Abstract
On 16 November 2018 a revision of the International System of Units (the SI) was agreed by the General Conference on Weights and Measures. The definitions of the base units were presented in a new format that highlighted the link between each unit and a defined value of an associated constant. The physical concepts underlying the definitions of the kilogram, the ampere, the kelvin and the mole have been changed. The new definition of the kilogram is of particular importance because it eliminated the last definition referring to an artefact. In this way, the new definitions use the rules of nature to create the rules of measurement and tie measurements at the atomic and quantum scales to those at the macroscopic level. The new definitions do not prescribe particular realization methods and hence will allow the development of new and more accurate measurement techniques.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The name International System (SI) was officially given to the system of units developed under the auspices of the Metre Convention in 1960 (CGPM 1960). The SI initially included six base units: the metre, the kilogram, the second, the ampere, the kelvin and the candela. The mole was added in 1971 as the seventh base unit (CGPM 1971). The SI is a system that has been changed whenever there has been consensus about the need for improvements. Although several base units had been redefined since the inception of the SI, there had never been any substantive change to the definition of the base unit for mass, the kilogram1. In this paper, we describe the changes to the system that were agreed in November 2018 that included the first to the kilogram since the agreement of the international prototype of the kilogram (IPK) as the basis for the unit of mass in 1889. The definitions of the ampere, the kelvin and the mole were also changed at the same time.
In the last 50 years, revolutions in atomic physics and quantum metrology have enabled the definitions of the second, the metre, and the practical representation of the electrical units to take advantage of atomic and quantum phenomena to achieve levels of accuracy limited only by our capacity to observe them. The changes agreed in November 2018 bring similar improvements to the definition of the kilogram. At the same time, the format used to write the definitions has been changed in order to emphasize the link between the definitions and the constants that underlie them. It is expected that this will be important in the field of thermometry particularly for the measurement of very high and low temperatures (see section 6).
It was recognized several decades ago that it would be beneficial to define the kilogram with reference to an atomic mass or a fundamental constant (Deslattes et al 1974, Olsen et al 1991, Taylor 1991) but it has only been in recent years that it has been possible to implement such concepts in practice with the accuracy required. It has been progress with the moving-coil watt balance experiment (now known as the Kibble balance) (Robinson and Schlamminger 2016) and the so-called 'x-ray crystal density (XRCD)' method (Fujii et al 2016), that have made a new definition of the kilogram a practical possibility. In this paper we review how collaborative work amongst a number of National Metrology Institutes (NMIs) around the world provided the experimental data needed to show that the new definitions provide an improved basis for the SI. The redefinitions will ensure that the SI continues to be both widely applicable and able to accommodate the most exacting measurement requirements.
In the following section we describe the process that led to the new definitions being agreed at the 26th meeting of the CGPM. The text of the resolution that officially adopted the revised SI is reproduced as an Annex to this paper. In section 3 we describe the motivation for the changes. The developments that have opened the way for this achievement are discussed in sections 4–7 for the fields of mass, electricity, thermometry and chemistry. The revision of the SI agreed in 2018 leaves open the possibility that at least one of the defining constants might be changed in the future. In section 8 we mention plans to re-define the second within the next 10 years.
2. The new definitions agreed by the General Conference on Weights and Measures
Progress towards the revision of the SI is well documented amongst the resolutions of the General Conference on Weights and Measures (CGPM) at which decisions are made internationally on matters of metrology of international scope and in particular on the SI. The decision taken in 2018 can be traced back to the 20th meeting of the CGPM in 1995 which reviewed the results of the third periodic verification of national kilogram prototypes against the IPK (Girard 1994). It recommended that NMIs should work on experiments that would open the way to a new definition of the unit of mass based upon fundamental or atomic constants (CGPM 1995). Subsequently, the CGPM has encouraged the continuation of the work needed to establish new definitions. The outcome of this long process has been the approval by the CGPM at its 26th meeting in November 2018 of the revision of the SI, which will come into force on 20 May 2019.
The SI is now based on a set of seven constants with exactly specified numerical values (table 1) (SI brochure). The attribution of numerical values to these constants is sufficient to define all of the SI base and derived units. The distinction between base and derived units has been maintained, largely for pedagogical reasons, but is no longer strictly necessary. These changes originate from proposals made in 2006 to adopt new definitions for some of the base units of the SI (Mills et al 2006). Since that first publication, the proposals have been refined and extended and have gained widespread support. The revision has changed the physical concepts underlying the definitions of the kilogram, the ampere, the kelvin and the mole. The principles underlying the definitions of the second, the metre and the candela were unchanged, although their wording has been changed. All definitions are now expressed in a form that specifies the numerical value of one of the seven constants when expressed in SI units ('explicit constant definitions' (SI Brochure, appendix 4, part 1)). The correspondence between the base units and the defining constants are indicated in table 1. As has been the case since 1960, the definitions of the units are not all strictly independent (for example, all of the units, except the mole, depend on the second). The wording of the definitions can be found in the 9th edition of the SI brochure (SI brochure) and in the CGPM resolution, reproduced in the Annex to this article. The definitions leave open the choice of the technique for the practical realization of the unit, thus allowing advantage to be taken of future developments.
Table 1. The seven constants and their values that define the base units of the SI. The hertz, joule, coulomb, lumen, and watt, with unit symbols Hz, J, C, lm, and W, respectively, are related to the base units according to Hz = s−1, J = kg m2 s−2, C = A s, lm = cd m2 m−2 = cd sr, and W = kg m2 s−3.
| Defining constant | Symbol | Numerical value | Unit | Base unit associated with the constant |
|---|---|---|---|---|
| Hyperfine transition frequency of Cs | ΔνCs | 9 192 631 770 | Hz | second |
| Speed of light in vacuum | c | 299 792 458 | m s−1 | metre |
| Planck constant | h | 6.626 070 15 × 10−34 | J s | kilogram |
| Elementary charge | e | 1.602 176 634 × 10−19 | C | ampere |
| Boltzmann constant | k | 1.380 649 × 10−23 | J K−1 | kelvin |
| Avogadro constant | NA | 6.022 140 76 × 1023 | mol−1 | mole |
| Luminous efficacy | Kcd | 683 | lm W−1 | candela |
3. The motivations for the changes
As discussed above, a long-standing motivation for the redefinition of the kilogram was that it was the last SI base unit to be defined by a material artefact, the IPK. As a result of its definition, if it were to be altered irreversibly through accumulation of contamination or mechanical wear (Quinn 1991, Davis 2003) it would nevertheless have a mass exactly equal to 1 kg. Thus it could not be used routinely because it had to be protected. The intervals of up to 50 years between uses of the IPK made it difficult for the BIPM to maintain traceability to the mass unit on its working standards. This became clear during its last use in 2014 when it was found that the mass unit disseminated by the BIPM had drifted away from the mass of the IPK since its previous use in 1992 (Stock et al 2015, de Mirandés et al 2016). As a consequence of the artefact-based definition, traceability to the SI kilogram was ultimately only available from the BIPM. A further limitation of the artefact-based definition was that standards for much lower mass values had to be linked to the kilogram through a chain of sub-divisions each contributing to the uncertainty.
Whilst the ambition for there to be a new definition of the kilogram was widely articulated, it was considered by the electrical metrology community that the simultaneous redefinition of the kilogram and the ampere in terms of fixed values of the Planck constant and the elementary charge, respectively, would be very beneficial. Since 1990, the practical realization of electrical units was based on the use of the Josephson voltage standard and the quantum Hall resistance standard, together with conventional values for the Josephson constant and the von Klitzing constant (Taylor and Witt 1989). The use of conventional values instead of SI values provided an independent unit system for electrical metrology that operated in parallel to the SI. The proposed simultaneous redefinition of the kilogram and the ampere would permit the conventional values to be abandoned and to make the methods used for the practical realization of electrical units fully consistent with the definitions of the units.
The thermometry community had realized that the planned revision of the SI would be an occasion to replace the definition of the kelvin based on the fixed value of the triple point temperature of water with a definition based on a fixed value of the Boltzmann constant. The former definition was not ideal because different water triple point cells showed different triple point temperatures, depending on their content of chemical impurities and the isotopic composition of the water (White et al 2003). The definition was also impractical for the realization of temperatures far away from that of the water triple point. Finally, to clarify the concept of amount of substance, which is related to numbers of microscopic entities such as atoms and molecules, and not to the mass of these entities, it was proposed to revise the definition of the mole, which made reference to 12 g of carbon 12.
4. The kilogram
4.1. The CCM requirements for the kilogram redefinition and the CCM-CCU roadmap
The redefinition of the kilogram presented a number of challenges for the field of mass metrology that led to some concern about how the continuity of the mass unit would be maintained and about the mutual consistency of future independent realizations of the kilogram. In 2010 the Consultative Committee for Mass and Related Quantities (CCM) formulated a number of conditions that should be met before the new definition could be adopted (Gläser et al 2010). These requirements were slightly reformulated in 2013 as follows (CCM 2013):
- R1: 'at least three independent experiments, including work from watt2 balance and XRCD experiments, yield consistent values of the Planck constant with relative standard uncertainties not larger than 5 parts in 108',
- R2: 'at least one of these results should have a relative standard uncertainty not larger than 2 parts in 108',
- R3: 'the BIPM prototypes, the BIPM ensemble of reference mass standards, and the mass standards used in the watt balance and XRCD experiments have been compared as directly as possible with the IPK',
- R4: 'the procedures for the future realization and dissemination of the kilogram, as described in the mise en pratique3, have been validated in accordance with the principles of the CIPM MRA' (MRA 1999).
To ensure that these conditions could be met before the planned target date of 2018, the CCM developed a roadmap which identified all essential pieces of work and their completion date. This roadmap was subsequently adopted by the Consultative Committee for Units (CCU) (Richard et al 2016). The main elements of the joint CCM-CCU roadmap are:
- the publication of a mise en pratique, to describe how the kilogram would be realized and disseminated after the redefinition,
- improved traceability to the IPK, to ensure continuity of the mass unit between the previous and the new definition of the kilogram, and to fulfill requirement R3,
- achieving consistent results for the Planck constant with sufficiently small uncertainty, to fulfill requirements R1 and R2,
- verification of the consistency of future realizations by a comparison, to fulfill requirement R4,
- a link of the BIPM ensemble of reference mass standards to the realization experiments and to the IPK (Stock et al 2017),
- the CODATA special adjustment of fundamental constants, based on all data available until 1 July 2017, to provide the numerical values of the defining constants used for the revision of the SI.
Progress with these pieces of work and an evaluation with respect to the CCM requirements are described in the following sections.
4.2. Improved traceability to the IPK
An important aspect of the change to the definition of the kilogram is that continuity should be maintained such that mass values from before and after the adoption of the new definition should be the same. This requires that the numerical value of the Planck constant, the basis of the redefinition, be consistent with the previous definition of the kilogram. The CCM had therefore imposed requirement R3 (section 4.1)—that improved traceability to the IPK should be provided to all NMIs that were involved in the experimental determination of the value of the Planck constant. In 2013 the CIPM authorized the BIPM to use the IPK in 2014 for an extraordinary calibration campaign which was the first use of the IPK since the 3rd Periodic Verification from 1988 to 1992.
During the first phase of this calibration campaign the IPK was used to recalibrate the six official copies and the BIPM working standards. The differences in mass between the IPK and the official copies had changed by an average of 1 µg since the 3rd Periodic Verification (Stock et al 2015). These results do not confirm the trend for the masses of the official copies to increase with respect to the mass of the IPK observed during the 2nd and 3rd Periodic Verifications (Davis 2003). The most likely conclusion is that the masses of the IPK and its official copies have remained stable since the 3rd Periodic Verification. The BIPM working standards were found to have lower masses than when they were calibrated during the 3rd Periodic Verification. A part of this mass loss had not been detected before and as a consequence, the mass unit disseminated by the BIPM (traceable to the use of the IPK around 1990) had been found to be offset by 35 µg with respect to the IPK (de Mirandés et al 2016).
In the second phase of the calibration campaign, mass standards of the LNE (France), METAS (Switzerland), MSL (New Zealand), NIM (China), NIST (USA), NMIJ (Japan), NRC (Canada) and PTB (Germany) were calibrated against the BIPM working standards. The calibration certificates were sent to the NMIs in April 2015, providing them with improved traceability to the IPK, with an uncertainty of 3.5 µg. This work contributes towards ensuring that determinations of the Planck constant made by these NMIs are traceable to the IPK, and as a consequence, that there should be continuity between realizations of the kilogram based on the past and on the new definitions.
One of the requirements of the CCM (R4, section 4.1) was that the procedures for the future realization and dissemination should be validated in accordance with the principles of the CIPM MRA. For this reason the 'CCM Pilot Study of future realizations of the kilogram' was organized by the BIPM in 2016. One of the objectives of this pilot comparison was to quantify the consistency of mass calibrations carried out with different realization experiments. The second objective was to verify in practice the continuity between the kilogram according to its artefact definition and the future definition.
An excellent agreement was found between the four NMIs with the smallest uncertainties (Stock et al 2018). The result with the largest uncertainty was somewhat offset, but was still in agreement with the others at the level of two standard deviations. The weighted mean of all results was in good agreement with the result traceable to the IPK.
4.3. Progress with determinations of the Planck constant
The CODATA Task Group on Fundamental Constants provided adjusted values of the four constants that would be used for the revised SI (table 1) (Newell et al 2018). In 2015 the CIPM had decided that results to be used for the determination of the defining constants for the revised SI should be accepted for publication by 1 July 2017. Relevant data for the adjustment of the Planck constant can be obtained from Kibble balance experiments (Robinson and Schlamminger 2016) and from the XRCD method (Fujii et al 2016).
4.3.1. XRCD method to determine h.
This method leads to a determination of the Avogadro constant NA that can be converted to a value of the Planck constant h by using an equation derived from the Bohr model of the hydrogen atom:

where c is the speed of light, Mu the molar mass constant, Ar(e) the relative atomic mass of the electron, α the fine structure constant and 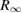 the Rydberg constant. Since the speed of light is specified exactly and, before the redefinition, the molar mass constant was also exact, the relative uncertainty of the combination of constants
the Rydberg constant. Since the speed of light is specified exactly and, before the redefinition, the molar mass constant was also exact, the relative uncertainty of the combination of constants 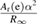 was 4.5 × 10−10, and did not depend on either the kilogram or the mole. Therefore, the value of h was calculated from the value of NA using (equation (1)) with essentially the same relative uncertainty as NA.
was 4.5 × 10−10, and did not depend on either the kilogram or the mole. Therefore, the value of h was calculated from the value of NA using (equation (1)) with essentially the same relative uncertainty as NA.
The following is a review of the most recent determinations of the Planck constant, which were the input data for the adjustment. Progress before 2014 has been described in Milton et al (2014). As will be shown later, the most recent results show a less good agreement compared to what was observed in the CCM Pilot Study described in section 4.2.
One of the principal issues with the 2011 determination of the Avogadro constant by the International Avogadro Coordination was that the 28Si-enriched spheres, AVO28-S5 and -S8, were found to be contaminated by a thin metallic surface layer composed of Ni, Cu and Zn (Andreas et al 2011). The spheres were therefore re-etched and re-polished in order to produce a clean surface. At the same time the sphericity was improved. In 2015 the International Avogadro Coordination reported a new result for the Avogadro constant with a relative uncertainty of 2.0 × 10−8 (Azuma et al 2015). From this, a value for the Planck constant can be derived with virtually the same relative uncertainty. The main improvements with respect to the 2011 result are related to the volume determination and the characterization of the surface layers.
In 2017 the NMIJ (Japan) made a new determination of the volume and the mass of the surface layers of the sphere AVO28-S5 after re-etching and re-polishing (now called AVO28-S5c). Combining these new results with those of the other parameters of the sphere which had been determined in 2015, resulted in a new determination of the Avogadro constant with a relative uncertainty of 2.4 × 10−8 (Kuramoto et al 2017).
In 2017 the International Avogadro Coordination published a new determination of the Avogadro constant using spheres made from a new crystal, named Si28-23Pr11, which has a higher enrichment of 28Si than those used for the former work, thus allowing smaller uncertainty of the molar mass determination (Bartl et al 2017). Two spheres were manufactured at the PTB from this crystal. Several new and improved methods were used for the measurements. The value for NA determined from the new crystal differs by 3.9 (2.1) × 10−8, relatively, from the results obtained in 2015 from the AVO28 crystal (Azuma et al 2015). The new determination has a relative uncertainty of 1.2 × 10−8.
4.3.2. Kibble balance method to determine h.
In 2017 the NRC (Canada) published a new determination of the Planck constant which included data of previous determinations in 2013 and of new determinations performed in 2016 (Wood et al 2017). The experiment had significantly improved over time resulting in lower noise and an improved uncertainty analysis. The relative uncertainty of 9.1 × 10−9 is the smallest published to date.
The NIST (USA) published a new result in 2017 using the NIST-4 Kibble balance (Haddad et al 2017). The uncertainty had been reduced by more than a factor of two to 1.3 × 10−8 due to a larger data set and a better understanding of the apparatus and its systematic uncertainties. This result includes data obtained in 2016 and supersedes this previous publication (Haddad et al 2016). The NIST 2015 result remains valid since it had been obtained with the NIST-3 Kibble balance apparatus (Schlamminger et al 2015).
The LNE (France) made a determination of the Planck constant using its Kibble balance operating in air (Thomas et al 2017). The relative uncertainty of 5.7 × 10−8 was dominated by effects related to the measurement in air. Future realizations of the kilogram will be made under vacuum and should reach substantially smaller relative uncertainties than the previous measurements of h made in air.
4.3.3. Synthesis of measured values of h.
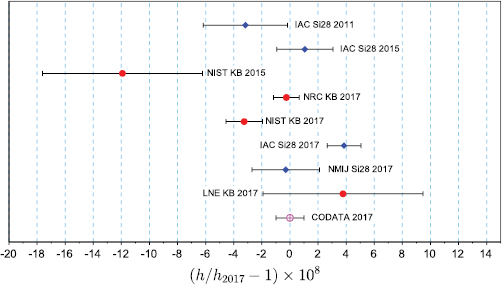
Following the closing date of 1 July 2017, the CODATA Task Group on Fundamental Constants considered all available data which had a significant impact on the determination of h, e, k and NA (Mohr et al 2018, Newell et al 2018). The eight input data for the Planck constant are those shown on figure 1. The least-squares adjustment of this data set resulted in several data having too large normalized residuals from the adjusted value of the Planck constant, which is an indication that the data set is not consistent. In particular the low uncertainty results NIST KB 2017 and IAC Si28 2017 are discrepant by four times their combined standard uncertainty. The Task Group applied therefore a multiplicative expansion factor of 1.7 to the uncertainty of all data, which reduced all normalized residuals to an acceptable value. After this uncertainty expansion, five of the results still have relative standard uncertainties of less than 5 × 10−8, and two of 2 × 10−8 or less. The data set with the expanded uncertainties still fulfills the CCM requirements R1 and R2. The value of the Planck constant obtained by the special adjustment is h = 6.626 070 150 × 10−34 J s, with a relative uncertainty of 1.0 × 10−8 (figure 1). In the revised SI, the Planck constant has the numerical value
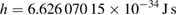
by definition, without uncertainty. At the same time the previous definition of the kilogram, m(IPK) = 1 kg, has been abrogated and the mass of the IPK became a quantity which needs to be determined by measurement. The effect is that at the time of the redefinition the mass of the IPK was still 1 kg, but with a relative uncertainty of 1.0 × 10−8, or 10 µg in absolute terms. This uncertainty propagates to any other mass value which is traceable to the IPK after the redefinition.
Figure 1. Determinations of the Planck constant included in the 2017 adjustment of the Planck constant by the CODATA Task Group on Fundamental Constants (Newell et al 2018). Red dots correspond to determinations carried out by the Kibble balance method, blue diamonds to determinations made using the XRCD method. Also shown is h2017, the recommended value of the Planck constant resulting from the adjustment.
Download figure:
Standard image High-resolution image4.4. Dissemination of the kilogram after the redefinition
Information on how to realize each of the SI base units in practice is summarized in a document known as mise en pratique (the French term for 'practical realization'). The mise en pratique of the definition of the kilogram (CCM MeP-kg) describes in a concise form the realization of the kilogram using as primary methods the Kibble balance and the XRCD method, and its dissemination. The mise en pratique can be extended in the future, when new practical realization methods become available. An important change with respect to the previous situation is that within the revised SI, in principle, any NMI that operates a Kibble balance or which carries out the XRCD technique can realize the kilogram (Stock et al 2017). The international recognition of mass calibrations based on these realization experiments at a particular NMI will be based on successful participation in international comparisons. It is planned that the BIPM will organize in coordination with the CCM an ongoing key comparison for laboratories with primary realization methods which will be similar to the CCM Pilot Study described at the end of section 4.2.
As can be seen in figure 1, the most recent results for the Planck constant are not consistent at the level of their standard uncertainties. The relative difference between the results NIST KB 2017 and IAC Si28 2017 is 7.1 × 10−8. If the experiments which led to these results would be used to determine the mass of a 1 kg weight, the difference would be 71 µg. A disagreement of this magnitude is unacceptable for mass metrology, as was made clear in CCM requirements R1 and R2 (section 4.1). This situation occurred about one year after the CCM Pilot Study, which had demonstrated good agreement between mass calibrations based on primary methods. These are indications that the primary methods had not yet reached the necessary level of consistency and stability in 2017.
The CCM reviewed this situation at its meeting in May 2017 and requested in recommendation G1 (2017) (CCM 2017) that those NMIs which had a future realization of the kilogram should 'avail themselves of the consensus value (as determined from an ongoing comparison) when disseminating the unit of mass according to the new definition, until the dispersion in values becomes compatible with the individual realization uncertainties, thus preserving the international equivalence of calibration certificates'. The consensus value will be the outcome of a statistical analysis of all comparison data from available realizations of the kilogram. It will be managed by the CCM to ensure stability and continuity, taking all new realizations and comparisons into account. It will in general be close to the reference value of the key comparison but would in practice be calculated using additional weighting factors. To ensure a smooth transition from the past traceability to the IPK to the new traceability to Kibble balances and XRCD experiments, it is foreseen to include mass artefacts traceable to the IPK in this analysis. During a transition phase, an NMI operating a realization experiment will disseminate the mass unit from the consensus value and not from its own unit realization. It will have access to the consensus value through its participation in the most recent key comparison. This coordinated dissemination scheme will ensure that mass calibrations made by institutes having realization experiments will be consistent. NMIs without a realization experiment can take traceability from an NMI operating such an experiment or from the BIPM (Stock et al 2017).
Once it has been established by comparisons that the realization experiments have become consistent and repeatable, the coordinated dissemination on the basis of the consensus value will be abandoned. From that time on, mass metrology will be in the same situation as other fields of metrology: traceability to the SI kilogram can then be obtained from a number of independent institutes. The principles of the CIPM MRA (MRA 1999): participation in key comparisons and internationally reviewed calibration and measurement capabilities (CMCs), will ensure consistency of the different realizations.
5. The ampere
Although the electrical base unit is the ampere, in practice the realization and dissemination of electrical units is based on the Josephson voltage standard and the quantum Hall resistance standard. Before the redefinition, the 1990 conventional values for the Josephson constant, KJ-90, and the von Klitzing constant, RK-90, respectively (Taylor and Witt 1989), were used. This led to the use of a parallel electrical unit system, which was close to, but not strictly identical to the SI. In the revised SI the ampere remains the electrical base unit, but there is no longer a need for the conventional values because the SI values of the Josephson constant KJ and the von Klitzing constant RK are calculated from the fixed numerical values of the defining constants e and h by using the relationships KJ = 2e/h and RK = h/e2.
The determination of the numerical value of the elementary charge e did not require any specific experiments, since it could be calculated from the experimental values for the fine structure constant α and the Planck constant h, together with the values of the speed of light c and the magnetic constant µ0, which were fixed by the definitions of the metre and the ampere before the redefinition (which is no longer the case for µ0 after the redefinition):

The numerical value for the elementary charge e has been determined by the special CODATA fundamental constants adjustment (Newell et al 2018) as e = 1.602 176 6341 × 10−19 C. The relative uncertainty of this value is 5.2 × 10−9. The uncertainty is dominated by the experimental uncertainty of the Planck constant.
In the revised SI, the elementary charge e has the numerical value
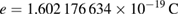
by definition, without uncertainty. At the same time, the previous definition of the ampere has been abrogated. The consequence is that the magnetic constant µ0, the numerical value of which was fixed as µ0 = 4π × 10−7 N A−2, became a quantity the value and uncertainty of which need to be determined experimentally from relationship (equation (2)). At the time of the redefinition its value remained unchanged within a relative uncertainty identical to that of the fine structure constant, 2.3 × 10−10. Further advances in the measurement of α would lead to a reduction in the uncertainty of the magnetic constant and potentially to a change of its value. The small deviation of the magnetic constant from its previously exact value and the related uncertainty are considered as being small enough to having no practical consequences (Mills et al 2006).
Another consequence of the introduction of the revised SI is that the conversion factors between the SI and the cgs system have changed. In the electrostatic cgs system the fine structure constant is given by α = e2/ћc. Since the fine structure constant is determined by measurement, this equation would be violated if e, h and c were simultaneously fixed by definition. The solution of this puzzle lies in the fact that although the elementary charge e has a fixed numerical value in the revised SI, this is not the case in the cgs system. The conversion factor between an electric charge expressed in the SI and in electrostatic cgs units depends on the square root of the magnetic constant and thus has an uncertainty, which is at present 1.2 × 10−10.
The Consultative Committee for Electricity and Magnetism (CCEM) has prepared a mise en pratique for the definition of the ampere and other electric units in the revised SI (CCEM MeP-A). It describes which methods can be used in practice to realize the SI ampere, volt, ohm, and other derived electric SI units with specific names and symbols. It also provides the values of the Josephson constant KJ and the von Klitzing constant RK, which are calculated from the fixed numerical values of the Planck constant h and the elementary charge e:
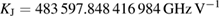
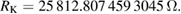
These values have been calculated to 15 significant digits. Should additional precision ever be required, one need only return to the definitions of KJ and RK and insert the exact numerical values of e and h.
These values depend on the assumption of the accuracy of the equations KJ = 2e/h and RK = h/e2. In the 2014 CODATA fundamental constants adjustment (Mohr et al 2016) the exactness of both relations has been investigated. It was concluded that the current data show the Josephson and quantum-Hall relations to be exact within 2 parts in 108. A number of 'quantum metrological triangle' experiments have been carried out to test this assumption. Within the present level of uncertainty, about 1 part in 106, no deviations from the equations have been observed (Scherer and Camarota 2012). The only theoretically predicted correction for the von Klitzing constant is at the 10−20 level (Penin 2009), which is negligible compared to the uncertainty with which the electric units can be realized in practice.
Although efforts have been made to avoid any significant changes between units realized from the past and the revised SI definitions, this has been unavoidable for the electric units. The reason is that in the past the volt and the ohm were realized using conventional, non-SI, values for the Josephson and the von Klitzing constant. The relative differences between the value of KJ and RK in the revised SI and the conventional values KJ-90 and RK-90 are
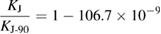
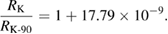
As a consequence, in the revised SI, voltage-related quantities are in relative terms 1.067 × 10−7 larger than before. Resistance-related quantities are 1.779 × 10−8 larger than before. This step change will not be noticeable for the vast majority of users because it will be hidden by the drift or instabilities of the widely used secondary standards (Fletcher et al 2014, Milton et al 2014). The most significant impact will be in the field of voltage metrology, when Josephson voltage standards are used to realize the volt. The CCEM has prepared an implementation note which establishes 'a minimal set of actions for the electrical community to provide a smooth transition through the implementation of the revised SI' (CCEM note 2017).
6. The kelvin
In the basic equations of physics, thermodynamic temperature T always appears multiplied by the Boltzmann constant k. The product kT is an energy whose coherent SI unit is the joule. The SI Brochure (SI brochure) points out that the seven defining constants are of different kinds, the Boltzmann constant being a factor that converts thermodynamic temperature to energy. This is reflected in the unit of the Boltzmann constant:
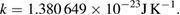
It is the fixed numerical value of this conversion factor, which has been determined by the 2017 special CODATA adjustment of fundamental constants (Newell et al 2018) (along with the definition of the joule) that defines the SI unit of thermodynamic temperature: K = 1.380 649 × 10−23 J k−1, where the joule is defined through the constant h ΔνCs.
6.1. The CCT recommendations and requirements for the kelvin redefinition
The following recommendations and declarations of the Consultative Committee for Thermometry (CCT) were all advisory documents for the CIPM, one of whose members is the CCT President. We compare what was asked for in these documents with what has transpired since.
6.1.1. The CCT conditions for the kelvin redefinition.
In June 2014 the CCT recommended that a fixed numerical value for the Boltzmann constant, as calculated by the CODATA Task Group on Fundamental Constants, be adopted when two conditions had been met (CCT 2014 T1):
- 'the relative standard uncertainty of the adjusted value of k is less than 1 × 10−6';
- 'the determination of k is based on a least two fundamentally different methods, of which at least one result for each shall have a relative uncertainty less than 3 × 10−6'.
These uncertainty requirements were motivated by the need to replace the definition of the kelvin then in force, namely that the temperature of the triple point of water TTPW was exactly 273.16 K. Experimenters would determine k = F/TTPWd where F is an experimental quantity measured in joules. The value of TTPW in kelvin was defined to be exact at that time, hence the subscript 'd' in the CODATA report of the 2017 adjustment (Mohr et al 2018). Mohr et al emphasize the dependence of F on constants that are either unaffected by the revision of the SI (in 2018) or whose changes were negligible compared to the experimental uncertainties of F. Therefore the relation could be inverted so that TTPW = F/kd and the numerical value of k is now defined to be exact, as noted by CODATA's use of the subscript 'd'.
Redefining the kelvin in terms of a fixed numerical value of k means that if the relative uncertainty of k prior to redefinition were 1 × 10−6 or less, as recommended by the CCT, then TTPW measured with the new definition of the kelvin would remain 273.16 K within a standard uncertainty of less than 300 µK. A comparison among NMIs and the BIPM compared water triple point cells (Stock et al 2006) and subsequent research leads to a conclusion that such cells are reproducible to 50 µK, with some laboratories claiming 30 µK (CCT MeP-K).
By the time of the CODATA special adjustment of the fundamental constants in 2017, the CCT recommendations had been substantially bettered: the relative uncertainty of the adjusted value of k was 0.37 × 10−6 (corresponding to a standard uncertainty of 100 µK in measurements of TTPW); the final CODATA adjustment was based on data obtained by three fundamentally different methods: acoustic gas thermometry (AGT (Moldover et al 2014)), dielectric constant gas thermometry (DCGT (Gaiser et al 2015)) and Johnson noise thermometry (see for example Qu et al (2017), each method achieving a relative uncertainty of less than 3 × 10−6 and two of the individual AGT experiments reporting relative uncertainties below 1 × 10−6 (Pitre et al 2017, de Podesta et al 2017). Acoustic gas thermometry determines the molar gas constant R from which the value of k is inferred from knowledge of the Avogadro constant. The DCGT experiment determines the ratio of the molar gas constant to the molar polarizability of helium 4 gas. The JNT experiment determines the ratio k/h from which k is inferred from knowledge of the Planck constant. The CODATA-2017 adjustment takes account of all known correlations between experimental determinations of k. The data, including their reported uncertainties are plotted in figure 2 (Newell et al 2018). The recommended value of k based on these input data is labelled CODATA 2017. An account of the world-wide effort which produced the experimental results is given in Fischer et al (2018).
Figure 2. Determinations of the Boltzmann constant included in the 2017 adjustment of the Boltzmann constant by the CODATA Task Group on Fundamental Constants (Newell et al 2018).
Download figure:
Standard image High-resolution image6.1.2. Defined temperature scales and the CCT Declaration of 2014.
In temperature metrology, practitioners almost always measure an approximation to thermodynamic temperature based on one of two defined temperature scales: the International Temperature Scale of 1990 (ITS-90) (Preston-Thomas 1990) and the Provisional Low Temperature Scale of 2000 (PLTS-2000) (Rusby et al 2002).
The lowest temperature of the ITS-90 is 0.65 K. The scale includes a number of fixed points, the highest being the freezing point of copper (~1358 K). One of these is the triple point of water. Most of the other fixed points are either the triple points or the freezing points (at 101 325 Pa) of chemical elements. In the years preceding 1990, the various fixed point temperatures were measured as well as possible with respect to TTPW, whose fixed value at that time defined the kelvin. Experimental differences between laboratory results were evident but these were reconciled in a pragmatic way—essentially by averaging discrepant data. The temperatures of the fixed points were then defined by convention to be exact in the ITS-90, making this scale an approximation to thermodynamic temperature over its range of applicability, except at TTPW. Interpolating and extrapolating thermometers calibrated in terms of the fixed points are used to determine any temperature on the scale. Uncertainties of temperatures measured on the ITS-90 are consequently smaller than for corresponding measurements of thermodynamic temperature and these temperatures can be determined reproducibly with far less effort and expense than by using absolute methods. This situation is gradually changing over some ranges of the ITS-90, as discussed below.
Many guidance documents on the use and interpretation of the ITS-90 are available on the CCT website (CCT website). As one of these documents states, '...the definition of the kelvin in terms of the Boltzmann constant has no effect on the temperature values or realization uncertainties of the International Temperature Scales' and that the ITS-90 will continue to remain in use 'for the foreseeable future' (CCT MeP-K). See also Fischer et al (2018).
After 1990, it was found that a scale could be defined where temperatures below 1 K are a closer approximation to thermodynamic temperature than can be achieved with the ITS-90. This scale for temperatures from 0.9 mK to 1 K is called the Provisional Low Temperature Scale of 2000 (PLTS-2000)—'provisional' because discrepancies between input data from two sources used to define the PLTS-2000 have yet to be resolved (Machin 2018).
The CCT Declaration of 2014 acknowledges the known short-comings of both defined temperature scales (CCT 2014)
- For the ITS-90: 'inherent weaknesses, including known discrepancies from T'.
- For the PLTS-2000: 'currently no resolution of its inherent discrepancy of ~6 % at the lowest temperatures'.
and concludes that new thermodynamic temperature determinations are required to support:
- 'In the short term: the introduction and implementation of the mise en pratique for the definition of the kelvin (MeP-K) through determining robust, reliable values of T–T90 and T–T2000'.
- 'In the medium term: facilitate direct dissemination of the redefined kelvin through developing robust and reliable methodologies to disseminate T, particularly at the extremes of temperature >1300 K and <1 K'.
- 'In the long term: generate the background data required for a new unified temperature scale of improved thermodynamic consistency compared to the currently defined scales'.
It is now evident from acoustic gas thermometry that (T–T90) is significantly different from zero except in the vicinity of TTPW, as illustrated in figure 3 adapted from (Moldover et al 2016) (only relatively recent results of acoustic gas thermometry from the complete set are presented in figure 3, although the complete historical data set, as taken from Fischer et al (2011) is also plotted and discussed by Moldover et al). Their selected data set, taken from many different laboratories, is remarkably consistent by comparison to the historical data. As Moldover et al (2016) emphasize, near the triple point temperature of water (dT90/dT) ≈ 1.0001 and this implies that all measurements of heat capacity are in error by 0.01% when using ITS-90 instead of thermodynamic temperature. However, the authors also remark that this error has not led to any known difficulties in technology or science.
Figure 3. Measurements of T–T90 by acoustic gas thermometry over the temperature range from 173 K to 373 K (courtesy of Michael Moldover). The data include the recent results of Underwood et al (2017), and of references cited therein.
Download figure:
Standard image High-resolution imageThe present state of the PLTS-2000 is discussed by Machin (2018) in his comprehensive review of the present and possible future of thermometry.
6.1.3. CCT recommendation T1 (2017) for a new definition of the kelvin in 2018.
In 2017, the CCT formally adopted Recommendation T1 for consideration by the CIPM (CCT 2017). Following introductory comments, the CCT recommends
- 'that the CIPM finalises the unit definitions through agreeing to fix the values of the fundamental physical constants, from which a fixed numerical value of the Boltzmann constant with 8 digits will be adopted for the definition of the kelvin',
- 'that the member states take full advantage of the opportunities for the realisation and dissemination of thermodynamic temperature afforded by the kelvin definition and the mise en pratique for the redefinition of the kelvin'.
Regarding the recommendation that the fixed value of the Boltzmann constant should have eight digits, it may be noted in section 6 that the fixed value ultimately approved by the CGPM has only seven digits. The reason, as explained in Mohr et al (2018), is that the eighth digit would have been zero and therefore the fixed value was truncated to seven digits.
The second point refers to a major benefit of the present definition of the kelvin, namely that the measurement of thermodynamic temperature does not require knowledge of any fixed point temperature (although fixed points can still be exploited). References (CCT MeP-K) and (Fellmuth et al 2016) distinguish between 'absolute primary thermometry' (where no a priori knowledge of any fixed point temperature is required) and 'relative primary thermometry' (where one or more fixed points are required, for which the thermodynamic temperatures T and their uncertainties are known a priori from previous absolute or relative primary thermometry).
6.1.4. The possible future of thermometry.
The standard uncertainty of TTPW is now u(TTPW) = 100 µK and the CCT sees no prospect for further improvement (CCT MeP-K). Nevertheless, they do foresee that the triple point temperature of water will continue to be important as the most accurately-known fixed point temperature.
Efforts are being directed to reducing the uncertainty of (T–T90) across the ITS-90. The definition of the kelvin makes absolute primary thermometry an attractive method to realize the kelvin at temperatures above 660 K by means of radiometric thermometry (Anhalt and Machin 2016, Moldover et al 2016), and relative primary thermometry in the same region through the addition of new types of high-temperature fixed points (Machin 2013), as discussed in greater detail in Annexes of the mise en pratique for the definition of the kelvin. Other primary techniques are being developed to measure temperatures in the range of the PLTS-2000. As primary methods begin to chip away at the extremes of the defined temperature scales, one can envision a day when thermodynamic temperature can be measured by practical means across the temperature range of the defined scales (Machin 2018).
7. The mole
The mole, unit of amount of substance, has been redefined as well by the 26th General Conference. Previously, the mole was defined as the amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon 12. This definition linked the mole to the unit of mass. In the past years, however, there has been great momentum to redefine the mole as a specified number of particles, independently of the unit of mass, therefore recognizing the stoichiometric nature of amount of substance, which is fundamentally a quantity that refers to an ensemble of entities. The present definition of the mole tells us, thus, that the number of entities in one mole is the Avogadro number, which is the fixed numerical value of the Avogadro constant when expressed in the unit mol−1.
This value of the Avogadro constant comes from the special 2017 CODATA adjustment of the fundamental constants (Newell et al 2018). As described in section 4.3, the Avogadro constant is linked to the Planck constant h by a relation which involves the Rydberg constant (equation (1)). The value of the Avogadro constant as adjusted by CODATA was NA = 6.022 140 758 × 1023 mol−1 with a relative uncertainty of 1.0 × 10−8. In the revised SI, the Avogadro constant has the numerical value
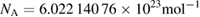
by definition, without uncertainty. Before the redefinition, the molar mass of carbon 12 had a fixed value of M(12C) = 0.012 kg mol−1. Now, in the present SI, the Avogadro constant has an exact value but the molar mass of carbon 12 needs to be measured to determine its value. At the time of the redefinition it had the same value as before, 0.012 kg mol−1, within a relative uncertainty of 4.5 × 10−10 (Mohr et al 2018). Equation (1) also governs this situation. Rearranging terms, this equation becomes
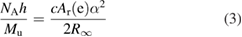
where the right-hand side is independent of the revisions that were approved on 16 November 2018. The relative uncertainty of the quantity on the right side is 4.5 × 10−10. The molar mass constant Mu remains exactly equal to M(12C)/12. When the value of Mu was exactly 0.001 kg mol−1, the relative uncertainty of the 'molar Planck constant', NAh was also 4.5 × 10−10. Now that the SI values of NA and h have no uncertainty, the relative uncertainty of Mu must be 4.5 × 10−10 despite its initial value remaining 0.001 kg mol−1.
In the revised SI, the definition of the mole is worded slightly differently than the definitions of the other units, which are strictly defined by the fixed numerical value of one or more fundamental constants when expressed in SI units. The definition of the mole, by contrast, while specifying the fixed numerical value of the Avogadro constant when expressed in mol−1, is redacted in a more pedagogical way, following the request of IUPAC and the chemistry community (Marquardt et al 2017, 2018).
8. The second
Aside from restructuring the definition of the second to be consistent with the definitions of the other base units approved by the CGPM in 2018, there has been no change to the definition adopted in 1967, which is based on a microwave transition frequency of the caesium 137 atom.
And yet, the improvements in clock technology during the past 30 years are arguably the most dramatic in any area of technology. The Consultative Committee for Time and Frequency (CCTF) discussed this during the recent years and the temporal evolution of the fractional uncertainty of realizations of the second with caesium atomic clocks and optical frequency standards is shown on figure 2 in Riehle et al (2018). In about 2008, the fractional frequency uncertainty of optical clocks surpassed that of the caesium microwave clock, which itself had seen dramatic improvements. One might infer from the figure that, taking optical clocks into account, overall clock technology has improved by four orders of magnitude during the past 30 years.
Riehle et al (2018) also proposes five principal achievements on the path to the redefinition of the second, as a tentative outline for achieving redefinition by the 28th meeting of the CGPM, which is assumed to be in 2026.
9. Conclusions
A revision of the SI was approved on 16 November 2018, and is to come into force on 20 May 2019. It is a major step towards some of the long-standing objectives of the measurement community. These date back to the late 18th century, when the advantage of a system of units that would be universally accessible was advocated, and to the 19th century, when James Clerk Maxwell advocated a unit system based on properties of 'imperishable and unalterable and perfectly similar molecules' (Maxwell 1870).
All of the units of the International System, the SI, are now defined by a set of seven constants the numerical values of which have been attributed by definition. The definitions of the base units are formulated in a way that will allow them to take advantage of future developments of more accurate measurement techniques. It is expected that the new definitions will allow the development of intrinsically accurate, quantum-based measurement techniques that can be used in research laboratories and to provide in situ traceability to the SI for a wide range of users.
However, these are not the final changes that will be made to the SI, it is expected that continued progress with the development of optical clocks will lead to a new definition of the second within the next decade. In the long term, the continuing ambition of metrologists to meet new needs means that the possibility will always remain that a consensus may emerge to change the SI again.
Annex. Resolution 1 of the 26th CGPM in 2018, adopting the revised SI
The following is the text of the resolution through which the revised SI was officially adopted by the 26th General Conference on Weights and Measures on 16 November 2018. It provides in the main body of text the numerical values of the defining constants, which are based on the special CODATA adjustment of fundamental constants (Newell et al 2018). The three appendices describe consequences of the revision. The new definitions of the seven base units are provided in appendix 3. The date of implementation is 20 May 2019.
Appendix. On the revision of the International System of Units (SI)
The General Conference on Weights and Measures (CGPM), at its 26th meeting,
considering
- the essential requirement for an SI that is uniform and accessible world-wide for international trade, high-technology manufacturing, human health and safety, protection of the environment, global climate studies and the basic science that underpins all these,
- that the SI units must be stable in the long term, internally self-consistent and practically realizable being based on the present theoretical description of nature at the highest level,
- that a revision of the SI to meet these requirements was proposed in Resolution 1 adopted unanimously by the CGPM at its 24th meeting (2011) that laid out in detail a new way of defining the SI based on a set of seven defining constants, drawn from the fundamental constants of physics and other constants of nature, from which the definitions of the seven base units are deduced,
- that the conditions set by the CGPM at its 24th meeting (2011), confirmed at its 25th meeting (2014), before such a revised SI could be adopted have now been met,
decides that, effective from 20 May 2019, the International System of Units, the SI, is the system of units in which:
- the unperturbed ground state hyperfine transition frequency of the caesium 133 atom ΔνCs is 9 192 631 770 Hz,
- the speed of light in vacuum c is 299 792 458 m/s,
- the Planck constant h is 6.626 070 15 × 10−34 J s,
- the elementary charge e is 1.602 176 634 × 10−19 C,
- the Boltzmann constant k is 1.380 649 × 10−23 J/K,
- the Avogadro constant NA is 6.022 140 76 × 1023 mol−1,
- the luminous efficacy of monochromatic radiation of frequency 540 × 1012 Hz, Kcd, is 683 lm/W,
where the hertz, joule, coulomb, lumen, and watt, with unit symbols Hz, J, C, lm, and W, respectively, are related to the units second, metre, kilogram, ampere, kelvin, mole, and candela, with unit symbols s, m, kg, A, K, mol, and cd, respectively, according to Hz = s−1, J = kg m2 s−2,
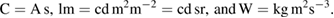
notes the consequences as set out in Resolution 1 adopted by the CGPM at its 24th meeting (2011) in respect of the base units of the SI and confirms these in the following Appendices to this Resolution, which have the same force as the Resolution itself,
invites the International Committee for Weights and Measures (CIPM) to produce a new edition of its Brochure entitled 'The International System of Units' in which a full description of the revised SI will be given.
Appendix 1. Abrogation of former definitions of the base units
It follows from the new definition of the SI described above that, effective from 20 May 2019:
- the definition of the second in force since 1967/68 (13th meeting of the CGPM, Resolution 1) is abrogated,
- the definition of the metre in force since 1983 (17th meeting of the CGPM, Resolution 1) is abrogated,
- the definition of the kilogram in force since 1889 (1st meeting of the CGPM, 1889, 3rd meeting of the CGPM, 1901) based upon the mass of the IPK is abrogated,
- the definition of the ampere in force since 1948 (9th meeting of the CGPM) based upon the definition proposed by the CIPM (1946, Resolution 2) is abrogated,
- the definition of the kelvin in force since 1967/68 (13th meeting of the CGPM, Resolution 4) is abrogated,
- the definition of the mole in force since 1971 (14th meeting of the CGPM, Resolution 3) is abrogated,
- the definition of the candela in force since 1979 (16th meeting of the CGPM, Resolution 3) is abrogated,
- the decision to adopt the conventional values of the Josephson constant KJ–90 and of the von Klitzing constant RK–90 taken by the CIPM (1988, Recommendations 1 and 2) at the request of the CGPM (18th meeting of the CGPM, 1987, Resolution 6) for the establishment of representations of the volt and the ohm using the Josephson and quantum Hall effects, respectively, is abrogated.
Appendix 2. Status of constants previously used in the former definitions
It follows from the new definition of the SI described above, and from the recommended values of the 2017 special adjustment of the Committee on Data for Science and Technology (CODATA) on which the values of the defining constants are based, that effective from 20 May 2019:
- the mass of the international prototype of the kilogram m(K) is equal to 1 kg within a relative standard uncertainty equal to that of the recommended value of h at the time this Resolution was adopted, namely 1.0 × 10−8 and that in the future its value will be determined experimentally,
- the vacuum magnetic permeability µ0 is equal to 4π × 10−7 H m−1 within a relative standard uncertainty equal to that of the recommended value of the fine-structure constant α at the time this Resolution was adopted, namely 2.3 × 10−10 and that in the future its value will be determined experimentally,
- the thermodynamic temperature of the triple point of water TTPW is equal to 273.16 K within a relative standard uncertainty closely equal to that of the recommended value of k at the time this Resolution was adopted, namely 3.7 × 10−7, and that in the future its value will be determined experimentally,
- the molar mass of carbon 12, M(12C), is equal to 0.012 kg mol−1 within a relative standard uncertainty equal to that of the recommended value of NAh at the time this Resolution was adopted, namely 4.5 × 10−10, and that in the future its value will be determined experimentally.
Appendix 3. The base units of the SI
Starting from the new definition of the SI described above in terms of fixed numerical values of the defining constants, definitions of each of the seven base units are deduced by taking, as appropriate, one or more of these defining constants to give the following set of definitions, effective from 20 May 2019:
- The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency ΔνCs, the unperturbed ground-state hyperfine transition frequency of the caesium 133 atom, to be 9 192 631 770 when expressed in the unit Hz, which is equal to s−1.
- The metre, symbol m, is the SI unit of length. It is defined by taking the fixed numerical value of the speed of light in vacuum c to be 299 792 458 when expressed in the unit m/s, where the second is defined in terms of ΔνCs.
- The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10−34 when expressed in the unit J s, which is equal to kg m2 s−1, where the metre and the second are defined in terms of c and ΔνCs.
- The ampere, symbol A, is the SI unit of electric current. It is defined by taking the fixed numerical value of the elementary charge e to be 1.602 176 634 × 10−19 when expressed in the unit C, which is equal to A s, where the second is defined in terms of ΔνCs.
- The kelvin, symbol K, is the SI unit of thermodynamic temperature. It is defined by taking the fixed numerical value of the Boltzmann constant k to be 1.380 649 × 10−23 when expressed in the unit J K−1, which is equal to kg m2 s−2 K−1, where the kilogram, metre and second are defined in terms of h, c and ΔνCs.
- The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number.The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
- The candela, symbol cd, is the SI unit of luminous intensity in a given direction. It is defined by taking the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency 540 × 1012 Hz, Kcd, to be 683 when expressed in the unit lm W−1, which is equal to cd sr W−1, or cd sr kg−1 m−2 s3, where the kilogram, metre and second are defined in terms of h, c and ΔνCs.
Footnotes
- 1
The wording of the original definition of the kilogram, adopted by the 1st CGPM in 1889, was clarified by the 3rd CGPM in 1901.
- 2
The watt balance is now called the Kibble balance in honour of Bryan Kibble, who invented it (Kibble 1976).
- 3
The mise en pratique for the definition of a unit is a set of instructions that allow the definition to be realized in practice at the highest level. Mises en pratique for the existing definitions are published on the BIPM web site (www.bipm.org) in appendix 2 of the SI brochure.