Abstract
Objective. In conventional radiotherapy, a single treatment plan is generated pre-treatment, and delivered in daily fractions. In this study, we propose to generate different treatment plans for all fractions ('Per-fraction' planning) to reduce cumulative organs at risk (OAR) doses. Per-fraction planning was compared to the 'Conventional' single-plan approach for non-coplanar 4 × 9.5 Gy prostate stereotactic body radiation therapy (SBRT). Approach. An in-house application for fully automated, non-coplanar multi-criterial treatment planning with integrated beam angle and fluence optimization was used for plan generations. For the Conventional approach, a single 12-beam non-coplanar IMRT plan with individualized beam angles was generated for each of the 20 included patients. In Per-fraction planning, four fraction plans were generated for each patient. For each fraction, a different set of patient-specific 12-beam configurations could be automatically selected. Per-fraction plans were sequentially generated by adding dose to already generated fraction plan(s). For each fraction, the cumulative- and fraction dose were simultaneously optimized, allowing some minor constraint violations in fraction doses, but not in cumulative. Main results. In the Per-fraction approach, on average 32.9 ± 3.1 [29;39] unique beams per patient were used. PTV doses in the separate Per-fraction plans were acceptable and highly similar to those in Conventional plans, while also fulfilling all OAR hard constraints. When comparing total cumulative doses, Per-fraction planning showed improved bladder sparing for all patients with reductions in Dmean of 22.6% (p = 0.0001) and in D1cc of 2.0% (p = 0.0001), reductions in patient volumes receiving 30% and 50% of the prescribed dose of 54.7% and 6.3%, respectively, and a 3.1% lower rectum Dmean (p = 0.007). Rectum D1cc was 4.1% higher (p = 0.0001) and Urethra dose was similar. Significance. In this proof-of-concept paper, Per-fraction planning resulted in several dose improvements in healthy tissues compared to the Conventional single-plan approach, for similar PTV dose. By keeping the number of beams per fraction the same as in Conventional planning, reported dosimetric improvements could be obtained without increase in fraction durations. Further research is needed to explore the full potential of the Per-fraction planning approach.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Following radiobiological and clinical evidence, the total dose in SBRT is generally administered in several fractions. Conventionally, a single SBRT plan is generated pre-treatment, based on the total intended target dose and constraints and objectives for healthy tissues. The total plan is then equally split among the fractions.
With this 'Conventional' approach, dose distributions are kept the same for every fraction, potentially limiting the degrees of freedom in plan optimization. Constraints may be required to avoid hotspots or undesired dose spikes, which could hamper minimization of organs at risk (OAR) doses to the full extent.
Several approaches to deviate from the Conventional single-plan convention have been proposed, with adaptive radiotherapy (ART) the most developed. The intention of ART is generally to adapt in each fraction the pre-treatment plan to the anatomy of the day or to compensate for problems in previous fractions (Sharfo et al 2016, Jagt et al 2018, 2019, Werensteijn-Honingh et al 2019, Sibolt et al 2021, Byrne et al 2022, Hoegen et al 2022, Kensen et al 2022, Mulder et al 2022, Oud et al 2022, Schiff et al 2022).
Another recent proposal for moving away from single-plan treatments is the spatiotemporal approach (Unkelbach et al
2016, 2017). In this planning technique, there seem to be no constraints for the single fractions; only cumulative doses are controlled while using the linear-quadratic model for dose addition in individual voxels. This can result in treatment fractions with doses much higher than  (N = number of fraction) in some parts of the tumor, and doses much lower in other parts. Although cumulative radiobiologically equivalent dose distributions may show impressive improvements compared to the conventional single-plan approach, especially the fraction dose distributions can strongly deviate from current treatments, making clinical introduction challenging.
(N = number of fraction) in some parts of the tumor, and doses much lower in other parts. Although cumulative radiobiologically equivalent dose distributions may show impressive improvements compared to the conventional single-plan approach, especially the fraction dose distributions can strongly deviate from current treatments, making clinical introduction challenging.
Many studies have demonstrated superior plan quality with automated planning compared to conventional manual trial-and-error planning (Sharfo et al 2016, Della Gala et al 2017, Heijmen et al 2018, Marrazzo et al 2019, Rossi et al 2019, Fiandra et al 2020, Bijman et al 2021, Fjellanger et al 2021, Naccarato et al 2022). Automated planning has also proven useful for comparing treatment techniques without bias from human planners (Sharfo et al 2015, Sharfo et al 2018, Bijman et al 2020, Fjellanger et al 2021, Rossi et al 2021, Leitão et al 2022, Redapi et al 2022). Automated planning also allows to increase the complexity of the optimization problem to further enhance plan quality (Breedveld et al 2009, Dong et al 2013, Sharfo et al 2016, Bijman et al 2020).
In this study, we used automated planning with integrated beam angle optimization to propose and investigate 'Per-fraction' SBRT planning to improve on the Conventional single-plan convention. In Per-fraction planning, the sequentially generated fraction plans can all be different to enhance the degrees of freedom for optimization of the cumulative delivered dose. For each fraction, the fraction dose, and the cumulative dose of the fractions up to and including the current fraction are simultaneously optimized. As proof of concept, Per-fraction planning was explored for prostate SBRT. For all fractions, (different) sets of patient-specific non-coplanar beam angles were automatically selected, taking into account dose delivered in previous fractions. To avoid increases in fraction durations, in each fraction the total number of beams was the same as the number used in Conventional planning. Per-fraction planning and Conventional planning were performed using the same automated planning solution.
2. Methods and materials
2.1. Patients and clinical protocol
Contoured CT scans of 20 prostate SBRT patients, previously treated with a robotic CyberKnife unit (Accuray Inc., Sunnyvale, USA), were used in the study. Patients were treated with four daily fractions of 9.5 Gy. Planning target volume (PTV) was defined as prostate contour with 3 mm isotropic margin. For the PTV, a planning aim was to obtain a V100% of 95% of the prescribed dose of 38 Gy at approximately the 60% isodose. Considered OARs were rectum, bladder, urethra and femoral heads (listed according importance priorities). Planning constraints were Dmax ≤ 38 Gy and D1cc ≤ 32.3 Gy for rectum, and Dmax ≤ 41.8 Gy and D1cc ≤ 38 Gy for bladder. Urethra dose was controlled by D5% ≤ 45.5 Gy, D10% ≤ 42 Gy and D50% ≤ 40 Gy, and femoral heads by Dmax ≤ 24 Gy (Aluwini et al 2010). Beyond fulfilling clinical constraints, further minimization of high OAR doses (first priority) and mean OAR dose (second priority), and maximization of minimum PTV dose and control of normal tissue dose was performed.
2.2. Conventional and Per-fraction planning for prostate SBRT
For the Conventional approach, a single 38 Gy treatment plan was generated for each patient, which was split into four equal 9.5 Gy dose distributions to be delivered in the four fractions. For each patient, this plan consisted of 12 patient-specific non-coplanar IMRT beams. Planning aims and priorities were in line with clinical planning (section 2.1). The applied automated plan generation is described below in section 2.3.
For the Per-fraction planning approach, the total dose of 38 Gy was equally divided between four sequentially generated, in principle different, fraction plans, each delivering 9.5 Gy to the PTV. For each fraction N > 1, both the to be established dose in fraction N, and the cumulative dose, defined as the sum of the already established doses for fractions up to and including N − 1, and the to be established dose in fraction N, were optimized. Both doses had to fulfill the respective PTV coverage objectives and OAR constraints. In all cumulative plans, also the dose bath constraints for healthy tissue outside the target and the entrance dose constraint had to be respected, while some violation was accepted in the individual fractions. The rationale behind this approach was to ensure for every fraction that none of the voxels could receive a cumulative dose higher than what was delivered in the Conventional approach, but that voxels which received low doses in previous fractions could get more in later fractions. Different 12-beam configurations could be automatically selected for each of the four fraction plans. See next section for technical details on plan generation.
2.3. Automated plan generation
The Erasmus-iCycle multi-criteria optimizer with integrated beam angle and fluence optimization (Breedveld et al 2012) was used for automatic generation of all Conventional and Per-fraction plans. With this system, the multicriteria optimization is steered by a wishlist, containing hard constraints which are always fulfilled and a list of prioritized objectives which are optimized in order of assigned priority. Generated plans are always Pareto-optimal. More details on Erasmus-iCycle plan generation can be found in (Breedveld et al 2012, Heijkoop et al 2014, Dirkx et al 2016, Buschmann et al 2018, Heijmen et al 2018, Rossi et al 2018, Rossi et al 2019, Bijman et al 2020, Bijman et al 2021, Leitão et al 2022, Redapi et al 2022). For beam angle optimization, beams could be selected from the 110 non-coplanar beams in the body node path of the M6 CyberKnife system.
Both Conventional and Per-fraction plans were generated using the wishlist reported in table 1. Apart from a constraint on the maximum PTV dose (a), there were OAR constraints for rectum (b), bladder (c) and urethra (d and e). Steering of the dose bath outside the PTV was performed with constraints f–k. Constraints l and m were used to control entrance dose. The applied seven objectives are ordered according to priority.
Table 1. Wishlist used for all plan generations for both Conventional and Per-fraction planning. N is the fraction number (which always equaled 1 for Conventional plan generation). '_cum' refers to planning aims for cumulative doses up to and including the dose in fraction N. '_fract' refers to dose added in a fraction N. 'Shellxxmm' are isostropic expansions of the PTV surface by xx mm. 'Entrance dose' is the dose in the 20 mm thick tissue layer below the patient's skin. EUD = Equivalent Uniform Dose.
| Constraints | ||||
|---|---|---|---|---|
| Structure | Type | Limit a | ||
| A | PTV_fract | Max | 61.5 Gy/4 | |
| B | Rectum_fract | Max | 36.5 Gy/4 | |
| C | Bladder_fract | Max | 39.5 Gy/4 | |
| D | Urethra_fract | EUD | 39 Gy/4 | k = 3 |
| E | Urethra_cum | EUD | 39 Gy/4 * N | k = 3 |
| F | Shell3mm_fract | Max | 38 Gy/4 * 1.8 | |
| G | Shell3 mm_cum | Max | 38 Gy/4 * N | |
| H | Shell30 mm_fract | Max | 20 Gy/4 * 1.8 | |
| I | Shell30 mm_cum | Max | 20 Gy/4 * N | |
| J | Shell50 mm_fract | Max | 16 Gy/4 * 1.8 | |
| K | Shell50mm_cum | Max | 16 Gy/4 * N | |
| L | Entrance dose_fract | Max | 18.5 Gy/4 * 1.2 | |
| M | Entrance dose_cum | Max | 18.5 Gy/4 * N | |
| Objectives | ||||
| Priority | Structure | Type | Goal | Parameters |
| 1 | PTV_fract | ↓LTCP b | 0.15 | (Dp, α, sufficient) = (9.5 Gy, 0.7, 0.15) c |
| 2 | PTV_cum | ↑Min | 30 Gy/4 * N | |
| 3 | Rectum_cum | ↓EUD | 0 Gy | k = 8 |
| 4 | Bladder_cum | ↓EUD | 0 Gy | k = 8 |
| 5 | Rectum_cum | ↓EUD | 0 Gy | k = 2 |
| 6 | Bladder_cum | ↓EUD | 0 Gy | k = 2 |
| 7 | Shell50 mm_cum | ↓Max | 0 Gy | |
a Some maximum dose constraints were set lower than clinical requirements to account for voxel sampling in the optimizations. b LTCP (Logarithmic Tumor Control Probability) minimization was used to enhance PTV coverage (Breedveld et al 2012). c See Breedveld et al (2012) for definition and use of α and sufficient. The wishlist was configured based on experiments for the first 5 study patients.
For Per-fraction planning, the wishlist was sequentially applied four times for each patient using N = 1, 2, 3 and 4 for generating the four fraction plans, automatically resulting in the final cumulative dose after generation of the plan for the last fraction (N = 4). The first fraction plans were always equal for Conventional and Per-fraction planning, since relaxations in dose bath and entrance dose were only acceptable in cumulative dose, as explained above.
For controlling both fraction doses and cumulative doses in Per-fraction planning, for all structures both 'structure_fract' planning aims and 'structure_cum' aims were used to steer on fraction and cumulative doses, respectively (table 1). The factors 1.8 in the limits for constraints f, h and j were used for relaxation of single-fraction planning aims for the dose bath, allowing to have 80% higher dose in a fraction. The factor 1.2 in constraint l was used to relax the single-fraction aim for the entrance dose. Objective 1 for PTV_fract aimed at adequate target coverage with 9.5 Gy in every fraction and thereby also with 38 Gy in cumulative plans. Constraint a ensured that the maximum PTV dose was approximately equal to  with Dp
equal to the prescribed dose, in line with clinical planning (section 2.1). Rectum_fract and bladder_fract Dmax constraints ensured adherence to Dmax constraints for both fraction- and cumulative doses. To ensure global optimality, only convex cost functions were used in the wishlist. This explains the use of EUDs for the urethra, while clinical planning aims were defined in terms of non-convex cost functions (section 2.1).
with Dp
equal to the prescribed dose, in line with clinical planning (section 2.1). Rectum_fract and bladder_fract Dmax constraints ensured adherence to Dmax constraints for both fraction- and cumulative doses. To ensure global optimality, only convex cost functions were used in the wishlist. This explains the use of EUDs for the urethra, while clinical planning aims were defined in terms of non-convex cost functions (section 2.1).
For Conventional planning, the wishlist was applied once using N = 1, which automatically rendered constraints f, h, j and l superfluous, since constraints e, g, I and k were already limiting more. The generated plan was then multiplied by four.
2.4. Plan evaluation and comparison
Prior to comparisons of total (cumulative) plans for the Per-fraction and Conventional approaches, generated plans were first evaluated for compliance with target coverage aims and OAR constraints.
Total plans were then compared in terms of PTV coverage, OAR sparing and dose bath in healthy tissues outside the PTV. Analyzed plan parameters were largely in line with the clinical protocol as described in section 2.1. For rectum and bladder, D0.03cc was used as a substitute for Dmax (Li et al 2018). The dose bath was evaluated with patient volumes (Vx%) receiving x = 10%, 30%, 50%, 70% and 100% of the prescribed dose. Femoral head doses were not reported as they were always far below the requirements.
Two-sided Wilcoxon signed-rank tests were used to analyze plan differences, using p < 0.05 for statistical significance.
3. Results
3.1. Plan acceptability
After minor rescaling to assure PTV coverage of at least 95%, the large majority of total cumulative plans fulfilled the PTV aims and OAR constraints, both for Conventional and for Per-fraction planning (table 2). This also held for the fraction plans in Per-fraction planning, as visible in table S1 of the supplementary material. There were exceptions for one patient with minor violations for bladder D1cc and urethra D10%, similarly for Conventional and Per-fraction planning.
Table 2. Dosimetrical parameters for cumulative dose distributions (38 Gy). All dose parameters are expressed in Gy while all volumetric parameters are expressed in %. Dmin' represents the near-minimum PTV dose, expressed as the dose that covers 100% of the PTV minus 0.03 cc. The last columns show percentual differences between Per-fraction and Conventional.
| Total plans | Conventional planning | Per-fraction planning | % Differences (Per-fract - Conv)/Per-fract *100 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Constraints | Mean | min | Max | mean | min | max | mean | min | max | p | |
| PTV | |||||||||||
| V95% | 95.0 | 95.2 | 95.0 | 96.9 | 95.2 | 95.0 | 96.5 | 0.0 | −0.4 | 0.2 | 0.3 |
| Dmin' | 28.0 | 24.8 | 30.4 | 27.5 | 23.1 | 30.8 | −2.1 | −9.8 | 4.7 | 0.02 | |
| Rectum | |||||||||||
| Dmean | 5.2 | 3.4 | 6.9 | 5.0 | 3.7 | 6.8 | −3.1 | −9.4 | 6.9 | 0.007 | |
| D1cc | 32.3 | 28.2 | 25.8 | 30.8 | 29.4 | 27.1 | 31.8 | 4.1 | 2.5 | 5.7 | 0.0001 |
| D0.03 cc | 38 | 35.0 | 32.6 | 37.4 | 36.0 | 33.6 | 38.0 | 2.9 | 1.8 | 3.8 | 0.0001 |
| Bladder | |||||||||||
| Dmean | 6.4 | 3.4 | 11.4 | 5.2 | 3.3 | 10.1 | −22.6 | −50.0 | −1.4 | 0.0001 | |
| D1cc | 38 | 35.2 | 33.5 | 38.9 | 34.5 | 32.4 | 38.8 | −2.0 | −4.3 | −0.3 | 0.0001 |
| D0.03cc | 41.8 | 39.5 | 37.8 | 41.8 | 39.1 | 36.9 | 41.7 | −1.0 | −2.9 | 1.2 | 0.001 |
| Urethra | |||||||||||
| D5% | 45.5 | 43.6 | 41.8 | 45.7 | 43.5 | 41.5 | 45.6 | −0.4 | −3.0 | 2.4 | 0.1 |
| D10% | 42 | 42.1 | 40.9 | 43.9 | 42.1 | 40.8 | 44.2 | 0.0 | −1.2 | 1.1 | 0.8 |
| D50% | 40 | 39.0 | 37.6 | 41.0 | 39.1 | 37.7 | 41.0 | 0.2 | −0.6 | 0.9 | 0.006 |
| Patient | |||||||||||
| V10% | 9.7 | 7.2 | 16.0 | 10.6 | 7.5 | 17.2 | 9.0 | 3.6 | 14.6 | 0.0001 | |
| V30% | 3.6 | 2.3 | 6.4 | 2.3 | 1.5 | 4.6 | −54.7 | −75.6 | −36.0 | 0.0001 | |
| V50% | 1.0 | 0.7 | 1.6 | 0.9 | 0.6 | 1.5 | −6.3 | −10.0 | −2.9 | 0.0001 | |
| V70% | 0.6 | 0.4 | 0.9 | 0.6 | 0.4 | 0.9 | −1.0 | −3.4 | 1.1 | 0.005 | |
| V100% | 0.3 | 0.2 | 0.5 | 0.3 | 0.2 | 0.5 | 0.3 | −0.5 | 1.3 | 0.009 | |
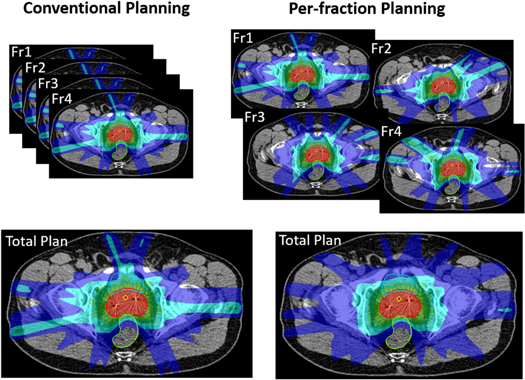
Although in Per-fraction planning, dose bath constraints and the entrance dose constraints were relaxed for the individual fractions, dose distributions for these fractions were considered acceptable, i.e. there were no unacceptable hotspots, spikes and entrance doses, as visible in figure 1 for an example patient.
Figure 1. Dose distributions for an example patient (patient with the difference between the two planning approaches in cumulative bladder Dmean most similar to the average difference in the population). For Conventional planning, the total plan is the sum of 4 identical plans, while for Per-fraction, the total plan is the sum of 4 different fraction plans. Displayed doses are 10%, 30%, 50%, 70% and 100% of prescribed.
Download figure:
Standard image High-resolution image3.2. Conventional versus Per-fraction—total cumulative dose
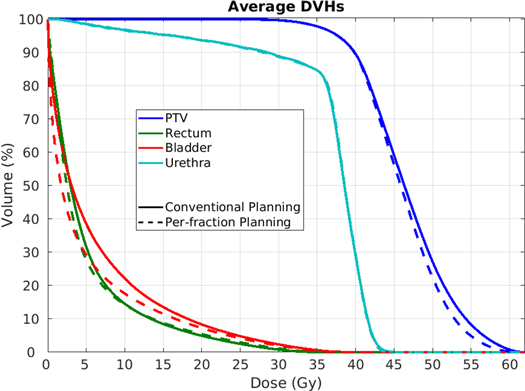
While obtaining comparable target coverage (table 2), Per-fraction planning significantly reduced bladder high and medium dose, as also visible in the average DVHs in figure 2. With Per-fraction planning, bladder D1cc and Dmean, reduced by 2% (p = 0.0001) and 22.6% (p = 0.0001), rectum Dmean reduced as well as by 3.1% (p = 0.007), see table 2. Rectum D1cc was 2.9% lower (p = 0.0001) in the Conventional approach, while no significant differences were observed for urethra (table 2).
Figure 2. Population average DVHs for the 20 patients for the Conventional approach (solid lines) and Per-fraction planning (dashed lines).
Download figure:
Standard image High-resolution imageTable 2 shows that Per-fraction planning resulted for one of the patients in a reduction in cumulative near-minimum PTV dose of 9.8%. The clinical aim in our center is that 95% of the PTV should get at least the prescribed dose. There is no planning aim for the dose in the remaining 5% of the PTV. This is also not evaluated in clinical planning. In this paper we have presented the near-minimum PTV dose to verify that overall the differences were acceptable with a mean reduction of 2.1%.
The dose bath got significantly improved with per-fraction planning; patient volumes receiving 30%, 50% and 70% of the prescribed dose reduced by 54.7%, 6.3% and 1%, respectively (all p < 0.005). Patient volumes receiving 10% and 100% of the prescribed dose were lower with Conventional planning by 9% (p = 0.0001) and 0.3% (p = 0.009). Figure 1 clearly shows reduced dose spikes in the total cumulative Per-fraction plan compared to Conventional planning.
3.3. Conventional versus Per-fraction—applied beams
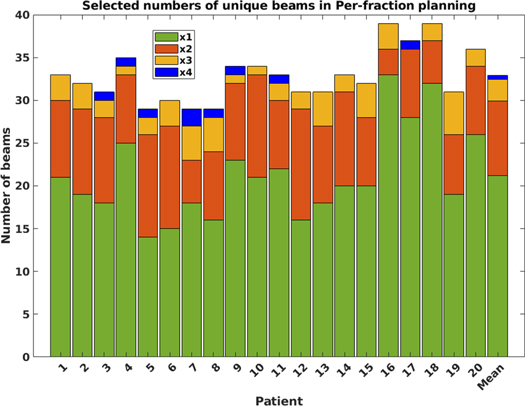
While in the Conventional approach, 12 unique patient-specific non-coplanar beams could be (and were) used for each patient, in the Per-fraction approach, a maximum of 48 unique beams could be used; 12 per fraction. Figure 3 summarizes the observed beam selections for all patients and the overall population mean (last bar). On average, 32.9 ± 3.1 [29;39] unique beams were selected per patient, meaning that some beams were used in more than one fraction. On average, out of the 48 selected beams, 21.2 beams were used in only one fraction, 8.8 in two, 2.5 in three and 0.5 in all four fractions. For 12 of the 20 patients, beams were used in at maximum 3 fractions. For the other eight patients, two beams (patient 7) or one beam (patients 3, 4, 5, 8, 9, 12 and 17) were used in all four fractions (figure 3). Differences in selected beams in the four fractions are also clearly visible for the example patient in figure 1.
Figure 3. Selected numbers of unique beams in the four-fraction Per-fraction planning approach for each of the 20 patients (maximum = 4 × 12), and the population mean (last bar). Green: beams selected for only one of the four fractions, orange: beams used in two fractions, yellow: beams used in three fractions, blue: beams used in all four fractions.
Download figure:
Standard image High-resolution image4. Discussion
In this study, we have proposed the novel Per-fraction treatment planning approach to increase degrees of freedom for plan optimization compared to the Conventional single-plan approach. In Per-fraction planning, the sequentially generated fraction plans are in principle all different. In each fraction, the fraction- and the cumulative dose up to and including the current fraction are simultaneously optimized. Some of the planning goals can be strictly controlled in all individual fractions e.g. a PTV coverage of at least 95%. For other planning goals, a relaxation can be applied in the individual fractions while cumulative doses always respect the total plan aims.
For proof of concept, Per-fraction planning was explored for four-fraction prostate SBRT. Different non-coplanar beam directions could be selected in the different treatment fractions to enhance the degrees of freedom in plan optimization. To not enhance fraction durations, the number of beams in each fraction was the same as the number used in the Conventional single-plan approach. Compared to Conventional, dosimetric improvements for bladder mean and high dose, rectum mean dose, and the dose bath and dose spikes going from the PTV outwards (figure 1) could potentially be clinically relevant. Rectum high doses slightly (but statistically significantly) deteriorated. As visible in table 2, rectum had the highest priority after target doses. In fact, as demonstrated in a previous study (Rossi et al 2018), with the applied automated planning approach very low rectum doses are obtained. In our current clinical practice, bladder dose is of serious concern.
In this study, plan generation for the current fraction was performed by simultaneous optimizations of the beam directions and the beam profiles. In principle, Per-fraction planning with only optimization of the fluence profiles while keeping the beam configuration fixed could also result in enhanced final cumulative plan quality. The latter approach was not investigated in this paper to not limit the degrees of freedom in plan optimizations.
The constraint violations that were allowed in the presented prostate SBRT planning study were rather conservative, upfront avoiding discussion on acceptability of fraction plans e.g. because of inadequate coverage or violations of critical OAR constraints in some of the fractions. Possibly, dosimetric gains of Per-fraction planning could be further enhanced by making less conservative choices, e.g. by allowing (slightly) reduced coverage in some of the fractions, while obtaining adequate coverage with cumulative dose. Such an approach could possibly also be used to mitigate the observed increases in rectum D1cc. This is a topic of future research that also needs to consider radiobiological impact of variable fraction doses for targets and OARs. Further work will also explore the impact of Per-fraction planning for other treatment protocols and treatment sites. In intensity-modulated proton therapy (IMPT), generally only a few treatment beams are used per patient in every fraction. Possibly, choosing different beams in every fraction could further enhance dosimetric quality of the plans.
In development of Per-fraction implementations, many-beam plans could possibly be used as benchmarks for finding optimal trade-offs between cumulative plan quality, quality of fraction plans and delivery time in Per-fraction planning. See figure S1 in the supplementary material for an example and further explanations.
In this study, the individual fraction plans in the Per-fraction approach were generated using for each patient a single, pre-treatment acquired planning CT in order to purely investigate the benefit of deviating from the single-plan approach. Application of the proposed cumulative Per-fraction planning methodology for adaptive radiotherapy with daily patient imaging is expected to be feasible and is a topic of further research.
We implemented Per-fraction planning using integrated beam profile and angle optimization. A limitation of the use of Erasmus-iCycle for beam angle optimization are the long calculation times (in this study 4.2 h (1.8–7.0) for a 12-beam plan on our busy computer cluster). Recently, it has been demonstrated that so-called total-beam-space beam angle optimization, TBS-BAO (Schipaanboord et al 2022) can significantly reduce computation times.
A practical limitation of Per-fraction planning may be that that the approach seems infeasible with conventional manual planning. It may not even be easy to implement with currently commercially available autoplanning systems. Erasmus-iCycle is not commercially available, but a very similar system for fully automated multi-criteria planning has been implemented in a commercial TPS and is currently being tested (Bijman et al 2021, Naccarato et al 2022).
Although also proposed spatiotemporal fractionation approaches (Unkelbach et al 2016, 2017) deviate from the Conventional single-plan approach, there are substantial differences with Per-fraction planning. Some of the main differences are that in the former, planning relies on voxel-wise radiobiological modelling, and tumor doses may be highly inhomogeneous, even with large parts of the PTV heavily underdosed in some fractions, which is then compensated in other fractions.
The study reported a RATING score of 86% (Hansen et al 2020), see supplementary material.
5. Conclusion
We have proposed and evaluated the novel Per-fraction treatment planning approach with different plans for all fractions, which are sequentially generated. Intention is to enhance the degrees of freedom for optimization of total delivered dose. Some planning goals are strictly controlled in each of the fractions, while for other goals a relaxation may be applied in individual fractions while cumulative doses still respect original aims. Per-fraction planning resulted for prostate SBRT in significant improvements in bladder doses and the dose bath.
Data availability statement
The data cannot be made publicly available upon publication due to legal restrictions preventing unrestricted public distribution. The data that support the findings of this study are available upon reasonable request from the authors.
Supplementary data (0.2 MB DOCX)