation is unable to achieve consistent responses at high rates due to the limited kinetics of opsins [19, 39]. Efforts to engineer opsins and improve trafficking of opsins to the plasma membrane have demonstrated improvements to nerve responses at high stimulation rates [19]. These improvements, however, have not been sufficient to achieve good spike probability at the rates of contemporary cochlear implants (e.g. 400 pps or greater).
A previous study investigating hybrid stimulation found adding subthreshold electrical stimulation to suprathreshold optogenetic stimulation improved entrainment rates at the level of the auditory midbrain (inferior colliculus) compared to optogenetic stimulation alone [20]. However, the responses evoked purely from the interaction of the combined stimulation (i.e. hybrid responses) could not be extracted from responses evoked by electrical-only nor optogenetic-only stimulation alone. Using compound action potential recordings, we were able to investigate the stability and fidelity of hybrid responses from the auditory nerve. In contrast to the Thompson et al [20] study, responses evoked using suprathreshold optogenetic stimulation and subthreshold electrical stimulation demonstrated poor fidelity, but good stability compared to optogenetic-only stimulation. On the other hand, suprathreshold electrical stimulation with subthreshold optogenetic stimulation could achieve both better stability and following ability than optogenetic-only stimulation. Single unit recordings confirmed this finding in a subset of neurons, however, the improvement to temporal precision and spike probability was limited. Further research to uncover the precise mechanism of hybrid facilitation is critical to unlock further improvements in this stimulation paradigm.
The probability of evoking sustained responses in chronic deaf mice was generally lower with compound action potentials showing poor percentage of elicited responses. This appears to be a consequence of prolonged deafness, affecting the ability of the nerve to sustain responses to optogenetic stimulation. As discussed above, the precise mechanism of this remains unclear.
Additionally, optogenetic responses in four of the six chronically deafened mice showed an increased latency and decreased response size when retested following 2–4 h of combined stimulation. Similar behaviour was seen in the acutely deafened animals, although only a subset was retested. The cause of this change is unclear but may be due to a number of factors including the surgical procedure or opsin deactivation following over exposure to light as seen with other opsins [40, 41].
3.4. Temporal precision
Not only high stimulation rates, but also high temporal precision is necessary to encode the fine temporal information of pitch and sound quality. There has been little success to date in achieving the temporal precision of electrical stimulation using optogenetic stimulation techniques. Indeed, in our study we observed a similar outcome, with optogenetic stimulation performing poorly at all measures of temporal precision compared to electrical stimulation, reflecting the slower kinetics of the opsin mediated responses. The opsin used in this study, ChR2-H134R, has relatively slow kinetics compared to other excitatory opsins, with many other opsins exhibiting much faster kinetics and better temporal precision [42]. However, even these 'rapid' opsins demonstrate poor temporal precision compared to electrical stimulation. In our study, we found hybrid responses demonstrated electrical-like temporal precision when the concurrent optogenetic stimulus was near threshold but worsened with increasing optogenetic stimulus power. Indeed, there appears to be a trade-off between facilitation and temporal precision, suggesting the best outcomes can be achieved at an intermediate level that both facilitates electrical activation without sacrificing temporal precision. The decreased temporal precision of hybrid responses with increasing light power suggests the optogenetic stimulation and opsin kinetics dominates the activation of the nerve at these higher powers.
Surprisingly, the responses of chronically deafened animals appeared to have better temporal precision compared to acutely deafened animals. This is possibly a reflection of the poorer ability of the nerve to sustain responses to the stimulus (i.e. reduced percentage of elicited responses) thus reducing the sample size, and/or reduced hybrid facilitation. Nevertheless, both good temporal precision and temporal fidelity could be achieved in chronically deafened animals, but required the use of higher optogenetic stimulation powers compared to the acutely deafened animals. This is likely a reflection of the poor facilitation seen in these animals.
In our single-unit recordings, we found optogenetic and hybrid stimulation paradigms resulted in increased jitter relative to electrical stimulation. Although this is described as poor temporal precision, jitter is normal in acoustic auditory nerve responses and may be an important cue for sound localisation. Further to this point, studies have implicated jitter as important for detection of interaural time differences and sound localisation in cochlear implant users [43–45].
3.5. Limitations and clinical translation
In this study, we directed our optical fibre towards the modiolus, illuminating as much of the auditory nerve as possible to improve the likelihood of overlap with the region of electrical stimulation, and hence the probability of successfully eliciting responses to hybrid stimulation. This simplified investigation of the temporal properties of the auditory nerve response to the three modes of stimulation. However, in a clinical translation of this technique, one would try to achieve a narrow region of activation. A previous study investigated the spread of activation of optogenetic, electrical, and hybrid stimulation in the cochlea using measures from the inferior colliculus. Hybrid stimulation not only achieved a reduced spread of activation compared to electrical and optogenetic stimulation, but also an improvement in the entrainment rates compared to optogenetic stimulation. Hence, as long as the region of overlap between the two stimuli—optogenetic and electrical—is sufficient, then hybrid stimulation can achieve both high spatial and temporal precision when the optogenetic stimulus is near activation threshold.
We only recorded compound action potential responses at a single rate of 100 pps, even though contemporary cochlear implants typically use rates of 400 pps or more. We were limited by the ability to consistently extract the auditory nerve response from the electrical artefact at rates higher than 100 pps. It would be ideal to test the temporal properties of the nerve to the three stimulation modalities at clinically relevant rates, but this was not possible with our setup, nor the opsin we used. Indeed, even the fastest opsins to date are unable to maintain good spike probability at rates beyond 100 pps, and this remains the limiting factor of optogenetic and hybrid stimulation.'Rapid' opsins with faster kinetics may combine with electrical stimulation to achieve even higher temporal precision. Future studies should consider the use of a variety of opsins to understand the effect of kinetics on the spatial and temporal precision of hybrid stimulation.
The transgenic mouse model used in this study expressed opsin in all auditory neurons, allowing for optogenetic and hybrid activation. However, clinical translation of optogenetic or hybrid stimulation would require the use of gene therapy techniques to deliver the opsin gene to the auditory nerve. Unfortunately, such techniques do not achieve complete transfection of the nerve, leaving some neurons that do not express the opsin and are incapable of optogenetic and hybrid responses. Indeed, a previous study demonstrated that hybrid stimulation did not have any consistent effect on the spatial precision, although subthreshold optogenetic stimulation did reduce the electrical activation threshold [46]. Techniques to improve transfection rates of gene therapy are likely to benefit not only optogenetic stimulation but also hybrid stimulation. Nevertheless, a hybrid stimulation technique would still allow for electrical activation of non-transfected neurons, and so does not necessarily limit the use of these neurons.
Finally, auditory nerve responses were recorded under anaesthesia, which has been previously shown to impact the behaviour of nerves and the auditory system [47–49]. As such, it is not clear how the results seen here will translate in the awake animal. Development of reliable recording techniques for awake animals will greatly improve our understanding of auditory nerve behaviour and be imperative in the investigation of hearing loss and alternative stimulation strategies.
4. Conclusion
Optogenetic and hybrid stimulation have both been demonstrated as potential alternatives to electrical stimulation of cochlear implants, overcoming the broad activation of the auditory nerve which limits user outcomes. However, optogenetic stimulation alone has many limitations for auditory processing, including low fidelity and temporal precision at high stimulation rates as shown in this and other studies. Hybrid stimulation—i.e. using both optogenetic and electrical stimulation concurrently—improves upon the limited temporal precision of optogenetic stimulation inherent from its reliance on opsins. Further investigation into the exact cause of these limitations, such as opsin kinetics, conductance, and expression quantity and quality, would be very valuable towards the advancement of optogenetics towards clinical use.
Although there is a lot of evidence available to understand how the chronic deaf condition impacts electrical stimulation, this is the first study to examine the performance of optogenetic and hybrid stimulation in a degenerated cochlea. We demonstrated that chronic deafness reduces facilitation in a hybrid stimulation paradigm compared to acute deafness. High temporal fidelity and precision can be achieved in both acutely and chronically deafened animals using a hybrid stimulation paradigm, although chronically deafened animals required higher power levels of concurrent optogenetic stimulation.
5. Methods
- Animals
Heterozygous ChR2-H134R-EYFP transgenic mice (n = 12 female, n = 16 male) were produced by breeding COP4-H134R/EYFP mice (Jax strain 012569: B6;129SGt(ROSA)26Sortm32(CAG COP4*H134R/EYFP)Hze/J [50]; backcrossed onto a C57BL/6 background) with Cre-parvalbumin mice (Jax strain 008069: B6;129P2-Pvalbtm1(cre)Arbr [51]). Use and care of experimental animals were approved by St. Vincent's Hospital Animal Ethics Committee (#18-003, #21-007), and followed the Guidelines to Promote the Wellbeing of Animals used for Scientific Purposes (2013), the Australian Code for Care and Use of Animals for Scientific Purposes (8th edition, 2013), and the Prevention of Cruelty to Animals Amendment Act (2015).
- Deafening procedure
All mice were unilaterally deafened prior to all electrophysiological recordings. Mice were placed under anaesthesia (gaseous isoflurane or combined ketamine/xylazine, details in the relevant methods sections) on a heat pad to maintain temperature at 37 °C. 1% lignocaine hydrochloride solution (v/v; 0.1 ml) was applied subcutaneously to the surgical site. A left side post-auricular incision was made and the soft tissues were dissected to expose the bulla. A scalpel was used to drill through the bulla, and forceps used to widen the bullostomy and expose the cochlea and round window. Care was taken to ensure the ossicle chain was kept intact, such that mechanical transduction of acoustic stimuli may still be transmitted via the middle ear. A hole was drilled into the middle-apex turn of the cochlea using a sharp probe. Mice were deafened by perfusing 10–15 µL of 10% neomycin sulphate (w/v) in 9% saline solution (w/v) through the round window while aspirating gently from the drilled hole over 20–30 min. Mice continued on to recordings immediately or 2–3 weeks following deafening, according to the experiment (details in the relevant methods sections).
- ABR recordings
The hearing status of adult mice (average age 78.8 ± 14.3 d old, range 55–90 d; n = 4; two male, two female) was first established using tone pip (8, 16, 32 and 36 kHz) and click-evoked ABRs under combined ketamine/xylazine anaesthetic (100 mg kg−1 ketamine, 10 mg kg−1 xylazine, 0.1 mL/10 g mouse administered intraperitoneally). Animals then underwent the deafening procedure (described above), followed by additional ABR recordings one hour after deafening (n = 4) and 2 weeks post deafening (n = 3). ABRs were recorded using subcutaneous electrodes positioned at the scalp, behind the left pinna and at the tail. The surgical site was closed with internal and external sutures at the conclusion of the procedure. Following final ABRs at 2 weeks post deafening, the cochleae of mice were extracted for histology.
- Micro-CT
The positioning of the optical fibre within the cochlea was imaged using micro-CT. A bare optical fibre (105 µm core, NA 0.22) was inserted 0.5–1.0 mm into the round window, directed towards the modiolus, and secured with cyanoacrylate. The mouse was terminated (cervical dislocation) and the cochlea extracted. The cochlea was fixed in 10% (v/v) NBF for 2 h and rinsed in phosphate buffered solution (PBS). The cochlea was positioned in a microcentrifuge tube filled with PBS and stoppered with gauze to prevent movement of the cochlea during micro-CT imaging (SkyScan 1276, Bruker). Images were taken at 0.004° intervals with 2.94 µm pixel size. The images were reconstructed (nRecon, Bruker) and the position of the optical fibre visualised (Ctvox, Bruker).
- Inferior colliculus recording
Immediately following deafening (described above), the surgical site was temporarily plugged with a saline soaked cotton ball, and the mouse placed in a stereotaxic frame. An incision was placed along the midline of the scalp, and skin displaced to expose the skull. A craniotomy was performed on the region overlying the contralateral inferior colliculus, and the dura gently removed using a sharp needle and forceps. A single shank multi-channel recording array (NeuroNexus Technologies, MI, USA) with 50 µm spacing between electrodes was positioned over the inferior colliculus and inserted using a micropositioner (David Kopf Instruments, USA) whilst monitoring responses along the recording array under optogenetic stimulation. Once positioned, a 1% (w/v) agar solution was applied over the insertion area and exposed brain to provide stability to recordings. The array was grounded through a steel needle in the right axillary region. A speaker was positioned approximately 10 cm from the right pinna, and multiunit responses to tone pips (100 ms, 1–52 kHz) were recorded. Following this, an optical fibre (105 µm core, NA 0.22) bonded to a platinum wire (100 µm, polytetrafluoroethylene insulated) was inserted into the round window and a second platinum wire inserted into the apical fenestration (created during the deafening procedure). Responses to optogenetic stimulation (1 ms, 0–32 mW), and electrical stimulation (25 µs phase, 8 µs interphase gap, 0–1.6 mA) in the left ear were recorded.
- Compound action potential recordings
Compound action potentials of the auditory nerve in response to light, electrical and hybrid stimulation were recorded in acutely or chronically deafened adult mice (acute: 66 ± 7 d old, two female, six male; chronic: 43 ± 4 d old, four female, two male). Mice were placed under gaseous isoflurane anaesthesia (1.5%–3%) and deafened via perfusion of neomycin sulphate through the cochlea, as described in the deafening procedure methods section. Deafening was conducted immediately before or 2–3 weeks prior to recordings, forming the acute deaf and chronic deaf groups, respectively. At the end of the deafening procedure, the round window and the hole drilled into the middle-apex turn were sealed with soft tissue dissected from the surrounding muscle for mice in the chronic deaf group, and internal and external sutures used to close the wound. On the day of recordings, the left cochlea of chronic deaf animals was re-exposed, scar tissue removed, and the middle-apex hole re-pierced. Following cochlea exposure (and deafening for the acute deaf group), the surgical site was temporarily plugged with a saline soaked cotton ball, and the mouse placed in a stereotaxic frame. Light pulses (1 ms, 452 nm) were delivered by an optical fibre (105 µm core, NA 0.22) inserted 0.5–1.0 mm into the round window. Electrical stimuli were delivered as biphasic current pulses (58 µs) delivered as wide bipolar across two 100 µm platinum wires inserted into the cochlea (round window and hole in the middle-apex turn). To improve the facilitation of electrical activation using optogenetic stimulation, current pulses were delayed by 942 µs so as to finish at the same time as the light pulse [20] (figure 2(B)). Stimuli were presented at 4 pps separately (electrical-only or optogenetic-only) or concurrently (combined). Combined stimuli were presented using a single power level of light pulses relative to the threshold for optogenetic stimulation (i.e. below threshold, at threshold (0 dB) or above threshold (1–26 dB)), while varying the electrical current.
Responses to stimuli were recorded using a 100 µm platinum wire positioned within the bulla between the stapedial artery and round window, and a steel needle inserted subcutaneously at the back of the neck and grounded through a steel needle in the right axillary region. Recordings were amplified 100× and recorded using custom software in Igor Pro (Wavemetrics, USA). Recordings were averaged over 50–100 repetitions and bandpass filtered (500–3000 Hz) before analysis. To reduce the size of the electrical artefact, electrical stimuli were presented as wide bipolar stimulation across the two platinum wires inserted into the cochlea, with half of recordings using the round window platinum wire as the stimulating electrode, and the other half using the round window platinum wire as the return electrode. This procedure was conducted for both electrical-only and combined stimulation recordings.
Activation thresholds were identified as the minimum power level to elicit a visually recognisable response. Hybrid responses were isolated from recordings by subtracting the optogenetic-only and electrical-only responses at the equivalent levels, prior to determination of thresholds.
- Single unit auditory nerve fibre recordings
Adult mice (average age 62.3 ± 4.2 d old, range 57–68 d; n = 6; threemale, three female) were placed under gaseous isoflurane anaesthesia (1.5–3%) on a heat pad to maintain body temperature at 37 °C. Mice were deafened according to the deafening procedure described previously. The surgical site was temporarily plugged with a saline soaked cotton ball, and the mouse placed in a stereotaxic frame. An incision was placed along the midline of the scalp, and skin displaced to expose the ear canals. Using microscissors the ear canals were cut close to the skull, and ear bars were inserted. The muscles overlying the parietal and interparietal bones were dissected and a small drill used to perform a craniotomy of the left interparietal bone. Sharp forceps were used to remove the dura mater underlying the skull, and gentle suction then applied to expose the DCN. Bleeding was controlled through the placement of cotton balls, and saline used to flush the site to prevent clotting from settling over the surface of the brain. The stereotaxic frame was rotated, and an optical fibre (105 µm core, NA 0.22) bonded with platinum wire (100 µm diameter) was inserted 0.5–1 mm into the round window for optogenetic and electrical stimulation, respectively. A steel needle inserted into the left axillary region formed the return electrode for electrical stimulation. A quartz glass micropipette (OD 1.0 mm, ID 0.7 mm) filled with 3 M KCL was inserted through the DCN towards the auditory nerve as optogenetic or electrical stimuli were applied. Single units were characterised as auditory nerve fibres if they demonstrated latencies less than 1 ms following stimulus completion.
Spike counts were recorded in the window immediately post stimulus until the start of the next stimulus. For electrical stimuli, the window was shortened to prevent overlap with stimulation artefacts.
- Stimulus waveforms
Stimulus waveforms were generated using custom software interfacing with Igor Pro (Wavemetrics, USA). Light stimuli were delivered via a custom 473 or 452 nm laser (OptoTech, Melbourne, Australia) coupled to the optical fibre by an FC connector. Light was presented as 1 ms pulses at 0–35 mW intensities. The laser was calibrated using a photodiode (PDA36A2, Thorlabs) connected via optical fibre. Electrical stimuli were delivered by a custom-built stimulator connected to the platinum wire, with a return electrode placed subcutaneously in the axillary region or the hole drilled in the cochlea (details in the relevant methods section). Biphasic current pulses with a phase of 25 µs or 100 µs (interphase gap of 8 µs and 30 µs, respectively) were delivered over a range of current levels (0–2 mA).
For hybrid stimulation, electrical stimuli were delayed with respect to the start of the optical pulse such that the end of the electrical pulse aligned with the end of the optical pulse.
- Histology
Following terminal recordings, mice were euthanised via cervical dislocation and the left and right cochleae were dissected and placed in 10% neutral buffered formalin for a minimum of 2 h. Following fixation, cochleae were rinsed in PBS and decalcified in ethylenediaminetetraacetic acid (0.12 M, EDTA) for 2–3 d. Cochleae were then embedded in optimum cutting temperature (Tissue-Tek, Saruka, Japan) and proceeded to either be snap-frozen for cryo-sectioning, or prepared for whole mount dissections. For the cochleae frozen for cryo-sectioning, 12 µm serial sections were collected through the entire cochlea on the NX70 Cryostar (Erpedia, MI, USA). Mid-modiolar sections were immunostained using standard immunofluorescent protocols using rabbit myosin VIIa (1:250, Proteus Biosciences, 25–6790) for hair cells and mounted with DAPI to visualise nuclei. Photomicrographs of the hair cells and CHR2-H134R protein present in auditory neurons (visualised via the EYFP tag) were taken from a fluorescent microscope and processed using ImageJ (National Institute of Mental Health, Maryland, USA). Cell body densities were assessed in Rosenthal's canal on 6–11 sections per turn (apical, middle, and basal).
The cochlea prepared for whole mount dissections were quickly snap frozen and bisected along the mid-modiolar plane. Half turns were then dissected from the bisections with the otic capsule, Reisner's membrane, and tectorial membrane removed with fine forceps [52]. Dissected cochlea pieces were then stained with standard immunofluorescent protocols using the same antibodies as above. Images of these turns were taken on the fluorescent microscope (Carl Zeiss, Germany), and inner and outer hair cells were quantified along the cochlea.
- Analysis and statistical tests
Numerical results stated are average ± standard error mean, except for box plot measures which state median ± interquartile range.
ABR recordings: statistical tests for threshold shifts used RM-ANOVA.
CAP recordings: statistical tests for change in electrical activation threshold used RM-ANOVA (within group) or two-tailed t-test (across group). Statistical tests for rapid stimulation measures (percentage of elicited responses, temporal spread, and response size ratio) used the Wilcoxon–Mann–Whitney test (two tailed).
Single-unit recordings: statistical tests use RM-ANOVA (within group) or two-tailed t-test (across group).
Neuron density: Statistical tests use two-tailed t-test across left and right sides.
Data availability statement
The data cannot be made publicly available upon publication because the cost of preparing, depositing and hosting the data would be prohibitive within the terms of this research project. The data that support the findings of this study are available upon reasonable request from the authors.
Funding
The research in this publication was supported with funding from the National Health and Medical Research Council (NHMRC) GNT#2002523 and by an Australian Government Research Training Program (RTP) Scholarship. The Bionics Institute acknowledges the support they receive from the Victorian Government through its Operational Infrastructural Support Program.