Abstract
In this letter, the feasibility of label-free in vivo GRIN-lens optical resolution photoacoustic micro-endoscopy is demonstrated. An image guide with 100 000 single-mode fibers in a 1.4 mm diameter bundle in conjunction with a 0.29 pitch GRIN lens is used in order to transfer a focused scanning spot through the image guide and refocus it into tissue. A high-repetition-rate (up to 600 kHz) ytterbium fiber laser is used in order to enable near real-time imaging capability. Phantom studies indicate 6 μm resolution. The system, with ∼2 mm working distance, overcomes the penetration depth limitation and hence improves the surface laser fluence of previously reported fiber based optical resolution photoacoustic microscopy (OR-PAM). The proposed system with a sub-mm probe footprint is very flexible and now has a significant penetration depth which is another step towards clinical applications.
Export citation and abstract BibTeX RIS
1. Introduction
Photoacoustic imaging is a unique imaging technique which takes advantage of optical absorption in order to provide imaging contrast. Acoustic resolution photoacoustic microscopy systems (PAM) rely on ultrasound transducer focusing in order to provide lateral spatial resolution [1]. In contrast, optical resolution photoacoustic microscopy (OR-PAM) can achieve micron-scale lateral resolution by using focused optical spot excitation [2]. OR-PAM can be used for various applications such as imaging capillary networks, capillary-scale blood oxygenation measurements, and neuro-vascular imaging in mice [2–4]. However, the limited penetration depth and large footprint of most OR-PAM systems restrict their noninvasive clinical applications to only superficial applications. For imaging of internal luminal organs, use of an endoscopic form is required [5–7].
In 2011, for the first time, we introduced the feasibly of optical resolution photoacoustic micro-endoscopy (OR-PAME) using a fiber bundle [5]. However, in the reported contact-mode operation, due to light divergence at the end of the image guide, the penetration depth was limited and deeper structures were not in-focus. In this letter, we introduce a gradient index (GRIN)-lens OR-PAME system for improving the lateral resolution and depth sectioning. The proposed system, with ∼2 mm working distance, extends the optically focused penetration depth to ∼1 mm, the transport-mean free path in tissue. Additionally, when focusing below the skin surface, surface fluence is significantly lower than our previous contact-mode OR-PAME system, offering improved safety. The small footprint of our novel system, ∼1.8 mm, gives this system the potential to be useful for clinical endoscopy applications when the thin fiber is inserted into body cavities.
GRIN lenses have been used in multiple modalities including two-photon microscopy, fluorescent microscopy, etc [8, 9]; however, this is the first report of a GRIN-lens-based OR-PAM system. OR-PAME is one of the few optical imaging techniques that can provide label-free absorption-contrast images in vivo. Other types of micro-endoscopy systems such as fluorescence and confocal fluorescence microscopy often use exogenous dyes in order to provide labeling.
Some endoscopic systems have employed micro-motors at the distal end of the endoscopic probe to provide light scanning, which increase the size of the endoscopy probe. In this letter galvanometer scanning mirrors are used at the proximal end of the probes where an imaging fiber bundle is used for transferring the focused light. The image guide consists of 100 000 individual fibers in a bundle 1.4 mm in diameter which provides transmission of a scanned focused laser spot in a compact, flexible fiber. The proposed C-scan GRIN-lens OR-PAME will bring our reported optical resolution photoacoustic micro-endoscopy (OR-PAME) system [5] one step closer to its introduction into clinical applications which can be used in longitudinal studies to examine tumor growth, angiogenesis, and anti-angiogenic drug efficacy.
The proposed GRIN-lens OR-PAME system is a label-free real-time imaging system with the capability of imaging small targets down to a capillary size. This system could have significant clinical impact for micro-endoscopic applications where the thin fiber can be inserted into body cavities. We demonstrate the capability of this technology with phantom studies and by imaging ears of hairless SCID mice.
2. Experimental setup
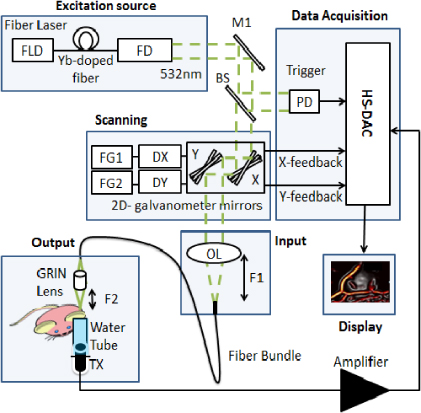
Figure 1 shows a simplified scanning GRIN-lens OR-PAME experimental setup. A diode-pumped pulsed ytterbium fiber laser (YLP-G, IPG Photonics Corporation) at 532 nm wavelength is used. This high pulse repetition rate (up to 600 kHz) fiber laser with ∼1 ns pulse width and up to 20 μJ per pulse enables real-time imaging. A photodiode is used to measure the pulse energy and trigger the data acquisition card. The laser beam is directed to an x–y galvanometer scanner (6230H, Cambridge Technology Inc.). The mirrors are controlled by analog sinusoidal signals from a two channel function generator (AFG3022B, Tektronix Inc.).
The speed and the angle of scanning are determined by the frequency and the peak-to-peak amplitude of the control signals respectively. In this experiment, the fast scanning rate of the 2D galvanometer system is fixed at 400 Hz while the slow scanning rate is fixed at 1 Hz, providing 2 C-scan frames per second. The pulse repetition rate (PRR) is fixed at 160 kHz. For a 400 by 400 μm field of view (FOV) the average step sizes for the X and Y directions are calculated as 2 and 1 μm, respectively.
The light is coupled to the 2 m long image guide fiber using an objective lens (f = 18 mm) focused on the fiber input and the beam is scanned by galvanometer scanning mirrors at the proximal end of the fiber. The light at the output of the fiber bundle is refocused using a 0.29 pitch GRIN lens (Thorlabs). We use an external 3.5 MHz focused ultrasound transducer to receive photoacoustic signals (19 mm focus, 6 mm active element, f# = 3.17, CD International Inc.). For each laser pulse, the photoacoustic signals are amplified using a 200 MHz computer controlled pulser/receiver (5900PR, Olympus NDT Inc). The amplified signals are then digitized using a 12-bit 8-channel PCI digitizer at a sampling rate of 125 Msamples s−1 (CS8289, Gage Cobra, Gage Applied Systems, Inc.). The x–y galvanometer feedback signals are also digitized and used to locate the position of each laser pulse at the sample. The peak-to-peak maximum amplitude from each A-scan is projected as a single pixel in a C-scan image. The image on a Cartesian grid is rendered using 2D interpolation.
Figure 1. Simplified scanning GRIN-lens OR-PAME experimental setup. FLD: fiber laser driver, Yb: ytterbium, FD, frequency doubling, M: mirror, BS: beam splitter, PD: photodiode, FG1: function generator channel 1, FG2: function generator channel 2, DX: X axis mirror driver, DY: Y axis mirror driver, OL: objective lens, F1: working distance of 19 mm, F2: working distance of 1.9 mm, HS-DAC: high speed data acquisition card, TX: ultrasound transducer.
Download figure:
Standard image3. Results and discussion
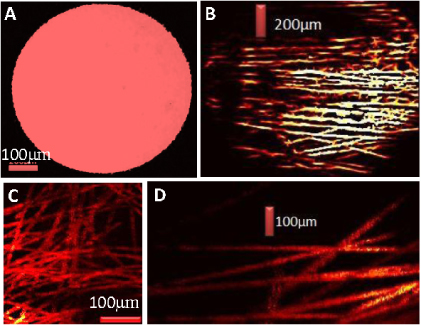
In order to demonstrate utilization of the entire aperture of the fiber (1.4 mm diameter field of view), we used black electrical tape as the imaging target, as shown in figure 2(A). We also imaged a network of carbon fibers with diameters of ∼7 μm (figures 2(B)–(D)), which are as small as capillary sized blood vessels. Figure 2(B) shows that the entire FOV of the 1.4 mm image guide is covered by the carbon fiber network.
Figure 2. (A) OR-PAME image demonstrating the entire field of view of the image guide. (B) A photoacoustic image of a carbon-fiber network using the GRIN-lens OR-PAME system. Each carbon fiber is ∼7 μm in diameter. (C), (D) An enlarged region of (B) which shows the fine details that can be achieved using this GRIN-lens OR-PAME system.
Download figure:
Standard imageThe full-width-half-maximum of the signal across the fiber was determined as ∼8.5 μm by fitting the carbon-fiber signal amplitudes to a Gaussian function. Taking into account the size of the fiber itself (7.5 μm), the lateral spatial resolution (FWHM) was estimated as ∼6 μm by performing numerical convolution of a Gaussian illumination spot with a rectangular region of width 7.5 μm. For in vivo studies, we imaged the ear of hairless SCID mice. A custom holder was engineered to connect the image guide fiber and GRIN lens in the right position.
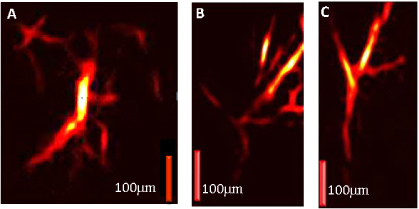
A customized ultrasound transducer holder was made to keep a small amount of water at the top of the transducer. The mouse ear was positioned on a thin transparent membrane stretched over this tube, and a drop of water was added for acoustic coupling. The distance between the mouse ear and the GRIN lens was about 1.5 mm. A focused 3.5 MHz transducer was positioned at the right distance inside the holder. All experimental animal procedures were conducted in conformity with the laboratory animal protocol approved by the University of Alberta Animal Use and Care Committee. The hairless SCID mouse was anesthetized using a breathing anesthesia system (E-Z Anesthesia, Euthanex Corp.) during image acquisition. Figure 3 shows different images of the microvasculature in a hairless SCID ear in vivo, and clearly shows a network of microvessels. Micro-endoscopy systems typically sacrifice wide field of view for high-resolution and small footprint flexible probes. A 2D-Hessian-based Frangi Vesselness filter [10] was used to filter C-scan maximum amplitude projection images to select tubular structures and reject noise. The acquisition frame rates for the GRIN-lens OR-PAME is 1–2 orders of magnitude faster than previous-generation systems thanks to our high-repetition-rate (600 kHz pulse repetition rate) fiber laser.
Figure 3. (A)–(C) GRIN-lens OR-PAME images of the microvasculature at various positions in a hairless SCID ear in vivo.
Download figure:
Standard imageThe power at the end of the fiber was measured as ∼45 mW with 160 kHz PRR, assuming that the depth of the laser focus was >200 μm below the tissue surface and that the numerical aperture of the lens was about 0.46. The calculated surface laser fluence was less than 1 mJ cm−2, which is much lower than the American National Standards Institute (ANSI) safety limit in the visible spectral region (20 mJ cm−2) [11]. We did not observe any tissue damage during our experimental sessions.
In the contact-mode operation of our fiber based OR-PAM without a GRIN lens, the signal-to-noise and lateral resolution degraded with imaging depth due to the large divergence angle at the output of the image guide fiber strands. However, using the GRIN-lens OR-PAME we can overcome this limitation. Future work could involve designing an all optical photoacoustic detector at the tip of the endoscopy probe in order to eliminate any external ultrasound transducer. The use of an external transducer, however, is not unrealistic as an ultrasound system could be used to both guide the positioning of the fiber and receive OR-PAME signals. Future enhancement may also include adding multi-wavelength capabilities for functional imaging. Mosaicing may expand the field of view [12].
4. Conclusion
In summary, the development of a GRIN-lens OR-PAME system with a 2 mm-footprint was demonstrated with the aid of an image guide (consisting of 100 000 individual fibers in a 1.4 mm bundle). The flexible and compact fiber bundle provided transmission of high optical resolution images. A fiber laser was employed to produce ∼1 ns pulses with energy up to 20 μJ and a repetition rate up to 600 kHz at a wavelength of 532 nm. The light was scanned using a high speed galvanometer mirror and coupled to the 2 m long fiber using an objective lens. The fiber bundle transferred the scanning focused light. The light at the output of the fiber bundle was refocused using a 0.29 pitch GRIN lens. The proposed setup maintains many of the powerful properties of bench-top OR-PAM systems and adds small footprint and high flexibility to them. We anticipate that this system and its future modifications will have significant clinical impact for endoscopic applications. The proposed system may find niches in almost every application where other micro-endoscopy systems are used currently. In future it could be used for even more applications because of its label-free imaging capability.
Acknowledgments
The first author gratefully acknowledges funding from an Alberta Innovates Graduate Student Scholarship and an SPIE Scholarship in Optics & Photonics. We also gratefully acknowledge funding from NSERC (355544-2008, 375340-2009, STPGP 396444), the Terry-Fox Foundation and the Canadian Cancer Society (TFF 019237, TFF 019240, CCS 2011-700718), the Alberta Cancer Research Institute (ACB 23728), the Canada Foundation for Innovation, Leaders Opportunity Fund (18472), Alberta Advanced Education & Technology, Small Equipment Grants Program (URSI09007SEG), Microsystems Technology Research Initiative (MSTRI RES0003166), University of Alberta Startup Funds, and Alberta Ingenuity/Alberta Innovates scholarships for graduate and undergraduate students.