Abstract
The rebound of impinging droplets is a defining characteristic of superhydrophobic surfaces; yet, such an intriguing interfacial phenomenon can be effectively suppressed by adding a tiny amount of flexible polymers to induce non-Newtonian viscoelastic properties. In this work, however, we demonstrate the promoting effects of surface heating on the rebound of impinging viscoelastic droplets on superhydrophobic surfaces. The underlying mechanism for the promotion is that the local heat transfer at the liquid–solid interface causes the fast evaporation of the liquid and thus the breakup of the formed viscoelastic filaments, which hinder droplet recoiling. Therefore, the lower threshold velocity for rebound increases while the upper threshold velocity for rebound suppression decreases with increasing surface temperature, resulting in a wider regime for droplet rebound in the impact phase diagram. The surface heating effect on liquid–solid interactions also leads to a nontrivial dependence of the contact time on the impact velocity and a linear decrease of the restitution coefficient with the Weber number for diverse bouncing viscoelastic droplets, which can be rationalized by coupling the interfacial force and energy analyses. We envision that these findings would be useful in technological processes requiring control the retention of viscoelastic liquids on solid surfaces.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Anti-wetting is an essential ability of biological systems living on the water-covered Earth [1], and various biosurfaces, including plant leaves [2, 3], insect wings [4], and animal feathers [5, 6], were found to be superhydrophobic. Micromorphology investigations revealed that these different biosurfaces have one characteristics in common: they are built of hydrophobic micro-/nanoscale structures, and thus can entrap a thin layer of air to separate the liquid from the solid [7]. As a result, an aqueous droplet beads up with a contact angle higher than 150° on the superhydrophobic surface, referring as the Cassie–Baxter state [8], and a slight inclination of the surface may cause the droplet rolling off [9]. The superhydrophobicity can even be maintained in a dynamic condition—an impinging droplet would rebound off the surface when its kinetic energy is sufficiently high to compensate the energy dissipated during impact, i.e. the impact velocity should be higher than a lower threshold [10, 11]. However, if the velocity exceeds an upper threshold, the separating air film breaks down upon impact, and the impinging droplet eventually sticks on the surface due to the wetting of the microscopic structures [12, 13], i.e. the droplet transits from the Cassie–Baxter state to the well-known Wenzel state [14].
The rebound of impinging droplets is an undesired phenomenon in agricultural spray since it reduces fluid transfer and thereby pesticide deposition [15, 16]. One remarkable strategy to prevent droplet rebound is by adding a tiny amount (down to ∼0.1 g l−1) of flexible polymers in the fluid [17, 18], which significantly slows down droplet retraction because of a few possible mechanisms under debate. The non-Newtonian extensional viscosity that originates from the orientation and stretching of polymer chains during impact was initially considered by Bergeron et al as the predominant factor hindering droplet motion, and hence suppressing the rebound [19]. However, this effect cannot explain the insignificant influence of polymer additives on droplet spreading, where strong extensional viscous dissipation is also expected. Later, Bartolo et al revised this mechanism and suggested that the non-Newtonian normal stress, which is induced by the stretching of polymer chains under the shear flow near the moving contact line, should be the main cause of the rebound suppression [20]. The generation of the normal stress can rationalize why there is a strong effect of polymer additives during retraction but not during spreading for impinging droplets on hydrophobic surfaces; yet, it fails to explain the impact behavior of polymer-laden droplets on structured superhydrophobic surfaces, where the normal stress is very small whilst the rebound suppression is still achievable [21, 22]. The negligible effect of the non-Newtonian normal stress on droplet impact dynamics was further confirmed by directly measuring the flow velocity within the impinging droplet, which also suggests that an effective friction arising at the retracting contact line is responsible for the suppression phenomenon [23]. Indeed, this mechanism is supported by the rejuvenated rebound of non-Newtonian droplets via nanoparticle enwrapping [22], and the direct visualization of viscoelastic ligaments as a polymer-laden droplet sweeps a superhydrophobic surface in the retraction stage [24].
Though adding polymer additives can effectively alter the outcomes of impinging droplets on solid surfaces, the maximum spreading radii of polymer-laden viscoelastic droplets are barely changed compared to that of Newtonian droplets [21, 24, 25], which are mainly controlled by the shear viscosity. As pointed out by Louhichi et al [26], both shear and non-Newtonian extensional viscous dissipations exist in the complex impact process of polymer-laden droplets on solid surfaces, and the dynamics is dominated by the shear rheology due to the liquid–solid contact. Indeed, the authors demonstrated the essential role of the extensional viscosity on the expansion of the polymer-laden droplets levitated on a liquid nitrogen thin layer and thus the enhancement of the maximum spreading due to the shear thinning behavior [26]. The dominant extensional effect was also identified during the droplet–droplet impact, where viscoelastic droplets exhibit less probability to rebound [27]. Moreover, Pack et al recently showed that a large increase of the extensional viscosity can significantly damp the capillary wave propagation during impact and thereby inhibit the droplet–surface contact on soft liquid films [28].
In industrial applications such as spray cooling, water-soluble polymers are normally employed to achieve high heat transfer efficiency [29]. Over the past decades, the influence of polymer additives on the impact dynamics on heated hydrophilic surfaces above the boiling temperature has been extensively investigated and well documented [30–33]. In particular, Bertola reported an increase of the maximum rebound height of aqueous polymer droplets on heated aluminium surfaces in the Leidenfrost regime [34], which might be ascribed to the enhanced storage of the impact kinetic energy as the recoverable elastic energy [35].
In this work, we report the impact dynamics of viscoelastic droplets of aqueous polymer solutions on heated superhydrophobic surfaces below the boiling temperature, which has received less attention so far. We demonstrate that surface heating does not influence droplet spreading but significantly speeds up droplet recoiling, and thus promotes the rebound behaviors of impinging viscoelastic droplets on superhydrophobic surfaces. Whereas the contact time of bouncing droplets was found to increase with surface temperature and show a nontrivial dependence on the impact velocity, a linear correlation between the restitution coefficient and the Weber number was identified. These phenomena can be explained by the reduced droplet–surface interaction as a result of the local heat transfer occurring at the liquid–solid interface, and the corresponding scaling models were proposed based on the interfacial force and energy analyses.
2. Experimental methods
Superhydrophobic surfaces were prepared by spray coating silicon wafers with a thin layer of hydrophobized nanoparticles (Ultra Glaco, Soft 99 Co., Japan), which form loose and porous structures (figure S1 (https://stacks.iop.org/NJP/22/123001/mmedia)) and thus own ultralow surface energy. Since the roughness of the solid surfaces would affect the contact line dynamics below the impinging droplets [36], it is essential to fabricate superhydrophobic surfaces with controlled surface roughness. In this work, our superhydrophobic surfaces were spray coated with Glaco nanoparticles for 3 times, which results in a nanoparticle layer with a thickness of ∼1.5 μm and a root-mean-square average of the height deviations of Rq
≈ 85 nm (Contour GT 3D, Bruker). We prepared viscoelastic solutions by dispersing 4M poly (ethylene oxide) (PEO, average molecular weight Mw = 4 × 106 g mol−1, Sigma Aldrich, USA) in pure water (18.4 MΩ cm, Millipore Synergy, Darmstadt, Germany). In order to identify the influence of liquid viscoelasticity on the droplet dynamics, the polymer concentration of the solutions should be sufficiently high [25]. Here we study aqueous PEO solutions with mass concentrations of cPEO = 0.1 g l−1, 0.5 g l−1, and 1.0 g l−1, whose extensional relaxation time is of the same order of the timescale of droplet impact  [28], with ρ, γ, R0 being the density, surface tension and radius of the impinging droplet respectively. Note that there could be a slight variability in the molecular weight of the PEO during the chemical synthesis and so do the aqueous PEO solutions. These solutions have comparable surface tensions as water, but exhibit non-Newtonian shear-thinning properties (figure S2). The static contact angles of 4 μl droplets of all aqueous PEO solutions on the superhydrophobic surface were measured to be ∼150°, while the contact angle hysteresis (i.e. the difference between the advancing and receding contact angles, θa − θr) was found to increase from ∼8° for 0.1 g l−1 PEO solution to ∼12° for 1.0 g l−1 PEO solution (table S1), suggesting an enhancement of droplet–surface interaction by adding polymer chains. In each impact event, a droplet of radius R0 ≈ 1.0 mm was released from a blunt needle, accelerated by gravity and eventually struck on the target superhydrophobic surface placing on a hot plate (figure S3). We investigated droplet dynamics at an impact velocity (V0) of 0.04–0.48 m s−1, in which the complete rebound happens, and the corresponding Weber number (
[28], with ρ, γ, R0 being the density, surface tension and radius of the impinging droplet respectively. Note that there could be a slight variability in the molecular weight of the PEO during the chemical synthesis and so do the aqueous PEO solutions. These solutions have comparable surface tensions as water, but exhibit non-Newtonian shear-thinning properties (figure S2). The static contact angles of 4 μl droplets of all aqueous PEO solutions on the superhydrophobic surface were measured to be ∼150°, while the contact angle hysteresis (i.e. the difference between the advancing and receding contact angles, θa − θr) was found to increase from ∼8° for 0.1 g l−1 PEO solution to ∼12° for 1.0 g l−1 PEO solution (table S1), suggesting an enhancement of droplet–surface interaction by adding polymer chains. In each impact event, a droplet of radius R0 ≈ 1.0 mm was released from a blunt needle, accelerated by gravity and eventually struck on the target superhydrophobic surface placing on a hot plate (figure S3). We investigated droplet dynamics at an impact velocity (V0) of 0.04–0.48 m s−1, in which the complete rebound happens, and the corresponding Weber number ( ) range is 0.05–7.5. The impact process was recorded by a high-speed camera (Phantom, V2012, USA) at 60 000 fps, and further analyzed using a MATLAB algorithm. All impact tests were performed under atmospheric pressure in the surface temperature range of T = 25 °C–95 °C, which is below the boiling point (100 °C) and well below the Leidenfrost temperature (∼193 °C) of water [37]. Therefore, the influence of liquid boiling and the Leidenfrost effect on the droplet dynamics can be safely neglected. Moreover, the high repeatability of the experimental results also indicates the negligible role of the slight variability of the molecular weights.
) range is 0.05–7.5. The impact process was recorded by a high-speed camera (Phantom, V2012, USA) at 60 000 fps, and further analyzed using a MATLAB algorithm. All impact tests were performed under atmospheric pressure in the surface temperature range of T = 25 °C–95 °C, which is below the boiling point (100 °C) and well below the Leidenfrost temperature (∼193 °C) of water [37]. Therefore, the influence of liquid boiling and the Leidenfrost effect on the droplet dynamics can be safely neglected. Moreover, the high repeatability of the experimental results also indicates the negligible role of the slight variability of the molecular weights.
3. Results and discussion
3.1. Rebound regime
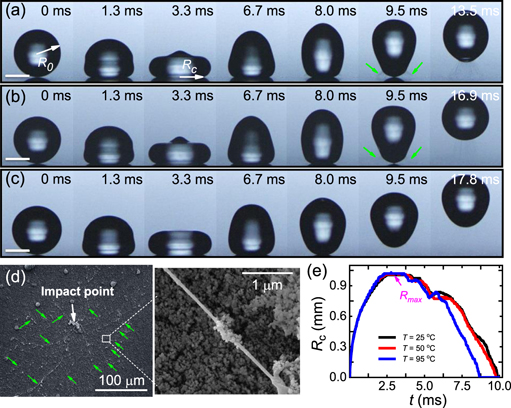
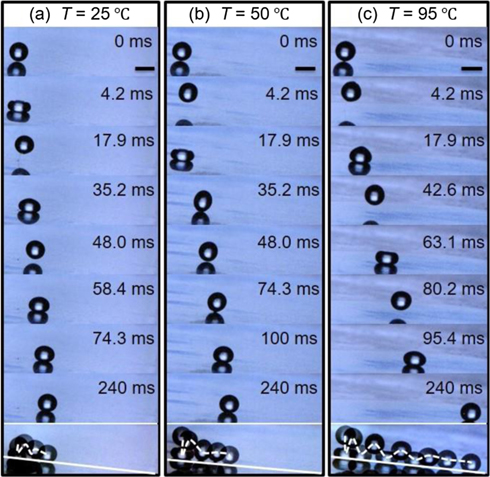
The promotion of the rebound of viscoelastic droplets on superhydrophobic surfaces via surface heating can be readily demonstrated by comparing the impact process and phenomena of 0.1 g l−1 PEO droplets with the same velocity of V0 ≈ 0.29 m s−1 at three different surface temperatures (T = 25 °C, 50 °C and 95 °C). After contacting the surface at 25 °C, the droplet spreads out with a violent capillary wave travelling from its bottom to top, resulting in complex deformations as that of low-viscosity Newtonian droplets [38, 39]. As a consequence, a pancake-like structure with a spire, as shown at ∼3.3 ms in figure 1(a), is formed at the maximal extension. For low-viscosity Newtonian liquids such as water, the spire subsequently punches deeply into the droplet due to its high velocity [38] and thus a cylindrical air cavity is created at the droplet center [40, 41]. With the recoiling of the droplet, the air cavity would be squeezed in the direction normal to the surface, and it may coalesce with the air film or bubble formed below the impinging droplet upon impact, which eventually suppresses droplet rebound on superhydrophobic-like soft surfaces since the liquid wets the surface [42, 43]. By contrast, if polymer chains are added in water, the downward motion of the spire can be significantly damped by the extensional viscosity near the droplet center, inhibiting droplet–surface contact [28]. In our experiments, this damping effect was also identified on superhydrophobic surfaces and it is more pronounced for impinging droplets with high PEO concentrations as the created air cavity is much shallower than of pure water droplets reported in previous studies [40–43]. Meanwhile, numerous viscoelastic filaments were observed near the contact line of the recoiling droplet (illustrated by green arrows at 9.5 ms in figure 1(a)). These filaments are anchored on the superhydrophobic surface and successively pulled out from the droplet once the liquid detaches (supplemental movie S1) [24]. This can be more clearly inferred from the scanning electronic microscopy (SEM) images of polymer residue after impact in figure 1(d), where numerous PEO nanofibers (denoted by green arrows) are deposited around the impact point. With droplet recoiling, they are further stretched, and eventually break up when their maximum limits are reached. Although the droplet can rebound off from the surface, the residual viscoelastic filaments restrict it to resume the spherical shape and be highly lifted up. The spreading characteristics described above were also observed for impinging PEO droplets on heated superhydrophobic surfaces at 50 °C and 95 °C, as evidenced by the snapshots in figures 1(b) and (c) and also the temporal evolution of the droplet contact radius (Rc) in figure 1(e). However, distinct droplet behaviors were found in the retraction stage. At any given time the formed viscoelastic filaments around the recoiling droplet are less on a hot surface than that on a cold surface (see 6.7–9.5 ms in figures 1(a)–(c), and supplemental movies S1–S3), and the corresponding retraction process is relatively faster (figure 1(e)). Consequently, on a hotter surface the droplet bounces off earlier with a shape comparably more close to a sphere, and the maximum height to which it can reach is relatively higher, particularly on the surface at 95 °C (figure 1(c)).
Figure 1. Snapshots of impinging droplets of 0.1 g l−1 aqueous PEO solution on superhydrophobic surfaces at 25 °C (a), 50 °C (b) and 95 °C (c). The scale bar is always 1.0 mm. The impact velocity V0 is ∼0.29 m s−1 and the corresponding Weber number We is 2.8. (d) SEM images of residual PEO fibers on the superhydrophobic surface acquired after the impact event in (a). (e) Temporal evolution of the contact radius Rc of impinging droplets in (a)–(c).
Download figure:
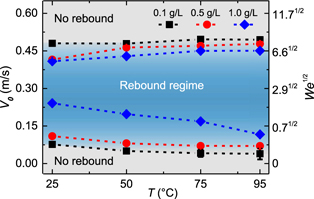
Standard image High-resolution imageFigure 2 plots the determined lower (VL) and upper (VU) threshold velocities of droplet rebound for those three aqueous PEO solutions we investigated. It is seen that the range of the impact velocity or equivalently the Weber number for rebound becomes wider either by increasing surface temperature or by decreasing polymer concentration. More specifically, VL of all aqueous PEO solutions decreases with increasing T or decreasing cPEO, while VU shows an increase trend when varying T or cPEO but less obvious.
Figure 2. Rebound regimes for viscoelastic droplets of 0.1 g l−1, 0.5 g l−1 and 1.0 g l−1 aqueous PEO solutions on superhydrophobic surfaces at different temperatures, defined by lower and upper threshold velocities or equivalently the Weber numbers.
Download figure:
Standard image High-resolution imageThe nontrivial dependence of the threshold velocities for rebound on the surface temperature and PEO concentration can be understood by an interfacial force analysis. The impact process and outcomes in figures 1(a)–(c) and 1(e) suggest that it is the viscoelastic filaments formed near the contact line leading to a large resistance force, slowing down droplet retraction, and thus determining the thresholds for rebound. Modeling each filament as a simple spring–mass–damper system (i.e. a point mass connected to a purely viscous damper and purely elastic spring in parallel), we derive the resistance force arising from stretching from the equation of motion [44],  . Here L is the length of the filament and is of the order of the maximum spreading radius of the impinging droplet Rmax (figure 1(e));
. Here L is the length of the filament and is of the order of the maximum spreading radius of the impinging droplet Rmax (figure 1(e));  represents the stretching speed and should be of the order of V0;
represents the stretching speed and should be of the order of V0;  denotes the acceleration rate and scales as
denotes the acceleration rate and scales as  ; c and k are the damping coefficient and the spring constant, both of which increase with increasing the impact velocity and the PEO concentration [24]; m is the mass of the liquid filament, which can be calculated by
; c and k are the damping coefficient and the spring constant, both of which increase with increasing the impact velocity and the PEO concentration [24]; m is the mass of the liquid filament, which can be calculated by  by assuming the liquid filament takes a cylindrical shape, where
by assuming the liquid filament takes a cylindrical shape, where  is the filament radius and Rfiber being the radius of the residual PEO nanofibers measured from the SEM image (figure S4). Substituting typical values of observed viscoelastic filaments (Rmax ∼ O(1.0 mm), V0 ≈ 0.1–0.5 m s−1, m ∼ 10−10–10−8 kg, c ∼ 10−4 Ns m−1 and k ∼ 1 N m−1 [24]), one finds that the viscous and inertial forces are at least 2 orders of magnitude smaller than the elastic force, and thus can be neglected. We further assume that the anchoring sites of viscoelastic filaments on the nanostructured superhydrophobic surface are uniformly distributed, then the total resistance force for the recoiling droplet can be expressed as, Fr ≈ 2πnfr
Rmax
2 ≈ 2πnkR0
Rmax
2, with n being the anchoring site number per unit area. It is noted that increasing the number of polymer chains in the solution or the liquid–solid contact area would cause a larger n and Fr. We also point out that the viscous force within the droplet [38, 45], which scales as πμRmax
2
V0/Hmax, is another resisting source of droplet motion, where μ is the dynamic viscosity and Hmax is the height of the droplet at maximum spreading. However, this term is also much smaller than the elastic force of viscoelastic filaments, and its effects on droplet motion can be ruled out as well. On the other hand, the driving force for droplet recoiling is the capillary force that originates from the impact-induced deformation [46], and it should be proportional to the inertial force of the impinging droplet, i.e.
is the filament radius and Rfiber being the radius of the residual PEO nanofibers measured from the SEM image (figure S4). Substituting typical values of observed viscoelastic filaments (Rmax ∼ O(1.0 mm), V0 ≈ 0.1–0.5 m s−1, m ∼ 10−10–10−8 kg, c ∼ 10−4 Ns m−1 and k ∼ 1 N m−1 [24]), one finds that the viscous and inertial forces are at least 2 orders of magnitude smaller than the elastic force, and thus can be neglected. We further assume that the anchoring sites of viscoelastic filaments on the nanostructured superhydrophobic surface are uniformly distributed, then the total resistance force for the recoiling droplet can be expressed as, Fr ≈ 2πnfr
Rmax
2 ≈ 2πnkR0
Rmax
2, with n being the anchoring site number per unit area. It is noted that increasing the number of polymer chains in the solution or the liquid–solid contact area would cause a larger n and Fr. We also point out that the viscous force within the droplet [38, 45], which scales as πμRmax
2
V0/Hmax, is another resisting source of droplet motion, where μ is the dynamic viscosity and Hmax is the height of the droplet at maximum spreading. However, this term is also much smaller than the elastic force of viscoelastic filaments, and its effects on droplet motion can be ruled out as well. On the other hand, the driving force for droplet recoiling is the capillary force that originates from the impact-induced deformation [46], and it should be proportional to the inertial force of the impinging droplet, i.e.  .
.
On the superhydrophobic surface at 25 °C, an impinging droplet is slightly deformed in the spreading stage (Rmax ∼ R0) at low impact velocity, and it only touches the top of microscopic surface structures during the whole impact process (i.e., it keeps an ideal Cassie state and n is almost a constant) [13, 24]. Therefore, balancing Fr with Fc yields the lower threshold velocity for rebound
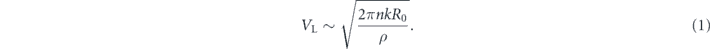
Based on the above equation, one can find that an increase in PEO concentration leads to a larger n and k, and thus a higher VL, agreeing well with the experimental data in figure 2. With the increase of the impact velocity, droplet deformation becomes significant, resulting in a larger spreading extent (Rmax > R0). Meanwhile, the liquid would impale into the microscopic surface structures if the wetting pressures exceed the anti-wetting pressure [10, 12, 13]. The wetting pressures are the liquid hammer pressure PH ≈ 0.2ρCV0 [47, 48], which lasts only a few microseconds on a square-micrometer-sized region around the liquid–solid contact point, and a dynamic pressure  [10, 13], which exerts on the droplet–surface contact area for the whole spreading process, where C is the sound speed in the liquid. Although the duration of the liquid hammer pressure is much shorter than the dynamic pressure, it has been demonstrated to play a dominant role for the transition from complete rebound to sticky state during the impact of liquid droplets on structured superhydrophobic surfaces [13, 49–51]. The anti-wetting pressures are the capillary pressures caused by the microprotrusions
[10, 13], which exerts on the droplet–surface contact area for the whole spreading process, where C is the sound speed in the liquid. Although the duration of the liquid hammer pressure is much shorter than the dynamic pressure, it has been demonstrated to play a dominant role for the transition from complete rebound to sticky state during the impact of liquid droplets on structured superhydrophobic surfaces [13, 49–51]. The anti-wetting pressures are the capillary pressures caused by the microprotrusions  and nanoprotrusions
and nanoprotrusions  with θa−f
being the advancing contact angle on the flat surface and Si
being the distance between two neighboring protrusions. By comparing these terms, we found that the impalement occurs on microprotrusions at V0 ≳ 0.02 m s−1 (SM ∼ 10 μm, θa−f
≈ 110°, C ≈ 1 495 m s−1) and on nanoprotrusions at V0 ≳ 0.40 m s−1 (SN ∼ 500 nm, figure S1), which enlarges the liquid–solid contact area, and thereby the number of filament anchoring sites n. As a result, the droplet rebound is inhibited above an upper threshold velocity VU of 0.45–0.52 m s−1 (figure 2), which is slightly higher than the impalement velocity on nanoprotrusions, and VU increases with increasing cPEO.
with θa−f
being the advancing contact angle on the flat surface and Si
being the distance between two neighboring protrusions. By comparing these terms, we found that the impalement occurs on microprotrusions at V0 ≳ 0.02 m s−1 (SM ∼ 10 μm, θa−f
≈ 110°, C ≈ 1 495 m s−1) and on nanoprotrusions at V0 ≳ 0.40 m s−1 (SN ∼ 500 nm, figure S1), which enlarges the liquid–solid contact area, and thereby the number of filament anchoring sites n. As a result, the droplet rebound is inhibited above an upper threshold velocity VU of 0.45–0.52 m s−1 (figure 2), which is slightly higher than the impalement velocity on nanoprotrusions, and VU increases with increasing cPEO.
We want to point out that when an impinging droplet approaches the target surface, the compression of the air between them would lead to the buildup of a large lubrication pressure, which deforms the droplet bottom into a dimple shape [46]. As a consequence, an air layer is always entrapped below the impinging droplet after contact with the surface, and it subsequently contrasts into an air bubble under the action of surface tension. Although surface roughness affects the initial contact between the droplet and surface [36], the shapes of the finally entrapped bubbles are barely influenced [52]. The entrapment of the air bubble has been theoretically investigated by Mandre et al [53], and experimentally resolved in finer details by van der Veen et al [54], and Li and Thoroddsen [55] on flat hydrophilic surfaces, and by van der Veen et al [52] and Langley et al [36] on structured superhydrophobic surfaces. The maximum pressure during the bubble formation  [56] serves as another anti-wetting pressure to prevent the liquid impalement, where Ca = μa
V0/γ is the capillary number with μa being the viscosity of air, and St = μa/ρV0
R0 is the Stokes number. However, Pmax (∼3.0 × 109 Pa) at the upper threshold velocity for rebound is 7 orders of magnitude higher than PD
(∼101–135 Pa) and 4 orders of magnitude higher than PH (∼1.4–1.6 × 105 Pa). This suggests that the entrapped air bubble would stay below the impinging droplet during the whole impact process, separating the liquid from the surface in a small region near the impact point (which has been experimentally observed in previous studies [36, 52, 56, 57]), and the liquid impalement (either in microprotrusions or in nanoprotrusions) triggered by the hammer pressure should occur at the rim of the entrapped bubble. Indeed, it was found that the residual PEO nanofibers after impact in figure 1(d) highly accumulate at a distance of ∼50 μm from the impact point, which is in the same order of magnitude with the bubble size Lb ∼ R0St4/9 ∼ 10 μm [58, 59], indirectly confirming the above analyses.
[56] serves as another anti-wetting pressure to prevent the liquid impalement, where Ca = μa
V0/γ is the capillary number with μa being the viscosity of air, and St = μa/ρV0
R0 is the Stokes number. However, Pmax (∼3.0 × 109 Pa) at the upper threshold velocity for rebound is 7 orders of magnitude higher than PD
(∼101–135 Pa) and 4 orders of magnitude higher than PH (∼1.4–1.6 × 105 Pa). This suggests that the entrapped air bubble would stay below the impinging droplet during the whole impact process, separating the liquid from the surface in a small region near the impact point (which has been experimentally observed in previous studies [36, 52, 56, 57]), and the liquid impalement (either in microprotrusions or in nanoprotrusions) triggered by the hammer pressure should occur at the rim of the entrapped bubble. Indeed, it was found that the residual PEO nanofibers after impact in figure 1(d) highly accumulate at a distance of ∼50 μm from the impact point, which is in the same order of magnitude with the bubble size Lb ∼ R0St4/9 ∼ 10 μm [58, 59], indirectly confirming the above analyses.
For impinging droplets on superhydrophobic surfaces at higher temperatures, local heat transfer happens at the solid–liquid contact area, and it is described by the heat conduction equation [60],  , where t is the time, α is the thermal diffusivity of the liquid and y is the distance of the liquid to the solid surface. As the impact process is radially symmetric, the one-dimensional heat conduction equation is thus employed for the analysis. A non-dimensional analysis of the equation provides a characteristic thermo-diffusion length
, where t is the time, α is the thermal diffusivity of the liquid and y is the distance of the liquid to the solid surface. As the impact process is radially symmetric, the one-dimensional heat conduction equation is thus employed for the analysis. A non-dimensional analysis of the equation provides a characteristic thermo-diffusion length  , in which the characteristic time τ should be on the timescale of droplet impact
, in which the characteristic time τ should be on the timescale of droplet impact  [46]. We found that LT is about 25 μm (α = 0.146–0.164 × 10−6 m2 s−1, τ ≈ 3.8 ms), which is comparable to the filament sizes (9–85 μm, figure S4) of diverse polymer solutions. It causes the fast evaporation of the water in viscoelastic filaments, significantly reducing their tensile strength, and thus they can easily break up during droplet recoiling (figures 1(a)–(c)). Consequently, a decrease of VL but an increase of VU with increasing T was identified in the experiments, regardless of the PEO concentration of impinging droplets (figure 2).
[46]. We found that LT is about 25 μm (α = 0.146–0.164 × 10−6 m2 s−1, τ ≈ 3.8 ms), which is comparable to the filament sizes (9–85 μm, figure S4) of diverse polymer solutions. It causes the fast evaporation of the water in viscoelastic filaments, significantly reducing their tensile strength, and thus they can easily break up during droplet recoiling (figures 1(a)–(c)). Consequently, a decrease of VL but an increase of VU with increasing T was identified in the experiments, regardless of the PEO concentration of impinging droplets (figure 2).
3.2. Contact time
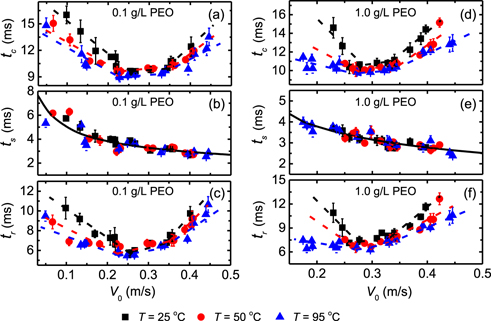
Among diverse characteristics of bouncing droplets, the contact time tc (i.e. the residence time of an impinging droplet after contact and before taking off the surface [61]) is a key parameter inferring the droplet–surface interaction during impact [17, 46]. For low-viscosity Newtonian liquids such as water, tc would increase at an impact velocity lower than a critical value, above which it approaches an asymptotic value of  [24, 61–63]. In figure 3, we comparatively show the contact time of bouncing droplets of 0.1 g l−1 and 1.0 g l−1 PEO solutions on superhydrophobic surfaces at three different surface temperatures. Evidently, tc of the same liquid droplets is shorter on hot surfaces than that on cold surfaces at similar impact velocities. By comparison, on superhydrophobic surfaces of same temperature, droplets of high PEO concentrations stay longer than droplets of low PEO concentrations before rebound (figure 3 and figure S5). Similar to low-viscosity Newtonian droplets [43, 63], tc of aqueous PEO droplets was found to increase with decreasing V0 at low impact velocities, and this trend stops at a critical velocity (∼0.27 m s−1), which is close to that of pure water droplets (∼0.26 m s−1) [24, 49]. However, at higher velocities, tc first starts to gradually increase (from a value close to τc, figure 3) with V0, and then an obvious increase is observed at V0 ≳ 0.35 m s−1, which corresponds to the impalement velocity of liquids into the nanoprotrusions of the superhydrophobic surfaces. By decomposing the impact process into the spreading and retraction stages, one can clearly see that the spreading time (ts) continuously decreases with the increase of V0 and the experimental data can still be well described by an empirical power-law for Newtonian droplets [38],
[24, 61–63]. In figure 3, we comparatively show the contact time of bouncing droplets of 0.1 g l−1 and 1.0 g l−1 PEO solutions on superhydrophobic surfaces at three different surface temperatures. Evidently, tc of the same liquid droplets is shorter on hot surfaces than that on cold surfaces at similar impact velocities. By comparison, on superhydrophobic surfaces of same temperature, droplets of high PEO concentrations stay longer than droplets of low PEO concentrations before rebound (figure 3 and figure S5). Similar to low-viscosity Newtonian droplets [43, 63], tc of aqueous PEO droplets was found to increase with decreasing V0 at low impact velocities, and this trend stops at a critical velocity (∼0.27 m s−1), which is close to that of pure water droplets (∼0.26 m s−1) [24, 49]. However, at higher velocities, tc first starts to gradually increase (from a value close to τc, figure 3) with V0, and then an obvious increase is observed at V0 ≳ 0.35 m s−1, which corresponds to the impalement velocity of liquids into the nanoprotrusions of the superhydrophobic surfaces. By decomposing the impact process into the spreading and retraction stages, one can clearly see that the spreading time (ts) continuously decreases with the increase of V0 and the experimental data can still be well described by an empirical power-law for Newtonian droplets [38],  , while the retraction time tr shows a similar dependence on V0, T and cPEO as tc, where ξ is a coefficient and β is the exponent. This finding further demonstrates that the effects of surface heating on bouncing viscoelastic droplets occur in the retraction stage as a consequence of the reduced droplet–surface interaction.
, while the retraction time tr shows a similar dependence on V0, T and cPEO as tc, where ξ is a coefficient and β is the exponent. This finding further demonstrates that the effects of surface heating on bouncing viscoelastic droplets occur in the retraction stage as a consequence of the reduced droplet–surface interaction.
Figure 3. Plot of the contact time tc, spreading time ts, and retraction time tr as a function of the impact velocity V0 for bouncing droplets of 0.1 g l−1 (a)–(c) and 1.0 g l−1 (d)–(f) aqueous PEO solutions at T = 25 °C, 50 °C, 95 °C. The solid lines are fittings with the equation  and the dashed lines show the trends of the experimental data.
and the dashed lines show the trends of the experimental data.
Download figure:
Standard image High-resolution image3.3. Restitution coefficient
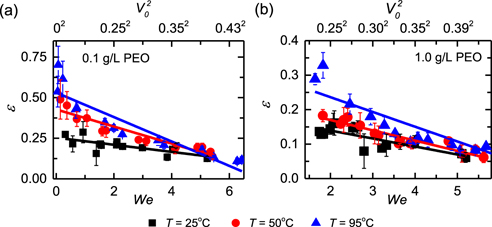
To quantitatively characterize the rebound phenomena, we have measured the restitution coefficient ɛ—another parameter reflecting the droplet–surface interaction in the dynamic conditions [11, 49, 64], and results of those two solutions in figure 3 are plotted in figure 4. Due to their nonspherical shapes (figures 1(a)–(c)), we traced the motion of the center of gravity of impinging droplets, and the restitution coefficient is defined as the ratio of the gravitational potential energy at the highest point of rebound and initial kinetic energy, i.e.  , where g is the gravitational acceleration and hmax is the height of the droplet gravity center with respect to the superhydrophobic surface. Obviously, ɛ is much smaller than 1 and decreases with increasing V0 or We for all bouncing droplets, implying strong energy dissipation during impact. The maximum restitution coefficient, which is found at the lower threshold velocity for rebound, decreases from ∼0.3 for 0.1 g l−1 PEO solution to ∼0.15 for 1.0 g l−1 PEO solution on superhydrophobic surfaces at 25 °C (figure 4 and figure S6). However, by increasing surface temperature, ɛ becomes larger for each aqueous PEO solution and a value up to ∼0.7 is observed for 0.1 g l−1 PEO solution at 95 °C. Most importantly, we found that the correlation between ɛ and We can be fitted by a linear function, ɛ = A − BWe, with A and B being two coefficients. The linear behavior is more pronounced for impinging droplets on superhydrophobic surfaces at T ≲ 50 °C and for impinging droplet at We ≳ 2.0 on superhydrophobic surfaces at T = 95 °C, regardless of the PEO concentration.
, where g is the gravitational acceleration and hmax is the height of the droplet gravity center with respect to the superhydrophobic surface. Obviously, ɛ is much smaller than 1 and decreases with increasing V0 or We for all bouncing droplets, implying strong energy dissipation during impact. The maximum restitution coefficient, which is found at the lower threshold velocity for rebound, decreases from ∼0.3 for 0.1 g l−1 PEO solution to ∼0.15 for 1.0 g l−1 PEO solution on superhydrophobic surfaces at 25 °C (figure 4 and figure S6). However, by increasing surface temperature, ɛ becomes larger for each aqueous PEO solution and a value up to ∼0.7 is observed for 0.1 g l−1 PEO solution at 95 °C. Most importantly, we found that the correlation between ɛ and We can be fitted by a linear function, ɛ = A − BWe, with A and B being two coefficients. The linear behavior is more pronounced for impinging droplets on superhydrophobic surfaces at T ≲ 50 °C and for impinging droplet at We ≳ 2.0 on superhydrophobic surfaces at T = 95 °C, regardless of the PEO concentration.
Figure 4. Restitution coefficient ɛ of bouncing viscoelastic droplets of 0.1 g l−1 (a) and 1.0 g l−1 (b) aqueous PEO solutions as a function of the Weber number We and impact velocity V0 at T = 25 °C, 50 °C, 95 °C. The solid lines are linear fittings.
Download figure:
Standard image High-resolution imageWe elucidate the inversely linear dependency of ɛ on We using a simple scaling argument. As discussed above, the main source dissipating the kinetic energy (Ek) of the impinging droplet is the viscoelastic resistance of the formed filaments, and the dissipated energy scales as  . Since the region over which the liquid hammer pressure applied is much smaller than that of the dynamic pressure, n is thus mainly determined by the wetting of surface microprotrusions under PD
. Making reasonable assumptions that these surface microprotrusions are constructed by columns of nanoparticles and the impalement depth of liquid into the microprotrusions increases with the dynamic pressure, one finds that the actual solid–liquid contact area [65] and thus the anchoring sites of viscoelastic filaments should be positively proportional to the Weber number, i.e. n ∝ We. Combining these correlations with the spreading law of impinging droplets on non-wetting surfaces, Rmax ∝ R0We0.25 [46], we obtain
. Since the region over which the liquid hammer pressure applied is much smaller than that of the dynamic pressure, n is thus mainly determined by the wetting of surface microprotrusions under PD
. Making reasonable assumptions that these surface microprotrusions are constructed by columns of nanoparticles and the impalement depth of liquid into the microprotrusions increases with the dynamic pressure, one finds that the actual solid–liquid contact area [65] and thus the anchoring sites of viscoelastic filaments should be positively proportional to the Weber number, i.e. n ∝ We. Combining these correlations with the spreading law of impinging droplets on non-wetting surfaces, Rmax ∝ R0We0.25 [46], we obtain  , and the restitution coefficient can be expressed as
, and the restitution coefficient can be expressed as
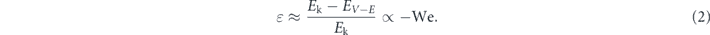
It is noted that droplet rebound only happens in an impact event that the input kinetic energy overweights the viscoelastic dissipation energy in the dynamic process. Therefore, the restitution coefficient would take a general form of ɛ = A − BWe as found in the experimental study for impinging droplets at T ≲ 50 °C. At surface temperature of 95 °C, the formed viscoelastic filaments quickly break up during recoiling, which significantly lessens the resistance force and thus leads to a high ɛ. As such, the linear fitting of the experimental data is compromised, especially for impinging droplets at We ≲ 2.0.
3.4. Droplet deposition after impact on inclined superhydrophobic surfaces
The promoted rebound of impinging droplets will reduce liquid retention on solid surfaces, and thus affect technological processes associated with droplet impact, e.g. pesticide deposition in agricultural spray [15, 16] and heat transfer in spray cooling [66]. To illustrate the influence of surface temperature on the deposition of aqueous PEO solutions on superhydrophobic surfaces, we further performed oblique droplet impact experiments, which are more commonly encountered in real-world applications [17, 46]. As displayed in figure 5(a) and supplemental movie S4, an impinging droplet of 0.1 g l−1 PEO solution with V0 ≈ 0.20 m s−1 rebounds two times on the inclined superhydrophobic surface (with tilting angle of ∼13°) at 25 °C, and it gets stuck on the surface at ∼3 mm away from the impact point. Although only two times of droplet rebounds were observed on the surface at 50 °C, the droplet is moved ∼5 mm due to its larger restitution coefficient (figure 4(a)) and then starts to roll down rather than stops moving (figure 5(b) and supplemental movie S5). In comparison, the number of droplet rebounds is increased to four times on the superhydrophobic surface at 95 °C, and the corresponding displacement distance along the surface is ∼12 mm (figure 5(c) and supplemental movie S6). Similar droplet behaviors were also found for other aqueous PEO solutions. These phenomena indicate an enhancement of the mobility of viscoelastic droplets on superhydrophobic surfaces by surface heating, which would reduce liquid deposition efficiency.
Figure 5. Snapshots of impinging droplets of 0.1 g l−1 aqueous PEO solution on superhydrophobic surfaces tilted for 13° with V0 ≈ 0.20 m s−1 at 25 °C (a), 50 °C (b), and 95 °C (c). The last row shows the superposition of successive images of droplet impact. The scale bar is always 1.0 mm.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, we experimentally investigated the impact dynamics of diverse viscoelastic droplets of aqueous PEO solutions on superhydrophobic surfaces at different temperatures. It was found that increasing surface temperature does not affect the spreading of impinging viscoelastic droplets, but can speed up droplet recoiling and thus promote the rebound behavior. This phenomenon is attributed to the evaporation and breakup of the formed viscoelastic filaments on the heated superhydrophobic surface, which hinder droplet retraction. As a result, the lower threshold velocity, above which droplet rebound occurs, is found to decrease with increasing surface temperature, while the upper threshold, above which liquid wets the microscopic surface structures and the rebound is inhibited, shows an increase trend with the surface temperature. The contact time is longer for impinging droplets with higher PEO concentrations on colder surfaces, and exhibits a non-monotonic dependence on the impact velocity: below a critical value the contact time significantly increases with decreasing the impact velocity, which is similar to Newtonian droplets, whereas above the critical velocity the contact time first gradually increases and then significantly increases due to the enhanced viscoelastic filament formation. We also found that the restitution coefficient of bouncing viscoelastic droplets linearly decreases with the Weber number, regardless of the PEO concentration. Simple scaling models exploiting the interfacial force and energy analyses are proposed to explain these results. We further demonstrate that the promoted rebound behavior by surface heating can reduce the deposition efficiency of viscoelastic liquids on inclined superhydrophobic surfaces.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 11772271) and the Opening fund of State Key Laboratory of Nonlinear Mechanics. BL and SWJ acknowledge the financial support from the National Research Foundation of Korea (Grant No. NRF-2018R1A2B3001246).