Abstract
Objective. Lack of sensation from a hand or prosthesis can result in substantial functional deficits. Surface electrical stimulation of the peripheral nerves is a promising non-invasive approach to restore lost sensory function. However, the utility of standard surface stimulation methods has been hampered by localized discomfort caused by unintended activation of afferents near the electrodes and limited ability to specifically target underlying neural tissue. The objectives of this work were to develop and evaluate a novel channel-hopping interleaved pulse scheduling (CHIPS) strategy for surface stimulation that is designed to activate deep nerves while reducing activation of fibers near the electrodes. Approach. The median nerve of able-bodied subjects was activated by up to two surface stimulating electrode pairs placed around their right wrist. Subjects received biphasic current pulses either from one electrode pair at a time (single-channel), or interleaved between two electrode pairs (multi-channel). Percept thresholds were characterized for five pulse durations under each approach, and psychophysical questionnaires were used to interrogate the perceived modality, quality and location of evoked sensations. Main results. Stimulation with CHIPS elicited enhanced tactile percepts that were distally referred, while avoiding the distracting sensations and discomfort associated with localized charge densities. These effects were reduced after introduction of large delays between interleaved pulses. Significance. These findings demonstrate that our pulse scheduling strategy can selectively elicit referred sensations that are comfortable, thus overcoming the primary limitations of standard surface stimulation methods. Implementation of this strategy with an array of spatially distributed electrodes may allow for rapid and effective stimulation fitting. The ability to elicit comfortable and referred tactile percepts may enable the use of this neurostimulation strategy to provide meaningful and intuitive feedback from a prosthesis, enhance tactile feedback after sensory loss secondary to nerve damage, and deliver non-invasive stimulation therapies to treat various pain conditions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Sensory feedback plays an integral role in everyday function, including planning and control of even simple movements, such as grasping an object [1]. Loss of sensory function caused by a life-changing event such as amputation after limb trauma or peripheral neuropathies after nerve injury can have substantial effects on work, leisure, social life, and daily living activities as well as on psychological well-being. Individuals with upper limb amputation may use a myoelectric prosthesis. However, despite recent technological advances, commercial prostheses are still limited in their ability to provide direct sensory feedback to users [2], thereby requiring an increased reliance on visual cues and attentional demand from the user [2]. This limitation can result in functional deficits and may hinder prosthesis embodiment, leading to dissatisfaction and abandonment of the prosthesis. Hence, sensory feedback is one of the most desired design priorities independent of the type of prosthesis and level of limb loss [3]. The provision of sensory feedback may enable the user to perform activities of daily living with enhanced control of the prosthesis [2, 4], and may also improve quality of life by promoting embodiment of the prosthesis [5, 6].
Several artificial feedback strategies have been explored to address the loss of sensory function. The simplest approach is to use non-invasive sensory substitution techniques such as mechanical [7, 8] or electro-tactile [9, 10] stimulation. These techniques activate cutaneous receptors on the user's skin to encode the missing sensory information (e.g. grasp force). This feedback is non-intuitive due to percept modality and location mismatch, thus increasing the user's cognitive load and response time [8, 11]. Alternatively, electrical stimulation of peripheral nerves can leverage existing neural feedback pathways to evoke somatotopically-matched, distally referred sensations. Implantable neuromodulation systems have been used to activate sensory fibers in the median and ulnar nerves to evoke graded distally referred tactile and proprioceptive sensations in the phantom hand of individuals with amputation [12–14]. These direct stimulation methods are characterized by high selectivity and sensation quality features that facilitate the delivery of more intuitive sensory feedback from prosthetic limbs. However, the invasive nature of device implantation procedures is not acceptable to all [15].
Non-invasive electrical stimulation uses surface electrodes applied on the skin to deliver transcutaneous electrical pulses to activate peripheral nerves. Earlier studies have shown that single-channel (SC) surface stimulation can be used to elicit distally referred sensations when targeting the median and ulnar nerves at the forearm [16] or at the elbow level [17]. However, standard methods for SC stimulation are hampered by poor selectivity and localized discomfort associated with large charge densities [16–19]. We hypothesized that surface stimulation could be used to elicit enhanced tactile percepts while avoiding the discomfort associated with localized charge densities by implementing a novel channel-hopping interleaved pulse scheduling (CHIPS) strategy. This multi-channel (MC) stimulation approach delivers interleaved current pulses from independent stimulation channels; i.e. stimulation hops across multiple strategically distributed surface electrodes. During CHIPS, stimulation pulses are scheduled to be delivered sequentially from different sources, with no overlap or additional temporal separation (i.e. one right after the other). By leveraging the combined influence of the interleaved current pulses, each independent channel could be set to stimulate at shorter pulse widths than SC stimulation, thus reducing the total charge per pulse delivered by any given electrode while maintaining net charge delivery to the target nerve at functional levels. In other words, as two short pulses are delivered sequentially at different locations on the skin, the target fibers in the nerve would experience one longer pulse. As a result, the stimulation would be sub-threshold for cutaneous activation near each electrode, but supra-threshold at the level of the nerve due to the spatiotemporal summation of the interleaved pulses [20, 21].
In this work, we developed the CHIPS strategy and evaluated its performance with able-bodied human subjects. Our findings show that the CHIPS strategy can evoke stronger, more comfortable, distally-referred sensations without local sensations, maintaining activation thresholds comparable to SC stimulation, while delivering shorter pulses on a given channel. This novel strategy has the potential to address some of the issues that have precluded wide adoption of surface stimulation as a viable alternative for chronic restoration of sensory function.
2. Methods
2.1. Able-bodied human subjects
Written informed consent was obtained from ten adult subjects (four males, six females, mean age ± SD: 34.9 ± 15.3) in compliance with the Institutional Review Board of Florida International University which approved this study protocol. All prospective subjects were screened prior to the study to determine eligibility. Subjects were able-bodied, with no sensory disorders or any self-reported condition listed as a contraindication for transcutaneous electrical stimulation (pregnancy, epilepsy, lymphedema, or cardiac pacemaker) [22].
2.2. Experiment setup
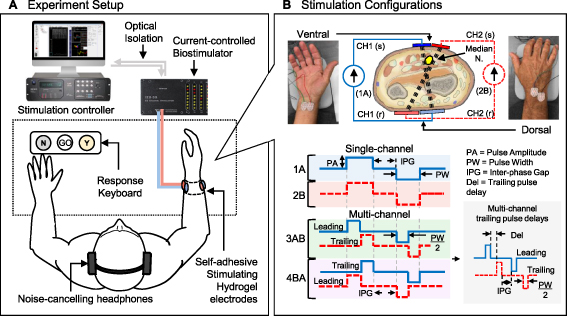
Subjects were seated on a chair with both arms on a table in front of them (figure 1(A)). Their right forearm was thoroughly cleaned with an alcohol wipe and placed on a support pad on the table, with their right hand's palmar surface parallel to the vertical plane. Subjects were encouraged to drink water before and during the experiment to ensure sufficient skin hydration.
Figure 1. Experiment setup and stimulation configurations for human studies. (A) Experimental setup schematic showing stimulation being delivered by an optically isolated, current-controlled biostimulator (TDT RZ5/IZ2H-16) through up to two surface electrode pairs placed around the subject's right forearm (∼3 cm from the distal radial crease). Percept responses (Yes/No) were collected using a custom keyboard. (B) Each electrode pair was assigned to an independent current source: CH1 (blue) and CH2 (red-dashed), to deliver charge-balanced biphasic pulses to the median nerve. Two stimulating (s) electrodes were placed on the ventral aspect of the wrist, and two return (r) electrodes on the dorsal aspect. In the SC configuration, only one channel (1A or 2B) delivered stimulation pulses with duration PW, followed by the inter-phase gap (IPG) and charge-balancing phase. In the MC configuration, stimulation was interleaved from 1A to 2B (3AB) or from 2B to 1A (4BA) so that pulses with the same polarity were delivered sequentially from each channel, with no overlap. In this case, the IPG was the interval between the end of the first trailing pulse and the start of the leading balancing pulse. For a given PW, half of the pulse was delivered by the leading channel and the second half was delivered by the trailing channel, followed by the charge-balancing sequence. The gray inset (bottom-right) illustrates an interleaved biphasic pulse with a delay (Del) between the leading and trailing pulses. The MC IPG was kept constant regardless of the trailing pulse delay used.
Download figure:
Standard image High-resolution imageEach subject received electrical stimulation from a set of four self-adhesive hydrogel surface electrodes (Rhythmlink International LLC, Columbia, SC) distributed around their right wrist to activate sensory fibers in the median nerve which emanate from the index, middle, and part of the ring finger. Two small stimulating (s) electrodes (15 × 20 mm) were placed on the ventral aspect of the wrist (∼3 cm from the distal radial crease) and two large return (r) electrodes (20 × 25 mm) on the opposite (dorsal) side (figure 1(B)). Each 's-r' electrode pair was assigned to an independent channel and configured such that their current paths would cross each other and intersect the median nerve transversely (figure 1(B)).
Placement of the first 's-r' pair was determined by fixing the return (dorsal) electrode near the styloid process of the ulna, and adjusting the stimulating (ventral) electrode near the median nerve while providing brief, 1 s long stimulation bursts (500 µs biphasic, anode-first pulses at 30 Hz) at various amplitude levels between 1.5 mA and 3 mA, in increments of 0.1 mA, until a distinct referred sensation was reported by the subject. The location that elicited a percept with the lowest amplitude was chosen. The dorsal electrode from the second 's-r' pair was placed lateral to the first one, near the dorsal tubercle of the radius. Similarly, the second ventral electrode was placed medial to the first one, keeping an approximately 1 mm gap between them. Again, short stimulation bursts at various amplitude levels were delivered from the second electrode pair until a distinct referred sensation was reported by the subject. Finally, the location of the ventral electrodes was adjusted further by shifting them together laterally so that both would elicit a distinct referred sensation with the lowest possible stimulation amplitude.
A custom three-button keyboard was placed on the table in front of the subject's left hand. Subjects used this keyboard to trigger the delivery of the electrical stimuli (Go) and provide percept responses (Yes/No). Subjects were fitted with a pair of noise cancelling headphones that delivered soft white noise to mask any ambient sounds that might be distracting as well as to play instruction sound queues at various stages of the study. Subjects were instructed to relax and maintain a fixed arm position throughout the experiment but were encouraged to stretch and move their hand during periodic breaks to avoid discomfort. Subjects were asked about their comfort levels, or if additional breaks were needed after each task.
2.3. Stimulation configurations
A MC programmable, optically isolated benchtop bio-stimulator (TDT IZ2-16H, Tucker-Davis Technologies, Alachua FL, USA) was used to deliver the electrical stimuli. A custom TDT Synapse stimulation control environment running on the TDT RZ5D base processor was used to schedule charge-balanced, current-controlled biphasic rectangular pulses with pulse amplitudes (PA) between ±3 mA per channel, with 1 µA/step resolution, and a pulse width (PW) resolution of 21 µs/step. Anode-first pulses were used throughout the study, as this waveform has been shown to activate orthogonally oriented fibers more efficiently than cathode-first pulses [23, 24] and resulted in lower percept thresholds than cathode-first pulses when stimulating the median nerve transversely during pilot studies. The TDT Synapse environment was interfaced to a custom MATLAB® program (v2018b, MathWorks® Inc., Natick, MA) designed to run and monitor the various study conditions and modulate the stimulation parameters based on subject responses.
A computational model of human median nerve afferents within the wrist was used to develop and characterize the CHIPS strategy before its implementation in human studies (see supplementary materials (available online at stacks.iop.org/JNE/18/055004/mmedia)). The model helped us visualize the potential outcomes of implementing this novel pulse scheduling scheme (figure S2). It provided the means to explore and narrow down the stimulation parameter space. These parameters were further refined during pilot experiments. We used the activation threshold results from the computational study to guide our electrode placement procedures used during human subject studies. For instance, electrode pairs from each channel used in CHIPS were configured such that their current paths would cross each other and intersect the median nerve transversely. In this configuration, electrode locations with the lowest SC thresholds would suggest that the target nerve is located between both stimulating electrodes. Based on the simulation results, this placement was expected to result in better targeting of the nerve when stimulating with the CHIPS strategy (figure S2(B)), avoiding unnecessarily larger activation regions for either of the channels.
Two stimulation configurations were used in this study (figure 1(B)). During standard SC stimulation, biphasic current pulses with a 100 µs IPG and a given PW were delivered to the median nerve from only one channel at a time (configuration pattern 1A or 2B). For the MC configurations used to test the CHIPS strategy, biphasic pulses were interleaved from two independent stimulation channels (from 1A to 2B, or from 2B to 1A) so that the anodic phases of each channel were delivered consecutively, followed by their respective charge-balancing phases after a 100 µs IPG. In this case, the PW for each channel was set to half of the PW used during SC stimulation. Both channels delivered identical pulses (with the same amplitude and duration), but stimulation from the second (trailing) channel was temporally shifted by the PW from the first (leading) channel to prevent pulse overlap. During some experiments, various delays (Del) were tested between the leading and trailing channels.
2.4. Experimental procedures
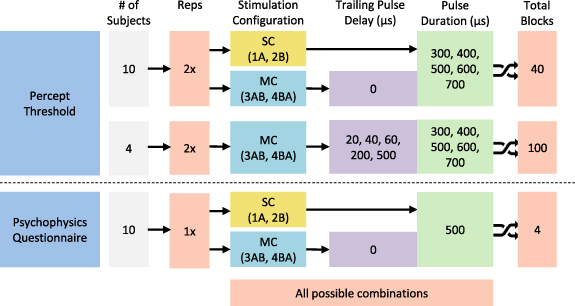
Performance for each of the stimulation configurations was assessed by comparing the percept threshold measurements and the results from the psychophysical evaluation of the elicited percepts. Figure 2 summarizes the experimental protocols completed in this study.
Figure 2. Experiment sequence diagram for the human study protocols. All subjects completed 40 threshold measurement blocks consisting of 2 reps of 5 PW and 4 stimulation configurations. A subset of randomly selected subjects completed 100 additional threshold measurement blocks, randomized across 2 reps of 5 PWs, 5 trailing pulse delays and 2 stimulation configurations. Each subject was assigned a unique (randomized) experimental sequence consisting of all possible combinations of the experimental conditions. Each threshold measurement block took between 30–45 s on average. Short breaks between blocks were at least 10 s, and extended as much as the subjects needed. Finally, all subjects completed four questionnaires for all configurations tested. SC = single-channel; MC = multi-channel. The order of the configuration used for the questionnaires was randomized across all subjects.
Download figure:
Standard image High-resolution image2.4.1. Percept threshold measurements
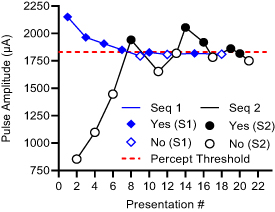
Percept thresholds were obtained from all subjects for each SC (1A, 2B) and MC (3AB, 4BA) configuration under five different PW values (300 µs to 700 µs, at 100 µs intervals). These PWs were consistent with values used in the literature to activate sensory fibers in the human peripheral nerves [25]. Additional MC stimulation trials were completed by a subset of subjects (n = 4) under various trailing pulse delay values to assess how much later could the trailing pulse be delivered after the leading pulse without negating the temporal summation of the pulses. To assess the effects of small delays on the CHIPS performance, we evaluated a set of short delays from 0 µs to 60 µs (20 µs intervals), while the effects of longer delays were evaluated using a delay of 200 µs, which is within the range of the leading pulse durations, and 500 µs, which is higher than the maximum leading pulse duration. The order of the stimulation configuration, PW and delays, was randomized across all subjects. All trials were completed twice under every condition. The percept threshold determination procedure used was a dual randomized parameter estimation by sequential testing (DR-PEST), which is a combination of the parameter estimation by sequential testing (PEST) method and a randomly alternating dual staircase method [26]. This combination was selected to reduce variability and subject bias, allowing for fast and accurate estimation of percept thresholds. An example of a stimuli presentation sequence is shown in figure 3.
Figure 3. DR-PEST procedure to determine percept threshold (example from one subject). Pulse amplitude is changed (y-axis) at each trial to elicit sensation based on randomly alternating dual staircase sequences (Seq 1 and Seq 2) while collecting Yes or No responses from the subject.
Download figure:
Standard image High-resolution imageA custom algorithm was designed and integrated into a MATLAB® program that controlled the delivery of electrical stimuli and collected information about the subject's sensory responses. Subjects triggered the delivery of the stimuli by pressing the 'Go' button, and then provided a positive or negative response by pressing the 'Yes' or 'No' button, depending on whether the stimulus was detected. Positive responses were followed by a decrease in PA while negative responses were followed by an increase in PA. The step size was halved after every positive response or doubled after two successive negative responses. The direction of the trials was always changed after a response reversal. The order of occurrence of the staircases was randomized in advance. The two sequences always started apart and eventually came together, crossing and re-crossing each other thereafter until six response reversals per sequence were reached.
The subject responses were analyzed for each sequence independently since they could be considered as two replicates of the same condition. Threshold values were computed by fitting the Wichmann and Hill psychometric function [27] and finding the stimulation amplitude value with a 50% probability of having a positive or negative response for each sequence. The final threshold amplitude for a given PW was computed by taking the average of the thresholds found from each sequence. The experimental percept threshold measures (two repetitions per PW) were fitted to the Lapicque–Weiss's theoretical model [28, 29] to compute individual strength-duration (SD) curves for each subject under each stimulation configuration. The collected percept threshold data were used to determine the sensory activation performance of each configuration, where lower threshold values meant better performance.
For trials comparing the SC (1A, 2B) and novel MC (3AB, 4BA) configurations, each subject's SD curves were normalized to the rheobase of configuration 1A, which was by definition the best-performing SC configuration (lowest overall threshold). This configuration was assumed to have been placed closer to the median nerve than '2B' and thus chosen as the comparison standard. Normalization was done in order to compare percept thresholds across participants while accounting for between-subject variability [29–31]. To compare activation performance across configurations, the normalized threshold values for each tested PW were first scaled to the % of the threshold from 1A on a per PW basis, then pooled across all PWs. A theoretical 'no summation' (NS) reference SD curve was calculated by assuming only half of the PW was delivered under configuration 1A. For trials comparing MC stimulation under various trailing pulse delays, each subject's SD curves under each delay were normalized to the rheobase of the tested MC configuration without any delay, and adjusted for PW. Furthermore, the configurations were assessed by comparing their normalized threshold measurements with a nonparametric one-way ANOVA (Kruskal–Wallis test, MATLAB® Statistics Toolbox 11.6). Multiple post hoc comparisons between configurations were made using the Dunn–Sidak test at an alpha level of 0.05 for significance.
2.4.2. Assessing elicited percepts: modality, quality, and location (MQL) questionnaire
To evaluate the characteristics of sensations evoked by stimulation, subjects were instructed to complete a multiple-choice psychophysics questionnaire about the MQL of the sensations under each configuration. This questionnaire was based on similar questionnaires used in other neurostimulation studies to provide sensory feedback [10, 32, 33].
While completing the questionnaire, subjects received 1 s long bursts (30 Hz, 100 µs IPG). For each configuration, the stimulation parameters were kept constant. Subjects were allowed to trigger the delivery of the short stimulation burst as needed to answer the questionnaire with confidence. Stimulation amplitude was set to 25% above the percept threshold (1.25 × PT) at a PW of 500 µs. This duration was chosen since it allowed for a wide range of amplitudes to be used. A trailing pulse delay of 0 µs was used during MC stimulation in this procedure. The order of the configuration used during this assessment was randomized across all subjects. There were short breaks in between each questionnaire to mitigate any possible effects of previous stimulation conditions on the subjects' responses.
The sensation modality was evaluated from a list of 16 pre-defined options (i.e. touch, pressure, needle prick, tingling, vibration, etc). The sensation quality was evaluated as comfortable or uncomfortable, as well as sharp, blunt, soft, mild or strong. The perceived location of the sensations was evaluated as local (at the stimulation site), spreading (from one site to another), or referred (in the hand). All options in the questionnaire were explained to the subjects before the experiment. Subjects were instructed to choose one or more options that best described the elicited sensation, or to report a different word if none of the options accurately described the sensation.
2.4.3. Assessing percept location
Subjects reported the percept location by drawing the localized region of the sensation on a paper form with printed outlines of the palmar and dorsal surfaces of the right hand. Subjects completed a percept map for each configuration, under the same stimulation parameters used during the MQL questionnaires. Each percept map was scanned and loaded into individual layers in Adobe® Photoshop® CS2 (Adobe, San Jose, CA). The percept regions were digitized by tracing a solid shade within the area drawn by the subjects with an Intuos Pro drawing tablet (Wacom Co., Ltd, Saitama, Japan). The same hand contour image provided to the subject was used as a base layer during the digitization process. All digitized percept areas from each configuration were stacked in MATLAB®, and overlapping pixels were aggregated to calculate the frequency of location reports for all subjects.
3. Results
Surface electrodes had impedance values (mean ± SD) of 26.4 ± 0.5 kΩ across all subjects and remained stable throughout the study (supplementary figure S3). No side effects like irritation or redness of the skin were observed in any of the subjects.
3.1. Percept thresholds
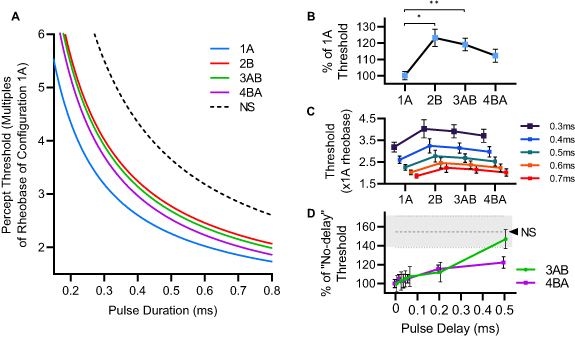
SD profiles obtained from the percept threshold measures of an individual subject under each stimulation configuration were normalized to the rheobase of configuration 1A, which was the best performing (lowest value) SC configuration, as compared to configuration 2B. On average, the mean rheobase current ± SD for 1A was approximately 695.7 ± 277.5 µA across all subjects. More specifically, for seven subjects with mean age 25 ± 5.5, the rheobase was approximately 570.1 ± 173.5 µA, while three subjects with mean age 55.6 ± 5.7 had a rheobase of around 988.9 ± 268.4 µA. Figure 4(A) shows the mean SD curves across all participants, where the pulse durations (x-axis) correspond to the total duration (temporal summation) of pulses from each channel. MC stimulation with the CHIPS strategy (configurations 3AB and 4BA) resulted in percept threshold values comparable to SC stimulation (between configurations 1A and 2B), and far below the NS reference (dashed-line) while delivering shorter pulses per channel.
Figure 4. Sensory activation thresholds in human subjects. (A) Mean SD curves from all participants (Weiss–Lapicque fit) normalized for each subject to the rheobase of the best performing (lowest percept threshold) SC configuration, 1A (blue) as compared to 2B (red). Pulse durations (x-axis) correspond to the total duration of delivered pulses (e.g. a 0.5 ms pulse from 1A or 2B is assumed to have the same duration as two 0.25 ms pulses, interleaved from 3AB or 4BA). A black-dashed reference SD profile represents the lowest theoretical percept threshold that would be seen if there was no temporal summation (NS) of interleaved pulses. (B) Mean normalized PT values adjusted to the % of 1A across all PW values tested (*p < 0.005, **p < 0.001 post-hoc Dunn–Sidak test). (C) Mean normalized PT values for each PW tested, across configurations. (D) Threshold differences under various trailing pulse delays. Mean percept threshold for 3AB (green) and 4BA (violet), normalized to their rheobase at 0 µs delay, and adjusted for all PW values tested. The gray dashed line and shade represent the lowest theoretical percept threshold (mean ± SEM) with no temporal summation (NS) based on the performance of 1A.
Download figure:
Standard image High-resolution imageThe plots in figures 4(B) and (C) compare the sensory activation performance of each configuration. Stimulation under configuration 1A resulted in significantly lower percept thresholds than configurations 2B (p = 0.0020) and 3AB (p < 0.001), while no significant differences were found between configurations 1A and 4BA, making 4BA the best-performing MC configuration.
The sensory activation performance of MC stimulation appeared to decrease with the introduction of delays between interleaved pulses, especially for large delays (i.e. 500 µs). As shown in figure 4(D), percept threshold values for both configurations 3AB and 4BA increased as delays were increased, suggesting an attenuation in the net charge delivery due to a reduction in temporal pulse summation. In general, both configurations appear to approach the NS threshold levels (gray dashed line), with 3AB reaching it at around 500 µs.
3.2. Elicited percepts
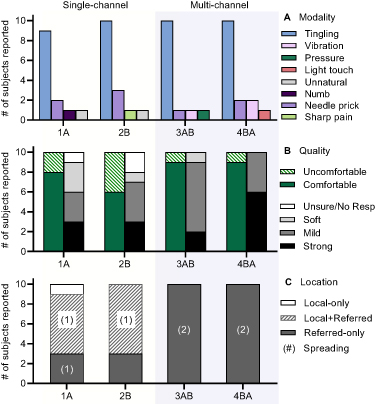
Results from the MQL questionnaire about percept modality (figure 5(A)) show that all stimulation configurations evoked sensations that were mostly described as 'Tingling', with only a few reports of 'needle prick'. Only SC stimulation resulted in numb, unnatural or painful sensations. In contrast, only MC stimulation evoked sensations of vibration, pressure or light touch. As shown in figure 5(B), most subjects (n = 9) reported comfortable sensations after MC stimulation, while up to four participants reported them as uncomfortable after SC stimulation. Percept location responses in figure 5(C) show that most participants felt referred sensations for all configurations, while local sensations (under the electrodes) were only reported after SC stimulation (n = 7).
Figure 5. Questionnaire responses across all stimulation configurations. The bar plots represent the number of subjects that reported a given (A) percept modality, (B) percept quality, and (C) percept location. The maximum possible number of reports for any given percept descriptor was 10.
Download figure:
Standard image High-resolution image3.3. Percept locations
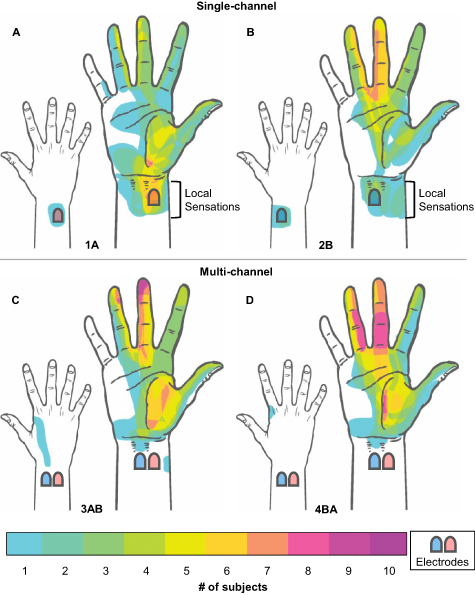
All participants reported distally referred sensations across the area of the hand including the ring, index, middle fingers and the thumb. In general, percept regions reported under each SC configuration were not identical. Percept regions reported under the MC configurations did not represent the spatial summations of percept regions from the SC configurations. Rather, they were often perceived as new percept regions that did not include the local sensations under the electrodes. As shown in figures 6(A) and (B), local sensation under the electrodes were reported by seven participants for both SC configurations only. Only one subject reported a tingle-like sensation on the lateral surface of the wrist (between electrodes, not under) with configuration 3AB (figure 6(C)). Finally, figure 6(D) shows that stimulation under configuration 4BA resulted in the most consistent reports of distally-referred sensations on the ring and middle fingers as well as the palm of the hand, without local sensations.
Figure 6. Location of the percept regions drawn by all subjects on diagrams of the palmar and dorsal surfaces of the right hand. All subjects reported distally referred sensations across the area of the hand including the ring, middle, index fingers and the thumb. (A) The color scale represents the number of subjects that reported a percept in any given location. (B) Local sensations under the electrodes were reported by seven subjects under configurations 1A and 2B. (C) A tingle-like sensation was reported by one subject on the lateral surface of the wrist under configuration 3AB (between electrodes, not under). (D) Sensations on the ring and middle fingers, and the palm of the hand were most consistently reported under configuration 4BA. The red/blue pads on the wrist represent approximate electrode locations for each stimulation configuration.
Download figure:
Standard image High-resolution image4. Discussion
This work evaluated the performance of a novel CHIPS strategy for MC surface stimulation to determine whether it could evoke distally referred sensations more comfortably than SC stimulation.
Overall, the results indicate that interleaved current pulses from multiple surface electrodes strategically distributed around the wrist result in more comfortable, distally-referred tingle-like sensations in the areas of the hand that are innervated by the sensory fibers in the median nerve, with lower incidence of local sensations near the electrodes than SC stimulation. They suggest that charge delivery using a distributed set of surface electrodes can activate deep nerve fibers without activating those close to the surface of the skin. This ability to elicit distally-referred sensations while avoiding the local sensations that can be distracting or uncomfortable suggests that the CHIPS strategy may be able to enhance the performance of surface electrical stimulation systems for delivering non-invasive sensory feedback.
4.1. Sensory activation performance in humans
MC stimulation with the CHIPS strategy resulted in percept thresholds that were within the range of thresholds found under both SC configurations (figure 4(A)), while delivering lower charges per pulse under any given electrode. We believe this is the result of the summation of interleaved pulses during the 'RC recovery time interval', in which the membrane still contains some of the charge of the leading pulse (bringing it close to the fibers' activation threshold), making it easier for the fiber to depolarize after the trailing pulse [20, 34]. Interestingly, the CHIPS strategy seemed to perform better when leading-trailing pulses were interleaved from high-threshold to low-threshold channels (worst-to-best), or from configuration 2B to 1A (4BA) as seen in figure 4(B). It is possible that the summation of the leading and trailing pulses is not complete. While the leading pulse's effect on the membrane potential could be momentarily sustained, there is likely some decay during the delivery of the trailing pulse. Since the trailing pulse ultimately drives the fiber across its activation threshold, the most effective sequence would be the one where the trailing pulse is delivered from the best channel.
The introduction of small delays between interleaved pulses did not strongly compromise the performance of the CHIPS strategy. Larger delays, however, seem to attenuate the effect of the leading pulse on the membrane potential at the time of arrival of the trailing pulse, resulting in increased percept thresholds (figure 4(C)), especially for the worst performing MC configuration (3AB). We initially expected a sharp transition in percept threshold toward the NS case after a sufficiently long trailing pulse delay. Instead, we saw a gradual threshold increase over the delay values tested. This is consistent with the results of the computational model (figure S2(E), supplementary materials).
We hypothesize that this rather gradual threshold increase reflects consequences of ion channel dynamics on electrical stimulation. The voltage-gated sodium and potassium channels responsible for the generation and propagation of action potentials are known to exhibit hysteresis [35]. For instance, their activation gates can respond much faster to stimulation than their inactivation gate [36], suggesting that some channels can remain open for some short time after the leading pulse is delivered. In addition, sub-threshold changes in membrane potential have been shown to alter the initial state of the channels in the membrane by partially engaging activation gate segments without reaching inactivation. This 'pre-conditions' the channels to open fully at a lower threshold potential, thus increasing their excitability to subsequent stimuli [36, 37]. This excitatory effect of sub-threshold pre-pulses has also been observed during transcutaneous stimulation studies [38].
Most surface stimulation studies have used cathode-first biphasic pulses. However, these studies typically utilize a pair of electrodes placed on the same aspect of the limb, but displaced longitudinally (i.e. along the long axis of the nerve); our approach utilizes pairs of electrodes placed transversely (across the wrist) and therefore uses anode-first pulses. During pilot studies, we observed that cathode-first pulses outperformed anode-first pulses (had lower thresholds) when targeting the median nerve with a proximal-to-distal electrode pair, placed along the ventral surface of the wrist. This is consistent with fibers hyperpolarizing under the distal anode (blocking efferent signals) and depolarizing under the proximally placed cathode (letting afferent information through). In contrast, anode-first pulses resulted in lower percept thresholds than cathode-first pulses when stimulating the median nerve transversely. The difference in sensory activation performance between the two waveforms could be a result of different electric field gradient orientations between these two electrode arrangements [23, 24].
4.2. Percept enhancement
The comfort and selectivity of surface stimulation are often associated with electrode size and charge density [18]. Large electrodes dissipate the charge over a larger region of the skin, which reduces discomfort, but the charge is also more widely dissipated in the underlying tissues, which reduces the ability to selectivity activate targeted nerves. Reducing the size of the electrode can help focus the stimulation, thereby enhancing the ability to activate nerve fibers, but may require charge densities that cause discomfort in the skin below the electrode. In recent studies, surface stimulation of the median and ulnar nerves also resulted in distracting local sensations due to the activation of the tactile afferents in the skin close to the electrodes [16, 17]. These sensations can be hard to ignore and therefore would affect the overall performance of the stimulation approach. In contrast, the CHIPS strategy allowed us to deliver focal stimulation to the median nerve using small surface electrodes while avoiding the large charge densities associated with local sensations and skin discomfort. In fact, analysis of the MQL questionnaire responses revealed that stimulation under configuration 4BA evoked the most consistent reports of stronger, more comfortable distally-referred sensations (figure 5) on the ring and middle fingers as well as the palm of the hand (figure 6) without local sensations. These results suggest that implementation of the CHIPS strategy allowed for focal activation of specific parts of the nerve (partial recruitment) resulting in sensations on the areas of the hand innervated by sensory fibers within the recruited section. More specifically, since the electrodes were placed so their current paths would interfere near the center of the wrist ventral surface, the median nerve would be expected to receive stimulation mostly near its ventral and medial aspect (the side closest to the ulna). Because of this, percepts were evoked more frequently on the ring and middle fingers as well as the palm of the hand, matching the expected somatotopy of the median nerve at this location [39]. Only one subject reported a percept on the back of the hand during CHIPS (configuration 3AB). It is possible that a branch of the ulnar nerve that innervates the back of the little finger, or a cutaneous branch of the radial nerve were inadvertently activated.
Percept intensity responses were found to vary across configurations. One potential source of variability in the reported intensity is electrode placement. Both SC configurations had different placements, presumably targeting the median nerve differently. This was probably also the case for both MC configurations, as they evoked different percept locations and intensities. It has been well documented that perceived intensity is a function of, among other things, population recruitment patterns [40]. The electrode placement used for each configuration could affect the number of afferents responding to the stimulus, consequently affecting the perceived intensity. The results presented here do not include information to account for the variability of reported intensities across subjects. Potential sources of inter-subject variability may include age, gender, skin conductivity, electrode impedance, etc.
4.3. Limitations
One limitation of this study is that the initial electrode fitting parameters were determined through trial and error. While computational modeling can provide some general guidance for electrode placement, it does not provide subject-specific electrode fitting parameters (i.e. absolute locations, current amplitudes, etc). During the electrode placement procedures, we performed coarse electrode location adjustments in order to reduce fitting procedure time. Because of this, it is possible that each individual channel's alignment with the median nerve was not optimal. This could explain the significant differences in percept thresholds found between the two SC configurations (figure 4(B)). To streamline and enhance the stimulation fitting process, a spatially distributed set of electrodes (an electrode array) could be used, in which subsets of electrodes are selected to optimize the stimulation effectiveness and comfort. The combinations and locations of active electrodes, as well as the characteristics of the stimulation pulses could be adjusted to reshape the spatiotemporal distribution of charge within the array [41, 42]. This would allow for spatial steering of the stimulation focus to target specific tissue regions to modulate percept areas and intensity, and to mitigate the effect of arm motion on the stimulation.
Another limiting aspect of this study is the long duration of the iterative processes used to determine percept thresholds. On average, it took about an hour for subjects to complete all basic threshold determination blocks using the modified dual staircase. While these procedures are designed to determine percept thresholds accurately for research objectives, they are not sustainable for stimulation parameter fitting in the real world. Accurate and efficient stimulation fitting could be achieved through interactive user-controlled fitting paradigms (user-in-the-loop) to help determine and optimize the stimulation parameter ranges, accelerate identification of the target nerve branches, and create user-specific stimulation profiles.
This work did not directly evaluate how different wrist position affects stimulation performance. Evidence from previous studies with transcutaneous stimulation [16, 17] show some degree of position dependence, where percept intensity, modality or location is affected by limb posture. Delivering focal stimulation with the CHIPS strategy could exacerbate position dependence, since the stimulation would be focused on smaller areas of the nerve and therefore percept areas on the hand would be more likely to change due to nerve motion. However, anecdotal reports during the evaluation of the CHIPS strategy suggests that percept intensity and location was less susceptible to wrist flexion and extension than with SC stimulation.
Other factors to consider in our percept assessments are the technical constraints of the stimulation system. For instance, MQL questionnaires were completed with PAs set to 25% above the mean percept threshold at 500 µs. This PW was selected because it afforded a wide range of suitable amplitudes below the maximum current output of 3 mA while avoiding some of the uncomfortable sensations associated with longer PWs. Shorter PWs would have required PAs much higher than the output limit of the stimulator. An additional constraint within the pulse sequencing algorithm used during our percept assessment procedures limited the stimulation frequency to 30 Hz. As a consequence, the percept characteristics reported in this study should be viewed in the context of these specific stimulation parameters. It is not yet known whether different percept characteristics would be reported between the stimulation approaches at various PWs and frequencies.
Lastly, this study evaluated the performance of our stimulation approach in able-bodied subjects at the wrist level. This location provides a flexible, yet stable setting for exploring the feasibility of the pulse scheduling strategy since the median nerve is typically approximately 1 cm under the skin of the volar wrist. This allows access to mostly afferent fibers that innervate the radial aspect of the palm, and the tips of the thumb, index and middle fingers, while avoiding most of the motor fibers within the median nerve. This stimulation approach was primarily developed to address the loss of sensory function after amputation. In its current configuration, this approach may be readily implemented to restore sensory function to individuals with distal transradial amputation or wrist disarticulation, given that the residual nerves are still accessible. However, it is unclear whether it could be translated to other individuals with amputations at other levels. It may be possible to implement the CHIPS strategy within an array of electrodes distributed around the upper arm, targeting the nerves along the medial side, beneath the short head of the biceps brachii. While stimulation near the elbow is more difficult in able-bodied subjects as it can cause muscle activation, individuals with elbow disarticulation and above-elbow amputations may not experience these side effects. Furthermore, patients undergoing pre-planned amputation could elect to have nerve reassignment procedures [43] to make the median and ulnar nerves more accessible via surface electrodes.
4.4. Implications and future directions
The novel CHIPS strategy proved to be a viable approach to deliver current pulses transcutaneously to selectively stimulate sensory fibers within the median nerve to elicit enhanced referred sensations, while avoiding the more superficial tactile afferents located under the electrodes. This addresses the primary issues hindering standard non-invasive neuromodulation approaches, making it a feasible alternative for individuals who may not be eligible, or chose not to undergo, surgical procedures for invasive neuromodulation, as the latter carries risks of adverse effects such as infection and persistent implant site pain [44]. Non-invasive neurostimulation with CHIPS could also be used to study the neural mechanisms of natural touch and develop advanced neuromodulation strategies in able-bodied subjects before deployment in implantable systems.
The fitting and targeting performance of the CHIPS strategy could be further improved by delivering the stimulation from an array of spatially distributed electrodes [30, 41, 45] in which subsets of electrodes are selected to optimize the stimulation effectiveness and comfort [17]. The distribution of currents within the tissue depends on the stimulation amplitude, electrode dimensions and tissue properties, among other factors. This distribution can be shaped by enabling additional electrodes within a single current source (virtually changing the surface area) and modulating the current amplitude to adjust the location of the interference region [46, 47], effectively steering the percept area. Computational modeling could be used to estimate the distribution of currents while user-controlled calibration routines could be used for sequential exploration of sensory responses from multiple combinations of stimulating electrodes within the array. These responses, combined with results from computational models could be used to optimize the active electrode selection, predict the most likely location of the target nerve within the treatment area, and create user-specific stimulation profiles.
It is possible that the CHIPS strategy could be also applied with extraneural interfaces such as cuff electrodes used for sensory stimulation and functional neuromuscular stimulation. The fascicular structure of the nerve and the insulating properties of its connective tissue are known to impair the ability of cuff electrodes to selectively stimulate small populations of fibers, albeit to a much lesser degree than surface stimulation. Some have attempted to overcome this limitation by reshaping the nerve, increasing the number of electrodes, or by selecting specific electrodes to shape the electric field [48]. The performance of the latter approach could be further enhanced by implementing the CHIPS strategy not only to avoid activating fibers closer to the electrode contacts, but also to reduce localized charge densities that could cause tissue damage and electrode degradation.
This strategy could potentially be used beyond prosthetics applications. For instance, the stimulation-evoked percepts could provide intuitive haptic feedback during manipulation and interactions within virtual, augmented, and real environments without the cumbersome restrictions of traditional haptic hardware. This could be useful for haptic feedback in games, teleoperation of unmanned vehicles, surgical procedure training, physical and neurological rehabilitation, and social interactions within virtual worlds. Another innovative aspect of this approach is the potential to deliver targeted neuromodulation therapies for peripheral neuropathies. Surface stimulation has been previously explored as a non-pharmacological alternative for patients with neuropathic pain symptoms secondary to nerve injury or amputation [49, 50]. Although the neural mechanisms underlying the analgesic effects of conventional surface stimulation are complex and incompletely understood, they are generally consistent with the gate control theory. In this context, our approach could be used to deliver focal stimulation to non-pain-related sensory fibers to prevent, or 'gate', nociceptive signals from being relayed from the spinal cord or brainstem to the brain.
5. Conclusions
This work evaluated the performance of a novel MC neurostimulation strategy against SC stimulation using electrodes placed on the skin. Able-bodied subjects reported enhanced distally-referred percepts when receiving interleaved current pulses from multiple channels strategically distributed around the wrist and most subjects never reported uncomfortable or distracting percepts under the electrode site. The results presented here demonstrate that our channel-hopping stimulation strategy avoids discomfort caused by unintended activation of skin afferents while allowing selective activation of deeper neural targets. Hence this work addresses some of the primary issues that have hindered the use of non-invasive neural stimulation to elicit meaningful sensations. This approach offers a potential alternative not only for delivering enhanced tactile feedback, but also for stimulation therapies to treat various pain conditions.
Acknowledgments
The authors would like to thank Kenneth Horch, Luis Herran and Heriberto Nieves for their valuable contributions during the development and testing of the stimulation approach. This work was supported by the Department of Biomedical Engineering Coulter Undergraduate Research Excellence Program to Luis Herran, FIU Graduate School Dissertation Year Fellowship to Andres Pena, and the Wallace H Coulter Eminent Scholar Endowed Chair in Biomedical Engineering to Ranu Jung.