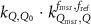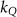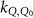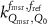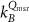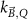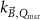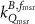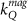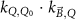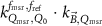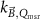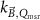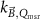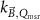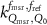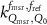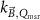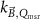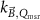Abstract
With the rapid increase in clinical treatments with MRI-linacs, a consistent, harmonized and sustainable ground for reference dosimetry in MRI-linacs is needed. Specific for reference dosimetry in MRI-linacs is the presence of a strong magnetic field. Therefore, existing Code of Practices (CoPs) are inadequate. In recent years, a vast amount of papers have been published in relation to this topic. The purpose of this review paper is twofold: to give an overview and evaluate the existing literature for reference dosimetry in MRI-linacs and to discuss whether the literature and datasets are adequate and complete to serve as a basis for the development of a new or to extend existing CoPs.
This review is prefaced with an overview of existing MRI-linac facilities. Then an introduction on the physics of radiation transport in magnetic fields is given. The main part of the review is devoted to the evaluation of the literature with respect to the following subjects:
• beam characteristics of MRI-linac facilities;
• formalisms for reference dosimetry in MRI-linacs;
• characteristics of ionization chambers in the presence of magnetic fields;
• ionization chamber beam quality correction factors; and
• ionization chamber magnetic field correction factors.
The review is completed with a discussion as to whether the existing literature is adequate to serve as basis for a CoP. In addition, it highlights subjects for future research on this topic.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
With the rapid increase in clinical treatments with magnetic resonance imaging (MRI) guided radiotherapy (MRgRT) based on MRI-linacs, a consistent, harmonized and sustainable ground for reference dosimetry in MRI-linacs is needed in the form of a Code of Practice (CoP). Since several CoPs for reference dosimetry in MV photon beams of conventional linacs exist (Lillicrap et al 1990, Almond et al 1999, Andreo et al 2000, Aalbers et al 2008), which have resulted in a good consistency in the applied methods (Saiful Huq et al 2001, Andreo et al 2002, 2013, Perik et al 2013), it is highly preferred to develop such a CoP for reference dosimetry in MRI-linacs as an add-on to the existing CoPs for conventional linacs.
In recent years, a vast amount of papers have been published in relation to this topic: reporting (magnetic field) correction factors for ionization chambers (Meijsing et al 2009, O'Brien et al 2016, Spindeldreier et al 2017, Malkov and Rogers 2017, Pojtinger et al 2018, van Asselen et al 2018, de Prez et al 2019b) and chemical detectors, such as alanine (Billas et al 2020) and Fricke-type (Trachsel et al 2020), development of primary standards (de Prez et al 2016a), beam quality specifiers (O'Brien et al 2016), as well as the characterization of effects uncommon in reference dosimetry for conventional linacs such as the air-gap effect (Hackett et al 2016, Agnew et al 2017).
The purpose of this review paper is twofold: to give an overview and evaluate the existing literature for reference dosimetry in MRI-linacs and to discuss whether the literature and datasets are adequate and complete to serve as a basis for the development of an add-on to existing CoPs. This review paper can then be used as starting point in the development of add-ons for reference dosimetry in MRI-linacs to existing CoPs. The following topics will be covered in this paper:
- description of MRI-linac facility characteristics relevant for reference dosimetry;
- basic physics of radiation transport in magnetic fields;
- beam characteristics of MRI-linac facilities;
- formalisms for reference dosimetry in MRI-linac facilities;
- characteristics of ionization chambers in the presence of magnetic fields;
- ionization chamber beam quality correction factors; and
- ionization chamber magnetic field correction factors.
Note that because most of the literature covers dosimetry for facilities with an orthogonal orientation of the linac beam with respect to the magnetic field, this review will mainly focus on these types of facilities. Where possible, dosimetry for inline facilities with a parallel orientation of the linac beam with respect to the magnetic field will be evaluated and discussed.
2. MRI-linac facility characteristics
2.1. Elekta Unity™
The Elekta Unity™ MRI-linac (Elekta Instrument AB Stockholm, Sweden) was invented by the UMC Utrecht and developed in collaboration with Elekta and Philips (Raaijmakers et al 2005, Raaymakers et al 2017). The system incorporates daily verification of variation in patient anatomy, i.e. variation in tumour position and healthy surrounding tissues by creating a new on-line adapted treatment plan for every treatment. A first-in-man study to demonstrate the safe and accurate delivery was performed in 2017 on the clinical prototype MRI-linac (Raaymakers et al 2017). The Unity™ system was clinically released in 2018 after CE-certification.
The device is a combination of a 1.5 T Philips 70 cm wide-bore MRI scanner, a 7 MV flattening-filter-free (FFF) beam generated with a standing-wave linear accelerator (linac) and an adaptive treatment software solution.
The active shielding method of the magnet provides a zone with a low magnetic field around the cryostat in which the magnetic-field sensitive linac equipment is located. The linac equipment is mounted on a gantry ring allowing for continuous rotation at 6 RPM. The source-to-isocenter-distance (SID) of the linac is 143.5 cm. The magnetic field inside the bore is directed towards −yf, IEC61217 (2011), see figure 6, perpendicular to the radiation beam. The radiation beam travels through the beam portal region in the cryostat (approximately 15 cm wide), which is free of the super-conducting coils and contains lower attenuating construction. Radiation field size ranges from 1 × 1 cm2 up to 22 × 57 cm2. The MLC, an adapted Agility design being compatible to the high-magnetic field, consist of 160 leaves (projected leaf width at isocenter is 7.2 mm) and two backup jaws at a fixed collimator angle.
The helium level in the cryostat is maintained high and constant to keep gantry angle dependent dose variations due to this level to less than 1%. Dose output variation due to local inhomogeneities of the cryostat (e.g. welds) and couch are characterised and taken into account in the TPS. The maximum dose rate at the isocenter is approximately 4.25 Gy min−1.
2.2. ViewRay MRIdian™
The ViewRay MRIdian™ system (ViewRay Inc. Oakwood, USA) was the first commercially available MRI-integrated radiation device (Mutic and Dempsey 2014). It obtained its Food and Drug Administration (FDA) approval in 2010 and the first patient was treated with this system in 2014 at Washington University (St. Louis, USA) (Acharya et al 2016).
This device has been described previously by Mutic and Dempsey (2014) and consists of a split bore 0.35 T MRI with a bore size of 70 cm. The magnetic field is directed towards +yf IEC61217:2011 (2011), see figure 6, perpendicular to the radiation beam. The MRI employs two superconducting solenoids, self-shielded gradient coils with gradient strengths and slew rates of up to 18 mT m−1 and 200 T m−1 s−1, respectively, a 75 cm diameter radiofrequency transmitting birdcage coil and a surface receiver coil with only 0.75% beam attenuation. In addition, the MRI allows planar as well as volumetric imaging with a field of view of up to 50 cm.
In the initial version of this device, a ring-gantry with three equally-spaced 60Co-sources (mounted within the gap between the two solenoids) was used, delivering a dose rate of about 5.5 Gy min−1 at the isocenter (Mutic and Dempsey 2014). This beam does not pass through any of the MRI components before entering the bore. The beam is shaped by double-focused multi-leaf collimators and the device allows delivery of 3D-conformal as well as step-and shoot intensity modulated radiotherapy. Recently, the three 60Co-cources have been replaced by a single standing-wave FFF 6 MV-linac (termed as MRIdian™ linac). All new installations are equipped with the linac-version (Klüter 2019). SID and maximum field size at the isocenter are 105 cm/27.3 × 27.3 cm2 for the 60Co and 90 cm/24.1 × 27.4 cm2 for the linac-version, respectively (ViewRay 2015, 2017). MR-imaging during treatment may be performed using either a single sagittal plane at 4 frames per second or three parallel sagittal planes at 2 frames per second and latency of image-based target tracking is specified to be 300 ms (Mutic and Dempsey 2014). The treatment planning system offers auto contouring, a fast Monte Carlo based dose calculation as well as inverse dose optimization and thus in principle the basic features required for adaptive radiotherapy treatments (Mutic and Dempsey 2014).
2.3. Other MRI-linac facilities
While this review mainly focusses on reference dosimetry for devices with a perpendicular orientation between the beam and the magnetic field, so-called orthogonal systems, for which two MRI-linac systems are commercially available (ViewRay MRIdian™ and Elekta Unity™), there are currently two projects studying MRI-linac designs in which the beam is parallel to the magnetic field, so-called inline systems. Both inline systems are under development and have not been clinically applied yet. For completeness, these systems are also briefly outlined here.
One project is the Australian Magnetic Resonance Imaging-linac program (Keall et al 2014), which investigated a prototype consisting of an ex-clinical, closed 1.5 T MRI scanner with an inline-oriented linac (with nominal beam energies of 4 MV and 6 MV) (Liney et al 2016). An advantage of this construction is the absence of attenuation and scatter radiation generated by the cryostat compared to orthogonal systems produced by a linac mounted outside a closed magnet. Apart from the general strategies of MR-guided radiotherapy, the project especially investigates potential advantages of an inline alignment over an orthogonal arrangement. A more recent prototype was realized as an actively shielded 1.0 T open-bore magnet (Jelen et al 2020). An obvious advantage of the inline design is the fixed position of the linac relative to the magnet, simplifying the linac operation, and the confinement of the secondary electrons along the beam direction by the magnetic field. This confinement leads to a reduced exit dose compared to the orthogonal systems. However, it may lead to a significantly increased in-field skin dose on the entrance side, which depends heavily on the fringe field outside of the magnet (Oborn et al 2012). A second advantage is the reduction of scattered electrons, because the beam does not pass through the cryostat. A further technical challenge is that the whole magnet-linac construction (or the patient) must be rotated to achieve different beam angles.
The second project which uses an inline system is located at the University of Alberta (Edmonton, Canada) (Fallone 2014). Here, the prototype is composed of a biplanar high-temperature superconducting open-bore magnet and a 6 MV linac, which is aligned either perpendicular or inline to the magnetic field. A field strength of 0.6 T was selected as the optimal trade-off between good image quality and minimized dosimetric effects of the secondary electrons. To achieve different beam angles relative to the patient, the whole construction consisting of magnet and linac is rotated.
3. Basic physics of radiation transport in magnetic fields
3.1. Charged particle transport in vacuum
The most important photon–electron interaction types in radiotherapy are photoelectric effect, Compton scatter and pair production. Generally, it is assumed that the impact of the magnetic field on these interactions is insignificant for field strengths used in MRI-linacs (Szymanowski et al 2015). Therefore, the energy transferred to secondary charged particles (kerma) remains unaffected by the magnetic field for a constant photon fluence. However, the secondary charged particles (electrons and positrons) produced by the primary photon beam traverse through the magnetic field and experience the Lorentz force. Therefore, the transport of charged particles and consequently the absorbed dose (distribution) will be affected by the magnetic field.
For the electric field free case, the Lorentz force,  , is given by
, is given by

where  (+ for positrons and − for electrons) with e the elementary charge and
(+ for positrons and − for electrons) with e the elementary charge and  the velocity of the electron moving in the magnetic field,
the velocity of the electron moving in the magnetic field,  . Since the proportion of secondary positrons is small compared to that of secondary electrons, in the remainder only secondary electrons will be considered.
. Since the proportion of secondary positrons is small compared to that of secondary electrons, in the remainder only secondary electrons will be considered.
According to equation (1), only the velocity component perpendicular to the magnetic field determines the Lorentz force, while the parallel component does not contribute. Therefore, in vacuum, an electron moving with an angle α between its velocity vector and the magnetic field vector will be forced to a helical trajectory with a gyroradius, rg ,

with the total energy, Etot
, defined as the sum of the kinetic, Ek
and the electron rest energy, E0
, and  being the velocity relative to the speed of light
being the velocity relative to the speed of light  . With α equal to 90°, the trajectory reduces to a circle (figure 1). rg
increases with increasing kinetic energy and decreasing magnetic field strength (figure 2). This corresponds with the fact that the larger the rg
, the more the electron trajectory resembles the trajectory of the magnetic-field-free case.
. With α equal to 90°, the trajectory reduces to a circle (figure 1). rg
increases with increasing kinetic energy and decreasing magnetic field strength (figure 2). This corresponds with the fact that the larger the rg
, the more the electron trajectory resembles the trajectory of the magnetic-field-free case.
Figure 1. The Lorentz force  bends an electron, moving in vacuum with velocity
bends an electron, moving in vacuum with velocity  perpendicular to a magnetic field,
perpendicular to a magnetic field,  , to a circular trajectory with radius
, to a circular trajectory with radius  .
.  ,
,  points into the plane.
points into the plane.
Download figure:
Standard image High-resolution imageFigure 2. Gyroradius of the circular trajectory of an electron moving in vacuum as a function of its kinetic energy with α equal to 90°.
Download figure:
Standard image High-resolution imageSince the secondary electrons generated in MV x-ray beams predominantly have the same direction as the x-rays, α will be small for inline MRI-linacs where the beam and magnetic field are parallel and hence the radius of the trajectory will be small. For the same reason in orthogonal MRI-linacs, rg will be considerably larger because α is close to 90°.
3.2. Charged particle transport in homogeneous media
The trajectory of the secondary electrons in media and with a magnetic field present depends on two interactions: firstly, on the electron interactions with the medium and secondly, on the interaction of the electron with the magnetic field by the Lorentz force. Neglecting energy loss by synchrotron radiation, the Lorentz force does not increase or decrease the electrons kinetic energy. Therefore, energy loss of electrons is completely dominated by the first interaction. Hence, electrons lose their energy over the same path length as in the magnetic-field-free case.
Their trajectory within the medium will however be different due to the Lorentz force, which depends on the electron velocity magnitude and direction relative to the magnetic field vector. Since the electron energy decreases due to interactions with the medium, the gyroradius of the electron trajectory also decreases along its trajectory. Therefore, in the continuous slowing down approximation (CSDA), in which scattering of the electron due to interactions with the medium is neglected, the circular or helical shaped electron trajectory in vacuum changes to a spiralling trajectory in a medium, as shown in figure 3.
Figure 3. Trajectories of electrons subject to a transverse magnetic field in a medium with a homogeneous mass density according to the continuous slowing down approximation. Different initial kinetic energies (2, 4 and MeV) of the electrons and different magnetic field strengths (0.35 T and 1.5 T) are plotted.
Download figure:
Standard image High-resolution imageWhen accounting for scatter and realistic energy loss by electrons in media, the trajectories show a stochastic behaviour. In this more realistic approach, the concept of dose kernels, i.e. a 3D dose distribution in a homogeneous medium of a 1D beam around a single interaction point, is more useful to qualitatively evaluate the impact of magnetic fields on electron transport. For orthogonal MRI-linacs, electrons will be swept aside in the direction perpendicular to the magnetic field. The dose kernels will therefore be tilted and shortened in the forward direction (Raaijmakers et al 2008, Gargett et al 2015). This also results in the dose being deposited closer to the interaction point. Therefore, in a homogenous medium the dose distribution will be more similar to the kerma distribution than without a magnetic field (van Asselen et al 2018).
For inline MRI-linacs (section 2.3) the electrons will be focused in the direction of the photon field as they will spiral along the magnetic field lines and therefore travel further away from the interaction point in the photon direction. In this case, the dose kernel will be stretched relative to a situation without a magnetic field (Gargett et al 2015).
3.3. Charged particle transport in density inhomogeneous media
As mentioned before, the trajectory of electrons in the presence of magnetic fields is determined by the interactions with medium and the interaction with the Lorentz force. Since only the former depends on the medium mass density, it is useful to approach the characteristics of electron trajectories in magnetic fields as a function of medium mass density. A helpful concept for this are the so-called mass thickness trajectories, which are particle trajectories in a coordinate system linearly scaled with mass density (Bouchard et al 2015b). When neglecting the stopping power density effect in the CSDA approximation, mass thickness trajectories of an electron in a homogeneous medium are independent on mass density with no magnetic field present (figure 4). As can be seen from this figure, this is not the case for the situation with a magnetic field present. Where the mass thickness trajectory of a 1 MeV electron in a medium density close to water hardly reaches a quarter of a circle for 1 T magnetic field strengths, the trajectory reaches more than 2 revolutions in a medium density 10 times lower. For lower medium densities the number of revolutions further increases. Note that the gyroradius of trajectories in a normal coordinate system will be similar for all densities.
Figure 4. Mass density dependence of the mass thickness trajectory of a 1 MeV electron slowing down in a uniform medium with varying mass densities (0.1 g · cm−3–1.0 g · cm−3) subject to transverse magnetic field: (a) B = 1 T, (b) B = 0.1 T, (c) B = 0.01 T. Reproduced from Bouchard et al (2015b). © 2015 Institute of Physics and Engineering in Medicine. All rights reserved.
Download figure:
Standard image High-resolution imageThis strong dependence of the electron trajectory characteristics on medium density is also demonstrated in the Monte Carlo simulation of figure 5. Therefore, interfaces between low- and high-density media will impact the electron trajectories considerably. This highlights the impact of the magnetic fields on the response of detectors especially for those with interfaces between low- and high-density media such as ionization chambers.
Figure 5. Monte Carlo simulation of electron trajectories emerging from a 1.5 MeV photon pencil beam in the presence of a magnetic field in water without (left) and with (right) an air cavity. Reproduced with permission from Pojtinger. Courtesy of Stefan Pojtinger, PTB.
Download figure:
Standard image High-resolution image3.4. Charged particle equilibrium in the presence of magnetic fields
An important aspect of Codes of Practice for reference dosimetry is the relation between the dose to the cavity and the dose to the (water) medium in the absence of the cavity. Codes of Practice base this relation on cavity theories, such as Bragg–Gray and Spencer–Attix cavity theory, which are valid under certain conditions. The condition for Bragg–Gray cavity theory states that the charged particle fluence,  , in the cavity is not perturbed by the presence of the cavity. To account for departure from this condition perturbation correction factors are applied (Bielajew 1986, Andreo 1992, Andreo et al
2000).
, in the cavity is not perturbed by the presence of the cavity. To account for departure from this condition perturbation correction factors are applied (Bielajew 1986, Andreo 1992, Andreo et al
2000).
An important concept related to the conditions for cavity theories is charged particle equilibrium (CPE). Full CPE is defined as

Considering energy conservation under the condition of CPE, absorbed dose equals collision kerma. In addition, based on the Boltzmann transport equation (BTE), Fano (1954) has shown that CPE remains unaffected by material density variations in a medium with uniform atomic properties (condition 1) and a uniform source scaled with mass density (condition 2). Hence, CPE in the absence of the ionization chamber assures that for ionization chambers of radiologically water equivalent materials, the Bragg–Gray condition is fulfilled and that perturbation corrections are unity (Bouchard et al 2015a).
Due to photon attenuation and scattering, CPE cannot be achieved in practice for reference fields. Only transient CPE (TCPE), which is defined as a constant (i.e. depth independent) non-unity ratio between dose and kerma, is achievable. Under TCPE conditions, perturbation corrections of ionization chambers are small and can be treated independently (Palmans et al 2017) which is the basis of the treatment of perturbations in CoPs (Almond et al 1999, Andreo et al 2000). For example, TRS-398 assumes the perturbation correction for the presence of the cavity, pcav, to be unity with an uncertainty of 0.1%.
Bouchard and Bielajew (2015) derived a modified BTE for charged particle transport in the presence of magnetic fields. Based on this modified BTE, Bouchard et al (2015a) and de Pooter et al (2015) have proven that the two Fano conditions, described above, are inadequate to establish CPE in a medium with mass density variations in the presence of magnetic fields. To achieve CPE, an isotropic angular distribution of charged particles is required. Because the production of secondary electrons in MV photon beams is dominated by Compton interactions, which produces an an-isotropic distribution of secondary electrons, this condition cannot be fulfilled in realistic radiation fields in the presence of magnetic fields.
Therefore, this condition of isotropy for the secondary electron field highlights a more fundamental aspect of the applicability of cavity theory for ionization chambers in the presence of magnetic fields. It shows that CPE in the absence of the chamber is insufficient to guarantee, that for ionization chambers of radiologically water equivalent materials, the Bragg–Gray condition is fulfilled and that perturbation corrections can be considered unity. To make this point clear, consider the following example with a uniform photon fluence in a medium of uniform atomic composition and mass density. When a small cavity of a different mass density but with the same atomic composition is introduced, the secondary electron fluence will not be perturbed in the absence of a magnetic field. In the presence of a magnetic field, however, this in general will not be the case because the secondary electron fluence will not be isotropic.
This point is supported by Monte Carlo simulations for a model with a water vapour cavity in a water medium and a uniform density scaled source of electrons (de Pooter et al 2015). Results for realistic angular distributions based on Compton electrons show a >10% deviation from unity for the ratio between dose to the cavity and kerma in the presence of a magnetic field (B = 1.5 T) compared to no deviation from unity without a magnetic field present. For non-realistic isotropic angular distributions, the deviation of the ratio from unity are insignificant and similar to those without a magnetic field. This suggests that for realistic radiation fields much larger perturbations can be expected for ionization chambers in the presence of magnetic fields than for radiation fields without a magnetic field present.
4. Beam characteristics and formalism
4.1. Radiation beam, magnetic field and chamber orientation
In commercially available MRI-linacs (Mutic and Dempsey 2014, Raaymakers et al 2017), the static magnetic field is parallel to the bore and the yf-axis (IEC61217). The linear accelerator is mounted on a gantry ring which can rotate around the yf-axis, as shown in figure 6. The direction of the magnetic field is different for the two commercially available MRI-linacs: +yf for the ViewRay MRIdian™ and −yf for the Elekta Unity™).
Figure 6. Front (A) and side view (B) of an MRI-linac geometry using the standard coordinate system of linacs (IEC61217) with the vector fluence  of the photon beam perpendicular to the magnetic field vector,
of the photon beam perpendicular to the magnetic field vector,  ,
,  (in A).
(in A).
Download figure:
Standard image High-resolution imageAs described in section 3.1, the direction of the Lorentz force is perpendicular to the magnetic field direction and the velocity of the electrons, and thus to the direction of the photon beam. Since the direction of the magnetic field is fixed and the photon beam axis rotates in the xf-zf plane, the Lorentz force vector is also rotating in the same plane orthogonal to the beam axis.
Detectors are usually placed along one of the three main axes of the facility (xf, yf or zf). Except for the axis aligned with the beam axis, the detector can be placed in two opposing directions: with the tip in the positive direction or with the tip in the negative direction. Therefore, there are five possible orientations for the detector relative to the beam and the magnetic field (represented by (a)–(e) in figure 7). In the most commonly used setup, the detector points in the direction of  (orientation b in figure 7). Most publications refer to this as the parallel orientation. For symmetry reasons there is no difference in response between the parallel orientation and the opposing orientation (orientation a in figure 7) for axisymmetric detectors. Also, the setup with the detector placed orthogonal to the magnetic field and in the direction of the Lorentz force, the perpendicular orientation, has been widely investigated. For this orientation the two opposing orientations are not symmetrically equivalent. Most papers refer to orientation d (figure 7) as the perpendicular orientation. In some papers the opposite orientation e is also investigated and referred to as the counter-clockwise perpendicular orientation.
(orientation b in figure 7). Most publications refer to this as the parallel orientation. For symmetry reasons there is no difference in response between the parallel orientation and the opposing orientation (orientation a in figure 7) for axisymmetric detectors. Also, the setup with the detector placed orthogonal to the magnetic field and in the direction of the Lorentz force, the perpendicular orientation, has been widely investigated. For this orientation the two opposing orientations are not symmetrically equivalent. Most papers refer to orientation d (figure 7) as the perpendicular orientation. In some papers the opposite orientation e is also investigated and referred to as the counter-clockwise perpendicular orientation.
Figure 7. The five different orientations of the ionization chamber relative to the magnetic field and the photon fluence vector of the beam: parallel to the magnetic field (a) and (b), in line with the photon beam (c), orthogonal to both the photon and magnetic field (d) and (e).
Download figure:
Standard image High-resolution imageOrientation c with the detector aligned along the beam axis has also been investigated but is not commonly used. Note that relating the orientation of the chamber with the direction of  requires relating the direction of
requires relating the direction of  to the machine coordinate system which is different for the two commercially available MRI-linacs (+yf and −yf).
to the machine coordinate system which is different for the two commercially available MRI-linacs (+yf and −yf).
4.2. Radiation field characteristics in the presence of magnetic fields
Generally, reference fields with field sizes of 10 × 10 cm2 are recommended in CoPs. In the presence of a magnetic field and for orthogonal MRI-linac facilities (figure 6), the following characteristics of a reference field change:
- build-up region;
- beam exit region;
- depth dose distribution; and
- penumbra.
The dose distribution in the build-up region changes due to the change in contaminant electron fluence and the change of electron trajectories in the phantom due to the Lorentz force. The contaminant electrons (generated in air, in the accelerator and in the cryostat) travelling towards the surface will be captured by the magnetic field and spiral around the magnetic field lines. As a result they will be swept out of the radiation field which reduces the surface dose in the field (Oborn et al 2009, Hackett et al 2018, Malkov et al 2019). Furthermore, electrons resulting from photon–electron interactions inside the phantom or patient will deposit their energy closer to the surface since their path is curved by the Lorentz force (section 3.2), which will increase the surface dose. As a result the build-up region will be shorter compared to no magnetic field (Woodings et al 2018), depending on the magnetic field strength (Oborn et al 2009, 2010), see figure 8. In addition, the electron streaming effect (Malkov et al 2019) contributes to out-of-field dose. Here, electrons backscattered from the build-up region into the air will spiral along the magnetic field and travel out-of-field. The effect depends on the curvature of the surface.
Figure 8. Depth dose profiles of reference fields for different magnetic field strengths (0.0 T, 0.35 T, 1.5 T and 3.0 T) for the same photon fluence. The profiles with a magnetic field present are normalized to the curve for B = 0.0 T at the position of maximum dose of this curve.
Download figure:
Standard image High-resolution imageAt the beam exit of the phantom, ejected electrons can return to the phantom and deposit their dose. This electron return effect (ERE) can result in a high dose area at the beam exit (Raaijmakers et al 2007, Ahmad et al 2016, Woodings et al 2018). The effect depends on magnetic field strength, field size and curvature of the exit surface (Raaijmakers et al 2008, Oborn et al 2009, 2010, Keyvanloo et al 2012).
The shape of lateral dose profiles becomes asymmetric (see figure 9) in the direction of the Lorenz force, i.e. perpendicular to the magnetic field direction (figure 6) (Raaymakers et al 2004, Ahmad et al 2016, Woodings et al 2018). The asymmetry depends on the magnetic field strength. In the direction of the magnetic field the lateral dose profiles will remain symmetric.
Figure 9. Crossline profiles with and without magnetic field (B = 1.5 T) present in a plane perpendicular to the magnetic field.
Download figure:
Standard image High-resolution imageIn reference dosimetry, the absorbed dose is usually measured for a reference field on the central axis downstream from the dose maximum. In this region the aforementioned changes in dose distribution will not play a role. At this point the dose changes slightly due to the curved path of the electrons. Since the energy is deposited closer to the photon interaction point, the absorbed dose will be closer to the kerma. As a result, the absorbed dose will be reduced in the presence of a magnetic field orthogonal to the photon field (see figure 8). For 1.5 T this reduction is around 0.5% in a 10 × 10 cm2 field for an Elekta Unity™ beam (O'Brien et al 2016, van Asselen et al 2018). For 0.35 T with the same beam this reduction is around 0.1%. For beams of experimental facilities, consisting of a clinical linear accelerator and an electromagnet, different values have been reported, 0.5% and 0.8% for B= 0.35 T and 1.5 T respectively (Delfs et al 2018).
4.3. Formalisms for reference dosimetry in magnetic fields
In current Codes of Practice such as TRS-398 (Andreo et al 2000), TG-51 (Almond et al 1999) and NCS-18 (Aalbers et al 2008) the magnetic field is not part of the reference conditions and the determination of the absorbed dose to water is based on the kQ formalism:

with  the absorbed-dose-to-water for beam quality
the absorbed-dose-to-water for beam quality  ,
,  the corrected reading of the dosimeter in beam quality Q and
the corrected reading of the dosimeter in beam quality Q and  the absorbed dose-to-water calibration coefficient at reference beam quality
the absorbed dose-to-water calibration coefficient at reference beam quality  , usually 60Co. To correct for the difference in calibration coefficient between the reference beam quality
, usually 60Co. To correct for the difference in calibration coefficient between the reference beam quality  and the beam quality
and the beam quality  , the beam quality correction factor
, the beam quality correction factor  , is used, with
, is used, with

The corrected reading,  , is given by
, is given by

where  is the raw dosimeter reading corrected for leakage,
is the raw dosimeter reading corrected for leakage,  the factor to correct the reading for temperature and pressure,
the factor to correct the reading for temperature and pressure,  for relative humidity,
for relative humidity,  for polarity effect and
for polarity effect and  for incomplete charge collection due to recombination.
for incomplete charge collection due to recombination.
The magnetic field affects the calibration coefficients of ionization chambers used in the CoPs (Meijsing et al 2009, Reynolds et al 2013, O'Brien et al 2016). To account for the difference in reference conditions (i.e. the presence of the magnetic field) between the beam used for the calibration of the chamber and the MRI-linac beam, the magnetic field correction factor is introduced by O'Brien et al (2016), van Asselen et al (2018) and Malkov and Rogers (2018), using slightly different notations of the measurement equation with different terminology and symbols. Van Asselen et al (2018) uses the following formulation:

with

which enables compatibility with the existing kQ,Q0
formalism in CoPs. Note that  is used as a vector, since the correction factor depends on the orientation of the magnetic field relative to the photon beam and the orientation of the detector. The
is used as a vector, since the correction factor depends on the orientation of the magnetic field relative to the photon beam and the orientation of the detector. The  directly corrects the calibration coefficient in beam quality Q for the presence of the magnetic field.
directly corrects the calibration coefficient in beam quality Q for the presence of the magnetic field.
The reference field in MRI-linacs in the absence of the magnetic field deviates from that in conventional linacs, because, among others, the MRI-linac FFF beam is filtered by the MRI-cryostat (Woodings et al
2018). For this purpose O'Brien et al (2016) have proposed to add the  factor to the formalism from Alfonso et al (2008) which is the basis of TRS-483 (Palmans et al
2017). This formalism adds a correction factor,
factor to the formalism from Alfonso et al (2008) which is the basis of TRS-483 (Palmans et al
2017). This formalism adds a correction factor,  , to the kQ,Q0
formalism, which accounts for the difference between reference fields in conventional beams and machine specific reference fields (msr). The complete measurement equation of O'Brien et al (2016) reads
, to the kQ,Q0
formalism, which accounts for the difference between reference fields in conventional beams and machine specific reference fields (msr). The complete measurement equation of O'Brien et al (2016) reads
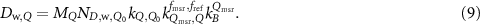
From the paper of van Asselen et al (2018) it is clear that  is implicitly defined for the MRI-linac machine specific reference field. Therefore, despite the apparent differences in the measurement equations (8) and (9) both equations have the same meaning, and
is implicitly defined for the MRI-linac machine specific reference field. Therefore, despite the apparent differences in the measurement equations (8) and (9) both equations have the same meaning, and  in the paper of van Asselen et al (2018) equals
in the paper of van Asselen et al (2018) equals  in the paper of O'Brien et al (2016). In addition, in a similar way, the product of
in the paper of O'Brien et al (2016). In addition, in a similar way, the product of  and
and  in O'Brien et al (2016) equals
in O'Brien et al (2016) equals  in van Asselen et al (2018). Furthermore, in O'Brien et al (2016) the correction factor
in van Asselen et al (2018). Furthermore, in O'Brien et al (2016) the correction factor  is defined as the product of
is defined as the product of  ,
,  and
and  . This can be written as a ratio of calibration coefficients by
. This can be written as a ratio of calibration coefficients by

This factor is similar to the  used in Malkov and Rogers (2018).
used in Malkov and Rogers (2018).
To avoid implicit definitions in the formalism and to make it generalizable, in the remainder of this paper the formalism of TRS-483 is used for the machine specific reference field of MRI linacs in the absence of the magnetic field. The formulation of van Asselen et al (2018) is used to make the step for this msr field in the presence of a magnetic field. To make clear that this correction applies for an msr field the subscript msr is added to Q, yielding  . This leads to the following measurement equation:
. This leads to the following measurement equation:

The relations between the used symbols in the three discussed papers and this paper is summarized in table 1.
4.4. Beam quality specifier
For clinical reference dosimetry the definition of a beam quality specifier for conventional MV photon beams is usually based on either %dd(10)x (AAPM TG-51) or TPR20,10 (TRS-398 and most national CoPs). %dd(10)x is defined as the percentage depth dose at 10 cm depth, %dd(10), in a 10 × 10 cm2 radiation field and at a source to surface distance (SSD) of 100 cm, for photons only (i.e. excluding the contribution from contaminant electrons). Therefore, the determination of %dd(10)x requires the application of a lead foil in the beam when measuring %dd(10), to exclude the effect of contaminant electrons (Almond et al 1999, McEwen et al 2014). This is not required for beams with nominal energies <10 MV and with a flattening filter for which %dd(10)x and %dd(10) are considered similar.
TPR20,10 is defined from the ratio of the measured absorbed doses (or corrected ionization chamber signals) at 20 cm and 10 cm water depth at a fixed source to detector distance (SDD), in a 10 × 10 cm2 radiation field. The determination of TPR20,10 requires only measurements at depths far beyond the build-up region, therefore TPR20,10 is not affected by electron contamination. Although the definition of TPR20,10 specifies an SDD of 100 cm, it turns out that the value of TPR20,10 is essentially independent of SDD provided that the field size 10 × 10 cm2 is maintained. Using the formalisms of section 4.3, and to avoid ramp-down of the MRI magnet, the beam quality specifier must be measured in the presence of a magnetic field. Therefore, it should be independent of the magnetic field for reference dosimetry in MRI-linacs.
The strong magnetic field in MRI-linac systems naturally tends to remove electron contamination from the beam. This has been demonstrated by O'Brien et al (2016), who calculated %dd(10) by Monte Carlo simulation of an Elekta Unity™ MRI-linac, based on a full head model, which includes electron contamination, and based on a point source model without contaminant electrons, for no magnetic field and for a magnetic field of 1.5 T. This study found that the dmax and %dd(10) is similar on both models with magnetic field present, confirming that most of the contaminant electrons in the full model never reach the point of dmax. When no magnetic field is present, the difference in %dd(10) between the full head model and the point source model is much larger (2.0%). Therefore, this data suggests that %dd(10)x and %dd(10) cannot be considered similar for MRI linacs when no magnetic field is present. Potentially this difference can be attributed to contaminant electrons from the part of the bore cover through which the beam passes.
Comparing %dd(10) with and without a magnetic field present shows differences of 0.7% and 1.7% for the full head model and the point source model, respectively. Because the point source model does not include contaminant electrons, the %dd(10) calculated with this model can be considered similar to %dd(10)x. The difference of 1.7% translates into a change of 0.3% in kQ,Q0 factors for typical ionization chambers. Therefore, it was concluded that the change in %dd(10) is resulting from the changing impact of the Lorentz force on secondary electrons between dmax and 10 cm depth and from the reduction in electron contamination. The same study showed that TPR20,10 remains unaffected by the presence of the magnetic field.
Malkov and Rogers (2018) have investigated the behaviour of TPR20,10 and %dd(10)x as a function of magnetic field strength (B = 0 T − 2.0 T) for 6 difference beam qualities (60Co, 7 MV (Elekta Unity™ beam), 6 MV and 25 MV (Elekta SL25 beams), 6 MV and 10 MV (Varian beams)). All models did not include contaminant electrons. They demonstrate that %dd(10)x reduces by 1.89% for the Elekta Unity™ beam quality at 1.5 T. Which is in agreement with the results of O'Brien et al (2016). For higher beam energies, the difference can be even higher than 6%. For the range B = 0 T − 0.35 T, the curve for %dd(10)x does not show differences larger than 0.2%. For TPR20,10 the difference with no magnetic field present is within 0.36% for all investigated beam qualities.
Therefore, the existing data suggests that TPR20,10 is the preferred beam quality specifier for reference dosimetry in MRI-linac facilities. Moreover, a technical difficulty in measuring %dd(10)x in an MRI-linac is the restricted range of possible SSD values, which would already make the direct use of TG-51 for reference dosimetry not feasible. Both O'Brien et al (2016) and Malkov and Rogers (2018) suggest to use a functional relationship between TPR20,10 and %dd(10)x (Kalach and Rogers 2003), to convert TPR20,10 to %dd(10)x for use with the TG-51 protocol.
4.5. Correction factors for charge readings
Equation (6) describes the general use of corrections for polarity, recombination, air density and humidity in the kQ,Q0 formalism. The magnetic field dependency of these corrections has been investigated by several papers (Smit et al 2013, de Prez et al 2019b). For two ionization chamber types (PTW 30013 and IBA FC65-G) ks and kpol were determined in anti-parallel and perpendicular orientation to a magnetic field of 0 T and at 1.5 T in an Elekta Unity™ (de Prez et al 2019b) using the same methods recommended by existing Codes of Practice (Weinhous and Meli 1984). A larger value for ks could be expected with a magnetic field, compared to no magnetic field due to the longer (curved) path length of the ions in the chamber cavity. However, even though ks at 1.5 T was slightly larger (0.06%), this was still within the expanded Type A uncertainty of 0.1%.
The application of corrections for volume averaging, kvol, were first explicitly mentioned in the addendum to TG-51 (Almond et al
1999) and adopted later on in TRS-483 (Palmans et al
2017). In TRS-483, kvol is included in  , in the TG-51 addendum it is included as a separate correction, Prp
, in the formalism. Andreo et al (2020) recently proposed kvol as a separate correction from kQ,Q0
, for the update of TRS-398. kvol is dependent on the beam profile over the chamber cavity and can be obtained by a simple integration of the beam profile over the length of the ionization chamber cavity or, for commonly applied FFF photon beams, by the equations given by TRS-483 Palmans et al (2017):
, in the TG-51 addendum it is included as a separate correction, Prp
, in the formalism. Andreo et al (2020) recently proposed kvol as a separate correction from kQ,Q0
, for the update of TRS-398. kvol is dependent on the beam profile over the chamber cavity and can be obtained by a simple integration of the beam profile over the length of the ionization chamber cavity or, for commonly applied FFF photon beams, by the equations given by TRS-483 Palmans et al (2017):
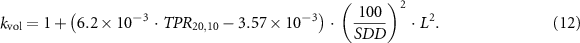
Here, kvol is dependent on beam quality Q (specified by TPR20,10), SDD and length, L, of the ionization chamber cavity (distances in cm). For the Elekta Unity™ the results of this equation were compared to those of the integration of its beam profile over the length of the ionization chamber and shown to agree within 0.02(5)% (de Prez et al 2019b).
This suggests that the impact of the magnetic field on kvol is small for reference fields and it does not affect  . Consequently, similar methods as those described in TRS-483 and the TG-51 addendum are applicable to implement this correction in the formalism. This can be done either by the TRS-483 approach, where kvol is considered as a contribution to the beam quality correction factor (or
. Consequently, similar methods as those described in TRS-483 and the TG-51 addendum are applicable to implement this correction in the formalism. This can be done either by the TRS-483 approach, where kvol is considered as a contribution to the beam quality correction factor (or  ), or by the TG-51 approach in which it is included as a separate correction factor to the ionization chamber reading.
), or by the TG-51 approach in which it is included as a separate correction factor to the ionization chamber reading.
5. Ion chamber characteristics
5.1. Chamber response curves as function of magnetic field
For reference dosimetry at commercially available MRI-linacs (sections 2.1 and 2.2) the knowledge of the ionization chamber's response is only needed for a few discrete magnetic field strengths (0.35 T for ViewRay MRIdian™ or 1.5 T for Elekta Unity™). Nevertheless, the investigation of the chamber response as a function of magnetic field strength has gained considerable interest to better understand the behaviour and characteristics of ionization chambers in magnetic fields.
For the experimental investigation of chamber response curves an adjustable magnetic field is needed, which is not available on commercially available MRI-linacs. Therefore, several papers investigate chamber response as a function of magnetic field strength experimentally using large electromagnets, or by applying Monte Carlo methods (see section 6.1). In most of these papers the relative response (i.e. the corrected ionization chamber reading, MQ
(equation (6), normalized to MQ
without magnetic field present) is determined as a function of magnetic field strength. Some papers calculate or measure  as a function of the magnetic field strength (e.g. Pojtinger et al (2018)).
as a function of the magnetic field strength (e.g. Pojtinger et al (2018)).
Generally these response curves do not monotonically increase with magnetic field strength but may show a maximum or minimum depending on the orientation between radiation beam, chamber axis and direction of the magnetic field, see for example figure 10. Furthermore, the shape of the curves depends strongly on the chamber type and the curves are typically asymmetric for the perpendicular orientation. Most probably this is a results from the direction of deflection of the secondary electrons which is either to the stem (orientation e, figure 7) or to the tip of the ionization chamber (orientation d, figure 7), see also section 5.3. Table 2 gives an overview of the maximum change of response for different types of ionization chambers and for different orientations between the radiation beam, chamber axis and direction of the magnetic field. This data is extracted from publications discussed in the following.
Figure 10. Example response change curves of three chamber types as a function of the magnetic field for a perpendicular orientation of the chamber w.r.t.  . Reproduced with permission from Pojtinger. Courtesy of Stefan Pojtinger, PTB.
. Reproduced with permission from Pojtinger. Courtesy of Stefan Pojtinger, PTB.
Download figure:
Standard image High-resolution imageTable 2. Overview of literature data on chamber response as a function of magnetic field strength for a perpendicular orientation of magnetic field direction with respect to beam direction from measurements (exp) and simulations (MC). The data is categorized by chamber type and by different orientations of chamber w.r.t. to beam and magnetic field direction. The values given for the maximum response change for positive magnetic fields (Extremum + and Extremum − respectively) and the magnetic field strength at which these extrema occur have been estimated from the plotted data. Positive magnetic fields and a perpendicular orientation (┴) of chamber w.r.t. B-field direction correspond to orientation e in figure 7. Unless indicated otherwise the tabulated data is for MV linac beams with beam qualities close to those of commercially available MRI-linacs.
| Chamber type | Paper | Orientation chamber w.r.t. B-field direction | Orientation chamber w.r.t. beam | MC/exp | Extremum + (%) | B-field (T) extremum + | Extremum − (%) | B-field (T) extremum − |
|---|---|---|---|---|---|---|---|---|
| NE2571 | Meijsing et al (2009) a | ┴ | ┴ | MC + exp | +8 | 1.0 | ||
| Reynolds et al (2013) a | ┴ | ┴ | MC | + 8 | 1.0 | |||
| Malkov and Rogers (2016) | ┴ | ┴ | MC | +8 | 1.0 | |||
| Pojtinger et al (2018) | ┴ | ┴ | MC | +6.5 | 1.0 | |||
| Pojtinger et al (2018) | ∥ | ┴ | MC | < + 0.01 | 1.0 | |||
| Meijsing et al (2009) | N.A. | ∥ | MC + exp | −10 | 1.0 | |||
| Reynolds et al (2013) | N.A. | ∥ | MC | −10 | 0.8 | |||
| PTW 30013 | Spindeldreier et al (2017) | ┴ | ┴ | exp | +8.3 | 0.9 | +7.2 | −0.9 |
| Spindeldreier et al (2017) | ┴ | ┴ | MC | +7.5 | 0.95 | +6.0 | −1.0 | |
| Agnew et al (2017) b | ┴ | ┴ | exp | +11 | 0.75 | |||
| Malkov and Rogers (2017) | ┴ | ┴ | MC | +6.5 ... + 7.0 c | 1.0 | |||
| Pojtinger et al (2018) | ┴ | ┴ | exp | +6.8 | 0.85 | |||
| Pojtinger et al (2018) | ┴ | ┴ | MC | +7.2 | 1.0 | |||
| Pojtinger et al (2019) | ┴ | ┴ | MC | +6.8 | 0.9 | +6.1 | −0.9 | |
| Pojtinger et al (2019) | ┴ | ┴ | exp | +6.8 | 0.9 | +6.3 | −0.9 | |
| Pojtinger et al (2018) | ∥ | ┴ | MC | < + 0.01 | 1.0 | |||
| PTW 31010 | Agnew et al (2017) b | ┴ | ┴ | exp | +5.1 | 1.0 | ||
| Malkov and Rogers (2017) | ┴ | ┴ | MC | +1.8 ... + 3.6 c | 1.0 | |||
| Malkov and Rogers (2017) b | ┴ | ┴ | MC | +5.6 ... + 6.5 c | 1.0 | |||
| PTW 31006 | Agnew et al (2017) b | ┴ | ┴ | exp | +7 | 1.75 | ||
| PTW 30015 | Pojtinger et al (2018) | ┴ | ┴ | MC | +6 | 1.0 | ||
| Pojtinger et al (2018) | ∥ | ┴ | MC | < + 0.01 | 1.0 | |||
| PTW 30016 | Pojtinger et al (2018) | ┴ | ┴ | MC | +6 | 1.0 | ||
| Pojtinger et al (2018) | ∥ | ┴ | MC | < + 0.01 | 1.0 | |||
| Capintec PR-06 C | Reynolds et al (2013) a | ┴ | ┴ | +9 | 1.0 | |||
| Reynolds et al (2017) | ┴ | ┴ | +9 | 1.0 | ||||
| Reynolds et al (2013) | N.A. | ∥ | −9 | 0.8 | ||||
| Exradin A1SL | Agnew et al (2017) b | ┴ | ┴ | exp | +8.8 | 1.5 | ||
| Malkov and Rogers (2017) | ┴ | ┴ | MC | +2.4 ... + 4.4 c | 1.0 | |||
| Shukla et al (2017) d | ┴ | ┴ | MC + exp | +3.7 | 1.1 | −1 | −0.8 | |
| Shukla et al (2017) d | ∥ | ┴ | MC + exp | +0.003 | 0.2 | 0.003 | 0.4 | |
| Exradin A12 S | Agnew et al (2017) b | ┴ | ┴ | exp | +12 | 1.0 |
a It is unclear whether the presented data correspond to positive or negative B-field. b Data is for measurements in a 60Co beam. c The data was for various sizes of the dead volumes (see section 5.3). d Data is for Exradin A1SLMR ionization chamber.
Meijsing et al (2009) were the first to investigate the impact of a magnetic field on the response of a Farmer NE2571 chamber experimentally and by means of Monte Carlo simulations for two orientations of the chamber axis with respect to the radiation beam. The Monte Carlo simulations (using the Geant4 toolkit) of the response of the Farmer chamber in the magnetic field match the measurements within the simulation precision of 2.5% standard deviation after a small misalignment of about 3° between the incident beam direction and the magnetic field has been considered. Furthermore, the paper directed the attention to the fact that the orientation of the chamber with respect to the magnetic field and radiation beam plays a crucial role for the response of ionization chambers in magnetic fields.
Reynolds et al (2013) extended these investigations by using the Monte Carlo code PENELOPE to model the responses of NE2571 and PR-06 C Farmer-type ionization chambers in the presence of orthogonal and inline magnetic fields (with respect to the photon beam) of varying magnitude. The simulated response curves for the NE2571 chamber agree very well with the curves obtained by Meijsing et al (2009).
This work was re-examined and extended in Reynolds et al (2015, 2017) who investigated the response of a Capintec PR-06 C ionization chamber in magnetic fields oriented either parallel or perpendicular to an incident photon beam. The ionization chamber response was calculated as a function of a number of polar and azimuthal angles. It was found that in a magnetic field perpendicular to the beam direction small angular deviations of the chamber axis of no more than 3° from its nominal directions either parallel or perpendicular to the beam can change the response by more than 1%—an effect which has also been observed unintentionally by Meijsing et al (2009). Malkov and Rogers (2016) calculated the response curve of an NE2571 ionization chamber as a function of the magnetic field strength. Their results largely agreed with previous results by Reynolds et al (2013) and Meijsing et al (2009).
A few papers have investigated response changes for orientations of chamber axis parallel to the beam (orientation c in figure 7) (Meijsing et al 2009, Reynolds et al 2017). The results show that the response changes in opposite direction compared to orientations a, b, d and e (i.e. response ratio < 1) with values down to 0.9.
It was found by Agnew et al (2017) that both the change of the chamber's response as well as the magnetic field strength at which the maximum change of response occurs depend on the chamber type. In their study measurements have been done in a 60Co beam, which might be a reason for the difference in maximum response change with other studies for the same chamber types in table 2. Malkov and Rogers (2017) used the EGSnrc Monte Carlo system to calculate the change of response in magnetic fields for the ionization chambers investigated experimentally by Agnew et al (2017). Similar to Spindeldreier et al (2017), they found that the experimental results could only be reproduced by Monte Carlo simulations when a dead volume close to the chamber stem is considered.
From table 2 it can be seen that the response changes for cylindrical chambers in a perpendicular orientation are significantly larger than for a parallel orientation. This is supported by the dataset (including a large set of different chamber types) of Malkov and Rogers (2018) which shows that for a parallel orientation at B = 1.5 T,  does not deviate from unity by more than 2% for reference type chambers. Note that this does not imply that the maximum response for any magnetic field strength is below 2%.
does not deviate from unity by more than 2% for reference type chambers. Note that this does not imply that the maximum response for any magnetic field strength is below 2%.
The previous overview and table 2 only focus on cylindrical chambers. Both Pojtinger et al (2018) and Malkov and Rogers (2018) investigated response changes for several plane-parallel chambers. It is clear from these studies that response changes larger than 10% can be found with significant variations between chamber types.
From table 2 it can be seen that the most data is available for the chamber types NE2571 and PTW 30013. The variation in the data set NE2571 chamber can be attributed to the fact that the measurements are strongly affected by small air gaps around the cavity. This effect is further discussed in section 5.2. Another reason is that not all simulation results for both chamber types have included the dead volume, which improves the consistency between measurements and simulations. The variation for the data set of the PTW 31010 chamber can be attributed to the fact that data set includes measurement and simulation results from both 60Co (Agnew et al 2017, Malkov and Rogers 2017) and linac beams (Malkov and Rogers 2017). In summary: response curves are affected by chamber type, chamber/magnetic field orientation, air gaps around the chamber and beam quality (60Co vs. linac beam).
5.2. Air gap effects
In reference dosimetry for conventional radiotherapy, non-waterproof chambers are often used in a water phantom in combination with a waterproof sleeve as recommended by several CoPs (Almond et al 1999, Andreo et al 2000, Aalbers et al 2008, McEwen et al 2014). Furthermore, solid phantoms are frequently used for QA measurements and sometimes even for absolute dose measurements. In both configurations, inserting of the ionization chamber requires that the insert or sleeve internal diameter is slightly larger (∼0.1 mm) than the ionization chamber's outer diameter resulting in thin air gaps between the ionization chamber and phantom.
While in dosimetry for conventional radiotherapy the influence of such thin air gaps on the response of the ionization chamber is negligible, it has been shown by several authors that in the presence of magnetic fields the response of ionization chambers is strongly influenced by these submillimetre air gaps (Hackett et al 2016, Agnew et al 2017, 2017a, O'Brien et al 2017b).
Hacket et al (2016) were the first to draw attention on this effect by measuring the responses of three waterproof Farmer-type chambers placed in a waterproof sleeve in a water phantom with the chamber axis, the radiation beam and the magnetic field pairwise perpendicular. A thin air gap of about 0.1 mm–0.2 mm existed between the chamber and the sleeve. On an MRI-linac (Elekta Unity™ prototype) the response of the chambers increased by 0.7%–1.2% when the thin air gap was removed by filling the sleeve with water. The experiment was replicated on a conventional linac without a magnetic field resulting in an insignificant response change (<0.2%).
Malkov and Rogers (2016) investigated the influence of 0.5 mm and 1.0 mm air gaps on the response of a NE2571 Farmer-type chamber in magnetic fields using the EGSnrc Monte Carlo code. In the simulations, two orientations of the chamber axis with respect to the radiation beam were considered (perpendicular and parallel) with the chamber axis always perpendicular to the magnetic field. In both configurations, the chamber response without a magnetic field changed negligibly (<0.2%) when a uniform air gap of 1 mm was introduced around the walls of the chamber. In the perpendicular orientation, an increase of the chamber response was found for magnetic fields below 1.0 T, whereas for higher magnetic fields a reduction of the response in the order of 1% was observed for a 0.5 mm air gap in good agreement with the findings by Hackett et al (2016). For the orientation with chamber parallel to the beam axis the situation is reversed; with a reduction in response for B < 1 T and an increase for B > 1 T.
The previous results were confirmed by O'Brien et al (2017b) who placed three Farmer-type ionization chambers in plastic phantoms with machine drilled holes congruent with the chamber shape. Each chamber was rotated axially, and the response was recorded as a function of rotation angle both with and without a magnetic field in an Elekta Unity™ system. Without a magnetic field a small response variation (<0.1%) was observed for all chambers. When a 1.5 T magnetic field was applied, variations of the response between ±0.2% and ±1.3% were observed depending on the chamber type. The larger variation of the response in the presence of a magnetic field was attributed to submillimetre air gaps whose thickness around the chamber changes during a rotation due to small misalignment of the rotation axis (wobbling).
Agnew et al (2017) investigated the influence of the location of the air gap around the chamber in greater detail. Several routinely used cylindrical ionization chambers were placed in custom-made PMMA (Poly(methyl methacrylate)) phantoms in a 60Co beam between the poles of a strong electromagnet, the chamber axis, beam direction and magnetic field being pairwise perpendicular. The phantom was designed to be tightly fitting and rotational symmetric around the ionization chamber except for a small recessed region 0.3 mm in depth comprising one quarter of the chamber's circumference. This recessed region, which provides an asymmetric air gap, could be positioned at different angles around the ionization chamber. Depending on the chamber type, variations of the response between 1.1% ± 0.1% (Exradin A1SL) and 8.5% ± 0.2% (PTW 31006) were observed in an applied magnetic field of 1.5 T when the asymmetric air gap was rotated around the chamber. For a Farmer-type chamber PTW 30013 a variation of the response of 3.8% ± 0.2% was observed. After water was added to the phantom cavity to eliminate all air gaps, the variation of response with position of the cavity recess was reduced to 0.20% ± 0.01%. By changing the magnetic field strength used for the measurements, it was found that the observed variation of response increases with increasing field strength. For a PTW 31010 chamber a variation of the response with the position of the recessed region of 0.25% was observed for B = 0.25 T, whereas it increased to 2.9% at 2 T. However, for both field strengths used in commercially available systems, 0.35 T and 1.5 T, the response with asymmetric air gaps and the response with water-filled air gap differed by 1.0% or more. Additionally, it was found that also the magnetic field strength at which the maximum of the response curve occurs (see section 5.1) as well as the slope of this curve depend on the size of the air gap and—to a lesser extent—on the location of the air gap around the chamber.
In order to find an explanation for the observed changes of response in the presence of small air gaps, O'Brien et al (2017a) investigated the effect of submillimetre gaps between a Farmer-type chamber and a solid phantom by means of Monte Carlo simulations. For symmetrical air gaps, calculated chamber responses were all within 0.5% for air gap thickness up to 1.4 mm and magnetic field strength up to 1.5 T. A similar result was obtained for asymmetric ('one-sided') air gaps without a magnetic field; here the response varied by less than 0.5% depending on the orientation of the air gap with respect to the chamber and radiation source. With B = 1.5 T, a variation of the response of more than 2.5% was found for 0.2 mm air gaps depending on its position around the chamber. By investigating the contributions to the total dose from regions outside, adjacent and inside the ionization chamber it was found that the observed variation of the response is not simply caused by the electron return effect (Raaijmakers et al 2005, 2007, 2008), as the gyroradius of most electrons is too large to be responsible for the observed effects. Instead, the change of response is attributed to the loss of dose contributions from electrons originating inside the air gap volume, which is not completely compensated by more distant electrons owing to their reduced range in the magnetic field.
In summary, the presence of small air gaps around ionization chambers increase the variation in response change because of small positioning variations that affect the size and symmetry of the air gaps. In addition, the air gap has a significant impact on the response change compared to no (or a water-filled air gap). While the former effect seems to be mainly relevant for 1.5 T MRI-linacs, the latter has a significant impact for both field strengths used in commercially available systems, 0.35 T and 1.5 T. Apart from the aforementioned factors, the influence of air gaps on chamber response depends on the orientation of the chamber axis, beam axis and magnetic field. Generally, it is concluded that the use of solid phantoms or waterproof sleeves is not adequate for reference dosimetry or QA measurements in MRI-linacs, due to the unavoidable presence of submillimetre air gaps and the unknown distribution of the air gap thickness. Instead, the sensitive volume of the ionization chamber should be wholly immersed in water in order to obtain reproducible results and the non-waterproof chamber seems less suited for reference dosimetry in MRI-linacs.
5.3. Dead volume effects in ionization chambers
McNiven et al (2008) have combined micro-CT images of ionization chambers with calibrations of these chambers. From this data it was possible to correlate the geometrical volume (from the micro-CT images) of the cavity with the 'electrical collecting volume' which is the volume derived from the produced charge and delivered dose to the cavity. The authors demonstrate that the volume of the cavity is significantly larger than the electrical collecting volume. Based on these findings, Ross (2009) suggested that the collecting volume could be obtained from finite element method (FEM) simulations of the electric field inside the ionization chamber and by excluding the volume in which the electric field lines from the chamber wall reach the guard ring instead of the electrode. In a more recent publication, Butler et al (2015) investigated the spatial response of ionization chambers with micro-beams. They demonstrate that the response of the ionization chamber cavity is not homogeneous and that Farmer-type ionization chambers exhibit a volume with a low sensitivity close to the chamber guard electrodes.
Malkov and Rogers (2017) used Monte Carlo simulations to calculate  for ionization chambers, for which the correction factors have been previously determined experimentally by Agnew et al (2017). In their simulations, part of the cavity close to the guard electrode was modelled as a cylindrically shaped volume of varying dimensions. The impact of these volumes on the calculation of
for ionization chambers, for which the correction factors have been previously determined experimentally by Agnew et al (2017). In their simulations, part of the cavity close to the guard electrode was modelled as a cylindrically shaped volume of varying dimensions. The impact of these volumes on the calculation of  was investigated by comparing simulations of response curves in which the dose scored in dead volumes is neglected with simulations in which it is included. It is shown that, depending on the chamber type, small dead volumes with an extent of at most 1 mm might change the response curves (e.g. Figure 10) by several percent. The influence of the dead volume is larger for chambers with a smaller collecting volume. Finally, the experimental results obtained by Agnew et al (2017) could be reproduced by a proper choice of the size of the dead volume.
was investigated by comparing simulations of response curves in which the dose scored in dead volumes is neglected with simulations in which it is included. It is shown that, depending on the chamber type, small dead volumes with an extent of at most 1 mm might change the response curves (e.g. Figure 10) by several percent. The influence of the dead volume is larger for chambers with a smaller collecting volume. Finally, the experimental results obtained by Agnew et al (2017) could be reproduced by a proper choice of the size of the dead volume.
A similar approach has been followed by Spindeldreier et al (2017) who determined the response curves of five custom-built and one commercial (PTW 30013) Farmer-type ionization chambers with increasing radius of the collecting volume, both experimentally and by Monte Carlo simulations using the EGSnrc toolkit. By varying the dead volume of the investigated chambers in Monte Carlo simulations the experimentally obtained response curves could be reproduced. These dead volumes were then used in Monte Carlo simulations to calculate magnetic field correction factors for orientations of the ionization chamber which could not be realized experimentally.
A more detailed treatment of the dead volume in the Monte Carlo calculation of correction factors was reported by Pojtinger et al (2019) who applied the idea formulated by Ross (2009). In the first step, the electric field inside a Farmer-type chamber PTW 30013 was calculated by FEM simulations. From these calculations the shape and size of the dead volume was deduced. It was found that the dead volume has a doughnut shape (see figure 11) which is different from the cylindrically shaped dead volumes assumed by Malkov and Rogers (2017) and Spindeldreier et al (2017). In the second step,  was calculated by means of Monte Carlo simulations, in which, the collecting volume of the ionization chamber was defined as the geometrical volume excluding the dead volume obtained from the FEM simulations. The calculated
was calculated by means of Monte Carlo simulations, in which, the collecting volume of the ionization chamber was defined as the geometrical volume excluding the dead volume obtained from the FEM simulations. The calculated  were then compared to experimentally obtained factors. When the dead volume was not considered, a discrepancy of more than 1% was found between measured and Monte Carlo calculated correction factors. After accounting for the FEM calculated dead volume the measured and simulated
were then compared to experimentally obtained factors. When the dead volume was not considered, a discrepancy of more than 1% was found between measured and Monte Carlo calculated correction factors. After accounting for the FEM calculated dead volume the measured and simulated  agreed on the 0.1% level which is well within the uncertainties of the experiments and simulations.
agreed on the 0.1% level which is well within the uncertainties of the experiments and simulations.
Figure 11. Calculated electric field lines in the vicinity of the guard electrode of a PTW 30013 Farmer-type chamber (left) and the dead volume excluded from the scoring region in Monte Carlo simulations (right, shown in red). The x coordinates correspond to the sagittal distance to the chamber axis and the y coordinates represent the longitudinal distance from the base of the guard ring. Reproduced from Pojtinger et al (2019). © 2019 Institute of Physics and Engineering in Medicine. CC BY 3.0.
Download figure:
Standard image High-resolution imageThese studies demonstrate that the dead volume must be considered in Monte Carlo simulations of detector response in the presence of magnetic fields. Based on the described literature above the following approaches in modelling the dead volume can be distinguished:
- Approach 1: in which the shape of the dead volume (e.g. cylindrical) is fixed. Dimensions are estimated based on the agreement between simulations and experiments.
- Approach 2: in which shape and dimension of dead volume is based on the electrical field line distribution from FEM simulations by assuming that electrical field lines that do not hit the collecting electrode are part of the dead volume.
The disadvantage of the first approach is that the Monte Carlo result is not completely independent from the measurements. In the last approach, it is assumed that the charge produced in the cavity has no kinetic energy and that the trajectory of the charge to the collecting electrode is not affected by the magnetic field. An approach in which these effects are also accounted for would require Monte Carlo simulations to a much lower energy range of the electrons than currently done in simulations of detector response, for which the cut-off energy is typically 10 keV, and in which the electric field is included. Given the good agreement between measurements and simulations it is anticipated that the impact of these improvements is small.
6. Correction factors
6.1. Monte Carlo based methods for determination of  factors
factors
6.1.1. Monte Carlo simulation of charged particle transport in magnetic fields
The two major aspects in the simulation of charged particle transport are the simulation of multiple soft scattering events and crossing of the boundaries between two bodies. Generally, modern Monte Carlo codes use a mixed scheme for particle transport in which condensed history (CH) steps alternate with hard discrete interactions (or hard events, HE). In a CH step multiple soft scattering events are modelled as a single soft event (SE) (figure 12).
Figure 12. Representation of a CH history step with step length, s. The position at which the soft event is simulated is determined by the random variable a which is sampled from a uniform distribution with a range of (0,1).  ,
,  and
and  are the initial particle velocity, the particle velocity just after the SE, and the particle velocity just before the HE or a new SE.
are the initial particle velocity, the particle velocity just after the SE, and the particle velocity just before the HE or a new SE.
Download figure:
Standard image High-resolution imageIn this approach first the step length, s, between two hard events is sampled from the path length probability distribution. Then the position of the SE is simulated by sampling a value for a from a uniform distribution with a range of (0,1) (figure 12). In the next step, the polar scattering angle, θ, of the soft event is sampled. The energy transferred from the particle to the medium before the SE is calculated using the CSDA approach and scored at the position of the SE. The energy transferred after the SE is calculated similarly and scored at the end of the CH step. Together with the energy scoring the velocity of the particle is reduced at these points, which results in three points of the trajectory where the velocity is known.
Most Monte Carlo codes that have implemented charged particle transport in static magnetic and electric fields base this implementation on the theoretical framework formulated in the work of Bielajew (1988). The Lorentz force acting on the charged particle depends on the velocity and position (if the magnetic field is not uniform) of the particle. Exact calculation of the impact of the Lorentz force on particle position and direction requires an integration of the Lorentz force along the particle trajectory (which reduces to an integration of the particle velocity for the case of a uniform magnetic field).
Although, the particle velocity is not known at each point of the particle trajectory, and consequently the Lorentz force acting on it, Bielajew (1988) has shown that under certain conditions and applying a first order integration of the Lorentz force along the particle trajectory, the simulation of the CH event can be performed by superimposing equations of motion of a charged particle under the influence of the Lorentz force in vacuum to the 'field-free' (i.e. without magnetic and electric field) equations of motion of charged particles in media based on those from statistical treatments such as by Moliere (1947) to describe the multiple soft scattering events.
The conditions described by Bielajew (1988) are represented as a set of constraints that restrict the relative change of the following quantities in a single CH step: particle energy and velocity, magnetic and electric field strength. All these constraints affect the maximum distance that a particle is allowed to travel in the CH event.
Although boundary crossing is treated differently in most Monte Carlo codes (Kawrakow et al 2013, Salvat et al 2014), they have one aspect in common. In each step made by the particle close to the boundary, the algorithm checks whether the particle crosses a boundary by calculating the intersection of the intended step with the nearest interface(s). In this check the intended step is assumed to be a straight path. Therefore, this method is not applicable for application in the presence of magnetic fields in which steps are curved paths. This might lead to missing boundaries between bodies and wrong registration of particle position and body and erroneous scoring of dose to the body.
6.1.1.1. EGSnrc
Simulation of charged particles in magnetic fields was implemented in EGS4. In the later releases of EGSnrc special actions were required to enable these simulations (Kawrakow et al 2013). The method in EGS4 and earlier EGSnrc releases is based on the same first-order integration method as Bielajew proposed, but differ in the implementation of the constraints (Kawrakow et al 2013). This is because EGS has only implemented a uniform magnetic field. Therefore, the change in particle direction is only proportional to the particle gyroradius of equation (2). This allows for the replacement of the set of four constraints to limit the step length, s, by a single constraint on the relative change in particle gyroradius along the CH step.
Malkov and Rogers (2016) proposed a further improvement in the implementation of the magnetic field in EGSnrc. Using the available velocity vectors at three points in a CH step (figure 12) they proposed a third order integration method, in which the velocity vector is integrated over the full CH step. Since the third order integration increases computation time, an adaptive algorithm is proposed that uses first order integration for small steps (low value of s) and third order integration for larger steps. The authors also describe how the constraint on the relative change in particle gyroradius along the CH step can be applied for the third-order integration method and show that this constraint becomes less restrictive.
In addition, Malkov and Rogers (2016) developed a boundary crossing algorithm compatible with simulations in magnetic fields. The algorithm transports the particle along the calculated step length by smaller steps in an iterative process. In each loop of the iteration the shortest distance from particle position to the nearest boundary is calculated. Then the particle is transported a small step with a step length equal to this shortest distance using an analytical solution for the particle trajectory in a magnetic field. This process is repeated until the shortest distance is smaller than a threshold or until the full step length has been reached. In the first situation, the particle is transported in a straight line to the other side of the body interface. The current release of EGSnrc has implemented the Malkov and Rogers (2016) third order integration method and boundary crossing algorithm.
6.1.1.2. PENELOPE
The PENELOPE (Salvat et al 2014) package contains routines for simulation of charged particle transport in both magnetic and electric fields. Implementation in the user codes requires only little software coding which is described in the routines. Similar to EGS4, PENELOPE uses first-order integration of the Lorentz force to simulate the direction change of charged particles in magnetic fields. However, in a single CH event this integration is performed twice; once before the SE and once after the SE (figure 12). In PENELOPE, in each step s is limited by constraints on the relative change in electric field, magnetic field, kinetic energy and velocity. In a homogeneous magnetic field this set reduces to the constraint on relative change in velocity or direction. Although this constraint has the same physical meaning as the one used in EGSnrc, due to the different implementation of the magnetic field in the code and due to differences in radiation transport, its value required for accurate simulations might be different.
6.1.1.3. GEANT4
Geant4 (Agostinelli et al 2003) uses a generic approach to calculate the curved trajectory of a charged particle in the presence of a field (Geant4 Collaboration 2017a, 2017b). This approach is applied to all fields implement in Geant4 (i.e. (in)homogenous electric and magnetic fields). This is done for each CH step by either using a numerical integration of the equation of motion (Runge-Kutta) or an approximate analytical calculation as for the case of a uniform magnetic field. In both approaches, the curved trajectory is approximated by a sequence of linear segments. These segments are used to determine a boundary crossing. A set of geometrical parameters can be used to constrain the deviation of the segments from the 'real' curved trajectory. This set of parameters is the following (see also Simiele and Dewerd (2018)):
- MissDistance: the maximum distance between the linear segment and the curved trajectory;
- δintersection: the maximum distance between the intersection of the segment with a boundary and the 'real' trajectory with the boundary. Because the linear segments are always on the 'inside' of the curved trajectory, this can introduce a bias in boundary crossing; and
- δone step: the accuracy for the endpoint of segments, which do not intersect a volume boundary.
In addition, several other parameters can be used in Geant4 to steer the accuracy of a full CH step by limiting the step length. A CH step will only be accepted if the constraints on the following parameters are fulfilled:
-
 max: maximum accepted relative error on particle position and momentum at the end of a CH step;
max: maximum accepted relative error on particle position and momentum at the end of a CH step; -
 min: minimum tolerated relative error on particle position and momentum at the end of a CH step;
min: minimum tolerated relative error on particle position and momentum at the end of a CH step; - maxStep: the maximum accepted CH step length for a particle in a magnetic field; and
- minStep: the minimum tolerated CH step length for a particle in a magnetic field.
6.1.2. Benchmarks for Monte Carlo simulation in the presence of magnetic fields
From the previous it is clear that, although the theoretical basis is the same, implementation of the magnetic field in charged particle transport is not trivial and the implementation in various Monte Carlo codes can differ significantly. This highlights the need for assessment of the accuracy of Monte Carlo simulation of detector response by benchmarks.
The most used theoretical benchmark for this is the Fano test based on the theoretical work of Fano (1954) which gives a solution of the Boltzmann transport equation in media with varying density under condition of CPE. CPE is established if the two Fano conditions are fulfilled; a medium with homogenous atomic cross sections (1) and a uniformly distributed source (2), see section 3.4. This solution has been applied by Smyth (1986) for the first time to validate the simulation of charged particles at density interfaces, which is one of the critical steps in the simulation of ionization chamber response. For simulation of ionization chamber response, the Fano test has been extensively used as the theoretical benchmark to validate the algorithm and to determine the optimal set of simulation parameters (Smyth 1986, Kawrakow 2000, Poon et al 2005, Sempau and Andreo 2006, Yi et al 2006). It was shown that for most of the Monte Carlo codes it is possible to pass the test within 0.1% for cavity geometries.
However, as shown by Bouchard and Bielajew (2015) the Lorentz force alters the Boltzmann transport equation in such a way that CPE cannot be achieved in non-zero magnetic fields. Bouchard et al (2015b) delivered a fundamental proof for two additional conditions, each of which is adequate to achieve CPE in the presence of a magnetic field:
- (a)primary charged particle fluence has an isotropic angular distribution; and
- (b)magnetic field scales with density of the medium.
Both conditions can be used to develop a Fano test in magnetic fields. The first condition was confirmed by de Pooter (2015) in an alternative proof and applied in a Fano test to benchmark the PENELOPE 2011 code for charged particle transport in the presence of magnetic fields using a slab geometry. By comparison with simulations with non-isotropic angular source distributions it was shown that the condition is essential to apply in Fano tests. Tests based on the first condition have been used in several papers (de Pooter et al 2015, Malkov and Rogers 2016, O'Brien et al 2016, Reynolds et al 2017, Simiele and Dewerd 2018, Lee et al 2018), but until now, no examples of tests based on the second condition are available in literature.
In general, tests based on the first condition are based on a Monte Carlo simulation of a geometry with a cavity for one or more magnetic fields strengths and energies of the primary particles. The published results for Fano tests are based on geometries that vary in complexity from a slab type geometry to a full ionization chamber geometry. In the latter situation the true material densities are used and the test can be performed for one or more of the materials used in the ionization chamber. In the former situation a material for the whole geometry can be chosen as well as the ratio between the density of the slab cavity and the density of the medium. Typically, a ratio of 1/1000, close to the ratio of air to water density, is used.
A critical point in implementing tests based on the first condition is to realize an isotropic fluence of the primary charged particles. The most straightforward way is to start with isotropically emitted electrons (mono-energetic or from a spectrum) as primary particles with a homogeneous intensity per unit of mass distributed over the whole geometry. Another method developed and applied by O'Brien et al (2016) is based on a sphere enclosing the cavity geometry with photons emitted isotropically from the sphere surface. Upon each photon interaction in the sphere, the scattered photon is discarded, and the original photon is regenerated on the spot. In this way an isotropic source of electrons that scales uniformly with mass density throughout the geometry is achieved.
Several authors have used the Fano test to find the optimal set of simulation parameters including the constraints related to the magnetic field implementation as discussed in section 6.1.1 (Malkov and Rogers 2016, O'Brien et al 2016, Lee et al 2018). Additionally, using the Fano test Malkov and Rogers (2016) have demonstrated that their new implementation of the magnetic field in EGSnrc is more accurate for less strict simulation parameters than the old implementation. In addition, the authors found different results for simulations with two different geometries (slab and cylindrical) using the same set of simulation parameters. This suggests that the outcome of the Fano test results are geometry dependent.
Simiele and Dewerd (2018) have investigated the accuracy of two versions of the Geant4code using the Fano test in a slab geometry. Their results highlight differences in agreement with the theory between the two investigated versions and between investigated Multiple Scattering models when no magnetic field was present. For the test in the presence of magnetic fields large discrepancies at specific combinations of magnetic field strength, particle energy and slab thickness have been observed. This suggests that Fano tests should be performed under conditions as close as possible to those for which the chamber response simulations are performed.
The publications on Fano test results for ionization chamber response simulations in magnetic fields are summarized in table 3. In this overview the different described aspects for implementing the test are categorized. Overall, it can be stated that Monte Carlo models pass the Fano test in magnetic fields on the level of 0.3% and in most cases on the level of 0.1%.
Table 3. Overview of published results and methods for Fano tests in detector response simulations in the presence of magnetic fields. In those cases where simulations have been performed for several values of simulation parameters, the accuracy listed in the last column represent the best achieved accuracy.
| Paper | Monte Carlo code | Geometry | Source and distribution | Material | B-range (T) | E-range (MeV) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| de Pooter et al (2015) | PENELOPE 2011 | Cylindrical | Mono-energetic electrons, isotropically and uniformly | Water | 0, 1.5 | 0.1–4.0 | 0.3 |
| Malkov and Rogers (2016) | EGSnrc a | Cylindrical, slab | Mono-energetic electrons isotropically and uniformly | Graphite | 0–5.0 | 0.01–10 | 0.1 |
| O'Brien et al (2016) | Geant4 v4.9.6p04 | PTW 30013 chamber | Poly-energetic photons spherically distributed, regeneration technique | Water, Graphite, Aluminium | 0, 1.5 | 7.0 b | 0.1 |
| Reynolds et al (2017) | PENELOPE 2011 | Slab | Mono-energetic electrons, isotropically and uniformly | Water | 1.5 | 2.0 | 0.4 |
| Simiele and Dewerd (2018) | Geant4 v10.02 and v10.04.p01 | Cylindrical, slab | Mono-energetic electrons isotropically and uniformly | Graphite | 0–1.5 | 0.01–10 | 0.2 |
| Lee et al (2018) | PENELOPE 2014, EGSnrc a , Geant4 v10.03, MCNP6.1 | Slab | Mono-energetic electrons isotropically and uniformly | Graphite | 0–3.0 | 0.01–3.0 | 1.0 c 0.2 1.7 0.4 |
a With new EEMF routines from Malkov and Rogers (2016). b Maximum energy of photon spectrum. c Respectively for the Monte Carlo codes listed in column 2.
Another type of benchmark specific for charged particle transport in magnetic fields was applied by Salvat et al (2014) and Malkov and Rogers (2016). This benchmark tracks the particle position in (near-) vacuum and compares this with the exact helically shaped particle trajectory which can be calculated from theory. Malkov and Rogers (2016) have shown that their third order integration method for EGSnrc better approaches the theoretical trajectory than the 1st order integration. The advantage of this benchmark is the sensitivity to small approximations in the numerical calculation of particle trajectories. The disadvantage is that deviations cannot be used as a quantitative estimate for the accuracy of detector response simulations.
6.1.3.
 data based on Monte Carlo simulations.
data based on Monte Carlo simulations.
The general applied method to calculate  factors is described in O'Brien et al (2016) and is similar to the calculation of
factors is described in O'Brien et al (2016) and is similar to the calculation of  factors as applied, for example, by Wulff et al (2008). According to this method, which is graphically explained in figure 13, four simulations using a detailed geometry model of the ionization chamber are performed: two simulations to calculate the dose to the air cavity of the ionization chamber, Dcavity, with and without a magnetic field; and two simulations to calculate the dose-to-water in the absence of the chamber at the reference point of the chamber with and without a magnetic field. The method of O'Brien et al (2016) is based on the assumptions that:
factors as applied, for example, by Wulff et al (2008). According to this method, which is graphically explained in figure 13, four simulations using a detailed geometry model of the ionization chamber are performed: two simulations to calculate the dose to the air cavity of the ionization chamber, Dcavity, with and without a magnetic field; and two simulations to calculate the dose-to-water in the absence of the chamber at the reference point of the chamber with and without a magnetic field. The method of O'Brien et al (2016) is based on the assumptions that:
- (a)the measured charge, M, is proportional to the dose to cavity; and
- (b)and this factor of proportionality is independent on magnetic field strength and chamber orientation.
Figure 13. Overview of the determination of  based on Monte Carlo simulations. The model includes a beam entering the phantoms (blue) from the top. Dw is scored in a thin disk and Dcavity is scored in the cavity volume of the ionization chamber.
based on Monte Carlo simulations. The model includes a beam entering the phantoms (blue) from the top. Dw is scored in a thin disk and Dcavity is scored in the cavity volume of the ionization chamber.
Download figure:
Standard image High-resolution imageSeveral papers describe Monte Carlo based methods to determine detector response changes as a function of magnetic field strength (Meijsing et al
2009, Reynolds et al
2013, O'Brien et al
2016, Shukla et al
2017, Spindeldreier et al
2017, Malkov and Rogers 2018, Pojtinger et al
2018). The earlier papers (Meijsing et al
2009, Reynolds et al
2013) focused on general parameters affecting ionization chamber response in magnetic fields such as their orientation. In these papers the simulations to calculate the dose-to-water in the absence of the chamber at the reference point of the chamber with and without the magnetic field are ignored. These results are not in line with the definition of the formalisms (section 4.3) and therefore the results of these papers are classified in table 4 as a ratio of readings. Several of the later published papers (O'Brien et al
2016, Spindeldreier et al
2017, Malkov and Rogers 2018, Pojtinger et al
2018) have described the calculation of  in line with the formalisms and with other further refinements in methodology due to improved understanding such as: inclusion of the dead volume (see section 5.1) and avoidance of air gap effects (see section 5.2). In most studies the simulations have been done for several cylindrical ionization chambers. An overview of papers on Monte Carlo based
in line with the formalisms and with other further refinements in methodology due to improved understanding such as: inclusion of the dead volume (see section 5.1) and avoidance of air gap effects (see section 5.2). In most studies the simulations have been done for several cylindrical ionization chambers. An overview of papers on Monte Carlo based  determination is given in table 4 together with the relevant characteristics of the applied methodology.
determination is given in table 4 together with the relevant characteristics of the applied methodology.
Table 4. Overview of papers on  simulations with chamber types investigated and the relevant characteristics of the applied methodology. The definition for the orientation of the chamber with respect to the magnetic field vector is in line with section 4.1 and figure 7.
simulations with chamber types investigated and the relevant characteristics of the applied methodology. The definition for the orientation of the chamber with respect to the magnetic field vector is in line with section 4.1 and figure 7.
| Paper | Chamber type | Chamber geometry | Dead volume | Beam model | B (T) | Orientation chamber | Monte Carlo code | Fano test | Experimental benchmark | Result |
|---|---|---|---|---|---|---|---|---|---|---|
| Meijsing et al (2009) |
| In-air with build-up cap | No | Spectrum 6 MV Elekta SL25 (parallel beam) | 0–2.0 | a or a b and d or e | Geant4.9.1.p01 | No | Yes |
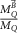
|
| Reynolds et al (2013) |
| In-water | No | Spectrum 6 MV Elekta SL25 (parallel beam) and Varian 600 C 6 MV | 0–2.0 | e | PENELOPE | No | Yes (up to 1.2 T) |
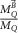
|
| O'Brien et al (2016) |
| In-water | No | Point-source, Elekta Unity™ spectrum | 1.5 | a, b, d and e | Geant4.9.6p04 | Yes | Yes |
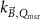
|
| Spindeldreier et al (2017) |
| In-water | Yes | PSF 6 MV Siemens Primus | 0–3.0 | d and e | EGSnrc c | No | Yes |
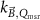
|
| Malkov and Rogers (2018) |
| In-water | Yes | Spectra of 60Co beam, Varian TrueBeam (6 MV, FFF), Elekta Unity™, Varian Clinical (6 and 10 MV). | 0.35, 1.5 | b and e | EGSnrc d | Yes | No |
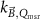
|
| Pojtinger et al (2018) |
| In-water | No | Head 6 MV Elekta FFF linac (0.35 T), Spectrum Elekta Unity™ (1.5 T) | 0.35, 1.5 | and e | EGSnrc d | Yes | Yes |
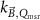
|
| Pojtinger et al (2019) |
| In-water | Yes | Head 6 MV Elekta FFF linac (0.35 T), Spectrum Elekta Unity™ (1.5 T) | 1.5 | e | EGSnrc d | Yes e | Yes |
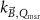
|
| Shukla et al (2017) |
| In-water | No | Spectrum 6 MV linac | 0–1.2 T | a, b, c and d | EGSnrc d | No | Yes |
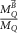
|
a The applied direction cannot be derived from the paper. b Non-waterproof chambers were modelled with a 1 mm thick layer of PMMA surrounding them, to represent a waterproof sleeve. The sleeves were assumed to be in full contact with the chambers with no air gaps in between. c With customised magnetic field macro. d With new EEMF routines from Malkov and Rogers (2016). e From Pojtinger et al (2018).
6.1.4.
Intra-type variability of

Monte Carlo studies result in type specific  correction factors. Application of type specific
correction factors. Application of type specific  correction factors is only feasible if intra-type variability of a chamber type is sufficiently low. Woodings et al (2019) investigated the intra-chamber-type variation of twelve PTW 30013 and thirteen IBA FG65-G Farmer-type chambers, for a parallel and perpendicular orientation (i.e. orientations a and d in figure 7). To measure
correction factors is only feasible if intra-type variability of a chamber type is sufficiently low. Woodings et al (2019) investigated the intra-chamber-type variation of twelve PTW 30013 and thirteen IBA FG65-G Farmer-type chambers, for a parallel and perpendicular orientation (i.e. orientations a and d in figure 7). To measure  for each individual chamber, two cross-calibrations were performed; one with (Elekta Unity™ MRI-linac) and one without a magnetic field (Elekta Agility™ linac) both with similar beam qualities, TPR20,10. From each set of ionization chambers, one chamber was selected as the reference chamber. It was demonstrated that beam quality corrections, recombination and polarity corrections are strongly correlated for each set of two chambers (ionization chamber to be cross-calibrated and reference chamber) in the cross-calibration, which yields a low uncertainty on the cross-calibration and
for each individual chamber, two cross-calibrations were performed; one with (Elekta Unity™ MRI-linac) and one without a magnetic field (Elekta Agility™ linac) both with similar beam qualities, TPR20,10. From each set of ionization chambers, one chamber was selected as the reference chamber. It was demonstrated that beam quality corrections, recombination and polarity corrections are strongly correlated for each set of two chambers (ionization chamber to be cross-calibrated and reference chamber) in the cross-calibration, which yields a low uncertainty on the cross-calibration and  for each chamber.
for each chamber.
The standard deviations of  for the two chamber types were: 0.19% (orientation a) and 0.13% (orientation d) for the PTW30013 and 0.15% (parallel) and 0.17% (perpendicular) for the FC65-G, which is smaller than the estimated standard uncertainty
1
(0.24%). This implies that it is valid to use a generic chamber-type magnetic field correction factor for the investigated chamber types, in the specific field-beam-chamber orientations.
for the two chamber types were: 0.19% (orientation a) and 0.13% (orientation d) for the PTW30013 and 0.15% (parallel) and 0.17% (perpendicular) for the FC65-G, which is smaller than the estimated standard uncertainty
1
(0.24%). This implies that it is valid to use a generic chamber-type magnetic field correction factor for the investigated chamber types, in the specific field-beam-chamber orientations.
6.2.
Measurement based methods for determination of

6.2.1. Based on primary standards
IAEA TRS-398 recommends the use of measured  factors by primary standards for Dw. Methods used for the primary realisation of Dw in high-energy photon beams, are: (1) standards using ionization chambers, (2) standards using ferrous sulphate (Fricke) solution, and (3) standards based on either graphite or water calorimetry (Seuntjens and Duane 2009, Renaud et al
2019a).
factors by primary standards for Dw. Methods used for the primary realisation of Dw in high-energy photon beams, are: (1) standards using ionization chambers, (2) standards using ferrous sulphate (Fricke) solution, and (3) standards based on either graphite or water calorimetry (Seuntjens and Duane 2009, Renaud et al
2019a).
Calorimeters are considered to be the most fundamental method of the available techniques for the measurement of Dw (Seuntjens and Duane 2009). Currently, several studies describe the development of calorimeters suited for application in the presence of a magnetic field (de Prez et al
2016a, Krauss 2019, Dsouza et al
2019, Renaud et al
2019b) and with the purpose to measure  . Apart from the fact that no conversion of dose-to-medium to dose-to-water is needed for water calorimeters, which usually depend on Monte Carlo calculations, they have the benefit of a minimum variation in material density around the point of measurement compared to graphite calorimeters, which generally use vacuum gaps to isolate the core from the remainder of the calorimeter (Seuntjens and Duane 2009). Based on section 5.2 it is known that this property makes a measurement device prone to response changes in the presence of magnetic fields. This effect can be reduced by applying isolation materials instead of vacuum gaps (Renaud et al
2018, 2019b, Bancheri et al
2019).
. Apart from the fact that no conversion of dose-to-medium to dose-to-water is needed for water calorimeters, which usually depend on Monte Carlo calculations, they have the benefit of a minimum variation in material density around the point of measurement compared to graphite calorimeters, which generally use vacuum gaps to isolate the core from the remainder of the calorimeter (Seuntjens and Duane 2009). Based on section 5.2 it is known that this property makes a measurement device prone to response changes in the presence of magnetic fields. This effect can be reduced by applying isolation materials instead of vacuum gaps (Renaud et al
2018, 2019b, Bancheri et al
2019).
Currently one water calorimeter has been successfully commissioned in the 7-MV photon beam of an Elekta Unity™ MRI-linac both with and without a magnetic field (de Prez et al
2019a). This study showed that a water calorimeter can be applied, and its response is independent on the presence of a magnetic field. Dw can be established with a standard uncertainty of 0.37%. This standard was used to measure ionization chamber  factors of two ionization chamber types (PTW 30013, IBA FC65-G) in a Unity™ MRI-linac (de Prez et al
2019b). In addition, it is currently the only study that describes the measurement of
factors of two ionization chamber types (PTW 30013, IBA FC65-G) in a Unity™ MRI-linac (de Prez et al
2019b). In addition, it is currently the only study that describes the measurement of  factors (see equation (11)) in an MRI-linac which can be compared to simulation studies.
factors (see equation (11)) in an MRI-linac which can be compared to simulation studies.  and
and  factors can be determined with a standard uncertainty of 0.42%.
factors can be determined with a standard uncertainty of 0.42%.
6.2.2. Cross-calibration against reference detector
Anine dosimetry offers an alternative for the determination of  . Compared to ionization chambers, the magnitude of response changes in the presence of magnetic fields is lower. Therefore, it offers the possibility to cross-calibrate ionization chambers against alanine as a reference detector in an MRI-linac. The benefits of the alanine as a reference detector have been identified by McEwen et al (2015) and a study focused on the characteristics of alanine in the magnetic field has been presented by Billas et al (2020).
. Compared to ionization chambers, the magnitude of response changes in the presence of magnetic fields is lower. Therefore, it offers the possibility to cross-calibrate ionization chambers against alanine as a reference detector in an MRI-linac. The benefits of the alanine as a reference detector have been identified by McEwen et al (2015) and a study focused on the characteristics of alanine in the magnetic field has been presented by Billas et al (2020).
To use alanine as a reference detector, its magnetic field correction factor,  , for MRI-linacs, must be determined.
, for MRI-linacs, must be determined.  factors for alanine have been determined by combining Monte Carlo simulations and alanine measurements for a set of beam qualities by Billas et al (2020). Alanine as a non-waterproof detector must be placed in a watertight holder for dosimetry measurements in water. This will produce unavoidable air gaps between the inner wall of the holder and the alanine pellets. This particular aspect of alanine dosimetry in the presence of magnetic fields has been quantified by Billas et al (2020) and can affect the alanine response by as much as 0.55%. In the same study, this effect, together with the randomness position of the pellets in the holder during irradiation, is included as a component in the measurement uncertainty. This specific uncertainty is magnetic field strength dependent and reaches 0.52% at 1.5 T.
factors for alanine have been determined by combining Monte Carlo simulations and alanine measurements for a set of beam qualities by Billas et al (2020). Alanine as a non-waterproof detector must be placed in a watertight holder for dosimetry measurements in water. This will produce unavoidable air gaps between the inner wall of the holder and the alanine pellets. This particular aspect of alanine dosimetry in the presence of magnetic fields has been quantified by Billas et al (2020) and can affect the alanine response by as much as 0.55%. In the same study, this effect, together with the randomness position of the pellets in the holder during irradiation, is included as a component in the measurement uncertainty. This specific uncertainty is magnetic field strength dependent and reaches 0.52% at 1.5 T.
A study by Billas et al (2017) determined ND
,w in an Elekta Unity™ MRI-linac, using alanine as a reference detector, for two Farmer-type chambers (PTW 30013 and IBA FC65-G). Alanine was traceably calibrated with the NPL's primary standard graphite calorimeter in a conventional Elekta linac. Their methods include the determination of the chamber  , for 0 T, at NPL (in a conventional linac beam) as a function of TPR20,10 and interpolated to the value for measured TPR20,10 in the MR-linac. For 1.5 T magnetic field strength, the
, for 0 T, at NPL (in a conventional linac beam) as a function of TPR20,10 and interpolated to the value for measured TPR20,10 in the MR-linac. For 1.5 T magnetic field strength, the  were obtained as the ratio of dose to water measured using alanine and the corrected chamber readings. In this study, the magnetic field correction factors for orientation b (figure 7) were found to be 0.996 and 0.997 for the PTW 30013 and IBA FC65-G chamber, respectively, with an uncertainty on
were obtained as the ratio of dose to water measured using alanine and the corrected chamber readings. In this study, the magnetic field correction factors for orientation b (figure 7) were found to be 0.996 and 0.997 for the PTW 30013 and IBA FC65-G chamber, respectively, with an uncertainty on  at 1.5 T of 1%. Billas et al (2017) concluded that the traceability provided through alanine dosimetry, which is compatible with existing measurement standards for radiotherapy, gives an alternative way of determining the
at 1.5 T of 1%. Billas et al (2017) concluded that the traceability provided through alanine dosimetry, which is compatible with existing measurement standards for radiotherapy, gives an alternative way of determining the  correction factor for Farmer-type chambers where an MR-safe primary standard water calorimeter is not accessible.
correction factor for Farmer-type chambers where an MR-safe primary standard water calorimeter is not accessible.
6.2.3. Monitor dependent method
A third method for  determination of ionization chambers, here coined as the monitor dependent method, is described in several papers (Smit et al
2013, van Asselen et al
2018, Pojtinger et al
2019). In contrast to the two previous described methods, for this method Dw is not measured separately in the presence and in the absence of the magnetic field. Instead, by means of simulations, it determines a ratio of Dw at the point of measurement between magnetic field present and magnetic field absent, under the condition of a constant photon fluence, φ. Next the reading of the chamber is measured in the presence and in the absence of the magnetic field. The beam photon fluence in the presence and in the absence of the magnetic field is thereby monitored,
determination of ionization chambers, here coined as the monitor dependent method, is described in several papers (Smit et al
2013, van Asselen et al
2018, Pojtinger et al
2019). In contrast to the two previous described methods, for this method Dw is not measured separately in the presence and in the absence of the magnetic field. Instead, by means of simulations, it determines a ratio of Dw at the point of measurement between magnetic field present and magnetic field absent, under the condition of a constant photon fluence, φ. Next the reading of the chamber is measured in the presence and in the absence of the magnetic field. The beam photon fluence in the presence and in the absence of the magnetic field is thereby monitored,  and
and  respectively, to correct for possible photon fluence variation with and without a magnetic field,
respectively, to correct for possible photon fluence variation with and without a magnetic field,  and
and  , during the ionization chamber measurements. Therefore, an underlying assumption is that the ratio
, during the ionization chamber measurements. Therefore, an underlying assumption is that the ratio  should be independent from the magnetic field.
should be independent from the magnetic field.
The measurement equation for determination of  is written as the product of two factors,
is written as the product of two factors,  and
and  as shown in the following equation:
as shown in the following equation:

where  is given by
is given by

 the absorbed dose-to-water for beam quality Qmsr with magnetic field present at the same reference point as the absorbed dose-to-water without the magnetic field present,
the absorbed dose-to-water for beam quality Qmsr with magnetic field present at the same reference point as the absorbed dose-to-water without the magnetic field present,  . This factor depends on the magnetic field strength. It can be determined by scoring the dose per history with Monte Carlo simulations in a homogenous water phantom. Therefore, details of the ionization chamber geometry and materials are not required, which simplifies the simulations.
. This factor depends on the magnetic field strength. It can be determined by scoring the dose per history with Monte Carlo simulations in a homogenous water phantom. Therefore, details of the ionization chamber geometry and materials are not required, which simplifies the simulations.
The factor  is the ratio of ionization chambers readings and is defined as
is the ratio of ionization chambers readings and is defined as

where  , the corrected reading in the absence of the magnetic field, and
, the corrected reading in the absence of the magnetic field, and  , the corrected reading obtained in the presence of a magnetic field are a measure for the fluence in both situations.
, the corrected reading obtained in the presence of a magnetic field are a measure for the fluence in both situations.  depends on the magnetic field strength and the ionization chamber orientation relative to the magnetic field and photon beam (sections 4.1 and 5.1).
depends on the magnetic field strength and the ionization chamber orientation relative to the magnetic field and photon beam (sections 4.1 and 5.1).
The  factor can be determined for any ionization chamber during ramp-up and ramp-down procedures. For an MRI-linac, this can be done for a single magnetic field strength for any orientation of the ionization chamber (Smit et al
2013, van Asselen et al
2018). For a radiation facility (linac or 60Co-source) combined with an electromagnet, this can be done for varying magnetic field strengths and, due to the limited space between the poles, for the perpendicular orientations ((d) and (e), figure 7) (Pojtinger et al
2018). The correction for possible variation of photon fluence output in both conditions (i.e. with and without magnetic field) can be based on an independent monitor in the beam in a low magnetic field area (Smit et al
2013) or on the linac internal monitor (van Asselen et al
2018, Pojtinger et al
2019).
factor can be determined for any ionization chamber during ramp-up and ramp-down procedures. For an MRI-linac, this can be done for a single magnetic field strength for any orientation of the ionization chamber (Smit et al
2013, van Asselen et al
2018). For a radiation facility (linac or 60Co-source) combined with an electromagnet, this can be done for varying magnetic field strengths and, due to the limited space between the poles, for the perpendicular orientations ((d) and (e), figure 7) (Pojtinger et al
2018). The correction for possible variation of photon fluence output in both conditions (i.e. with and without magnetic field) can be based on an independent monitor in the beam in a low magnetic field area (Smit et al
2013) or on the linac internal monitor (van Asselen et al
2018, Pojtinger et al
2019).
6.2.4.
Evaluation of measurement-based methods for determination of
 and
and
 factors.
factors.
The previous sections describe three measurement methods to determine the ionization chamber magnetic field correction,  . Methods based on a primary standard with and without a magnetic field is the most direct way. In addition, it allows for measurement of
. Methods based on a primary standard with and without a magnetic field is the most direct way. In addition, it allows for measurement of  for the same ionization chamber and facility in the same experiment. However, these measurements are cumbersome and time consuming.
for the same ionization chamber and facility in the same experiment. However, these measurements are cumbersome and time consuming.
Alternatively,  can be measured by crosscalibration of ionization chambers against a reference detector, with a low dependency on the magnetic field strength. The most suited candidate for this now is alanine. The advantage of alanine dosimetry is that its response change in magnetic fields is less than for ionization chambers. However, this change partially depends on alanine pellet holder design and needs to be quantified by the user.
can be measured by crosscalibration of ionization chambers against a reference detector, with a low dependency on the magnetic field strength. The most suited candidate for this now is alanine. The advantage of alanine dosimetry is that its response change in magnetic fields is less than for ionization chambers. However, this change partially depends on alanine pellet holder design and needs to be quantified by the user.
The monitor dependent method was proposed by van Asselen et al (2018). The advantage is that this can in principle be performed by any radiotherapy department. However, in contrast to the two other methods, this method requires a stable and magnetic field independent monitoring method of the photon fluence. Also, it requires the application of  , which depends on the reliability of the used dose calculation algorithm.
, which depends on the reliability of the used dose calculation algorithm.
6.3.
Evaluation of available data for chamber-type specific
 and
and
 correction factors
correction factors
6.3.1.
Data for

Since 2009, many papers have been published which investigate quantitatively the change of response of ionization chambers in magnetic fields or determine the magnetic field correction factors,  , needed for absorbed dose measurements (see section 4.3)—either experimentally or by means of Monte Carlo simulations. From today's perspective, most of the early publications on this topic have some flaws caused by the lack of knowledge about peculiarities of dosimetry in magnetic fields which have been realized at a later stage. Such flaws are, for instance:
, needed for absorbed dose measurements (see section 4.3)—either experimentally or by means of Monte Carlo simulations. From today's perspective, most of the early publications on this topic have some flaws caused by the lack of knowledge about peculiarities of dosimetry in magnetic fields which have been realized at a later stage. Such flaws are, for instance:
- the use of waterproof sleeves or solid phantoms without considering the air gap effect (see section 5.2);
- the use of Monte Carlo algorithms or simulation parameters which have not been benchmarked thoroughly for their use in magnetic fields (see section 6.1.2); and
- the negligence of the influence of the dead volume on the results of Monte Carlo calculations (see section 5.3).
Furthermore, the orientation of the chamber, magnetic field and beam direction is not always specified in sufficient detail and sometimes it is not clearly stated if the correction factors  or
or  are determined (see sections 4.3 and 5.1), i.e. whether the change of the absorbed dose due to the magnetic field
are determined (see sections 4.3 and 5.1), i.e. whether the change of the absorbed dose due to the magnetic field  is considered or not (see equation (13)). Such flaws, together with a large variety of measurement setups and conditions (phantom, SSD, field size, beam quality etc.), make it difficult to compare the results from earlier and recent publications and to judge their applicability for reference dosimetry in MRI-linacs. Moreover, in most cases a proper uncertainty evaluation of the determined correction factor is lacking.
is considered or not (see equation (13)). Such flaws, together with a large variety of measurement setups and conditions (phantom, SSD, field size, beam quality etc.), make it difficult to compare the results from earlier and recent publications and to judge their applicability for reference dosimetry in MRI-linacs. Moreover, in most cases a proper uncertainty evaluation of the determined correction factor is lacking.
The first measurement of magnetic field correction factors in a (prototype) Elekta Unity™ MRI-linac has been reported by Smit et al (2013) for a set of NE2571 ionization chambers in a polystyrene slab phantom with a perpendicular orientation, using (partially) the monitor dependent method (section 6.2.3). Although it is mentioned in the publication that the doses applied to all NE2571 ionization chambers with and without magnetic field were equal, the  factor is not used in the determination of
factor is not used in the determination of  . The correction factors determined here are therefore
. The correction factors determined here are therefore  . Due to the use of the solid phantom, an influence of the air gap effect on the results (see section 5.2) cannot be excluded. For the four ionization chambers investigated here, values for
. Due to the use of the solid phantom, an influence of the air gap effect on the results (see section 5.2) cannot be excluded. For the four ionization chambers investigated here, values for  between 0.951 and 0.955 (average: 0.953) were obtained, which suggests a relatively small intra-type variation for chambers of type NE2571 and a neglectable influence of the air-gap effect in this experiment. Furthermore, because the four chambers were positioned at three different depths in the phantom, it can also be concluded that the magnetic field correction factors are largely independent on depth. The correction factors obtained by Smit et al (2013) agree with the results obtained by Meijsing et al (2009) and Reynolds et al (2013); a preliminary relative uncertainty of 0.5% is estimated for the correction factor.
between 0.951 and 0.955 (average: 0.953) were obtained, which suggests a relatively small intra-type variation for chambers of type NE2571 and a neglectable influence of the air-gap effect in this experiment. Furthermore, because the four chambers were positioned at three different depths in the phantom, it can also be concluded that the magnetic field correction factors are largely independent on depth. The correction factors obtained by Smit et al (2013) agree with the results obtained by Meijsing et al (2009) and Reynolds et al (2013); a preliminary relative uncertainty of 0.5% is estimated for the correction factor.
The first correction factors for Farmer-type ionization chambers (PTW 30013 and IBA FC65-G chamber) based on measurements using a water calorimeter as a primary standard for absorbed dose to water in an Elekta Unity™ MRI-linac have been reported by de Prez et al (2016b). No measurements in the Elekta Unity™ MRI-linac without magnetic field have been done, therefore the calibration coefficient in a 60Co beam quality has been used which results in the product  . From these values,
. From these values,  was determined using a generic
was determined using a generic  value taken from IAEA TRS-398 (Andreo et al
2000) for
value taken from IAEA TRS-398 (Andreo et al
2000) for  . In a later publication de Prez et al (2019b) presents updated data for
. In a later publication de Prez et al (2019b) presents updated data for  which have now been determined based on calorimeter measurements in an Elekta Unity™ MR linac with and without a magnetic field. These correction factors agree within the uncertainty with the previously reported values; the deviation between both sets of correction factors for the two types of chambers investigated in both studies is 0.2% and 0.4%, respectively.
which have now been determined based on calorimeter measurements in an Elekta Unity™ MR linac with and without a magnetic field. These correction factors agree within the uncertainty with the previously reported values; the deviation between both sets of correction factors for the two types of chambers investigated in both studies is 0.2% and 0.4%, respectively.
Wolthaus et al (2016) and van Asselen et al (2018) report results for  using the monitor dependent method (section 6.2.3). The obtained results agree well with the calorimeter-based correction factors obtained by de Prez et al (2016b, 2019b).
using the monitor dependent method (section 6.2.3). The obtained results agree well with the calorimeter-based correction factors obtained by de Prez et al (2016b, 2019b).
Monte Carlo calculated and experimental data are also presented in a study described by Shipley et al (2019). This study calibrated a Famer-type ionisation chamber (PTW 30013) in a conventional 8 MV Elekta Synergy beam, in an electromagnet and at different magnetic field strengths, using alanine as a transfer standard. Alanine was traceably calibrated against the NPL's primary standard of absorbed dose to water, a graphite calorimeter, and corrected for the effect of the magnetic field based on Billas et al (2020) (see section 6.2.2). In addition, Billas and Duane (2018) determined  data with the same method as described in section 6.2.2.
data with the same method as described in section 6.2.2.
Tables 5 and 6 present a selection of published  data for orthogonal MRI-linacs. Some publications publish
data for orthogonal MRI-linacs. Some publications publish  or
or  factors (e.g. Iakovenko et al (2020)). Recalculation to
factors (e.g. Iakovenko et al (2020)). Recalculation to  would require the selection of a
would require the selection of a  value and makes the resulting
value and makes the resulting  value dependent on the selected data set (de Prez et al
2016b). Therefore, these publications are not included in tables 5 and 6.
value dependent on the selected data set (de Prez et al
2016b). Therefore, these publications are not included in tables 5 and 6.
Table 5.
 data for orthogonal MRI-linacs with B = 0.35 T fulfilling the selection criteria. Orientations are defined according to section 4.1 and figure 7. The bold-faced values in the column Parallel orientations are values for orientation b, all other values are for orientation a. References with (MC) indicate that the
data for orthogonal MRI-linacs with B = 0.35 T fulfilling the selection criteria. Orientations are defined according to section 4.1 and figure 7. The bold-faced values in the column Parallel orientations are values for orientation b, all other values are for orientation a. References with (MC) indicate that the  has been determined with Monte Carlo simulations.
has been determined with Monte Carlo simulations.
| Chamber type | Reference | Perpendicular orientations | |||||
|---|---|---|---|---|---|---|---|
| Orientation d | Orientation e | Parallel orientations | |||||

|
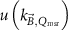
|

|
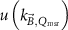
|
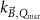
|
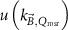
| ||
| PTW 30013 | Spindeldreier et al (2017) (MC) | 0.9675 | 0.31% | 0.9830 | 0.31% | 0.9970/0.9965 | 0.30% |
| Pojtinger et al (2019) | 0.9649 | 0.15% a | 0.9770 | 0.15% a | |||
| Malkov and Rogers (2018) (MC) | 0.9957 | 0.10% | |||||
| Shipley et al (2019) (MC) | 0.9750 | 0.10% | 0.9770 | 0.10% | |||
| Shipley et al (2019) | 0.9780 | 0.20% | 0.9730 | 0.20% | |||
a
The uncertainty on the calculated  was estimated to be similar to the uncertainty on the Monte Carlo calculated value for
was estimated to be similar to the uncertainty on the Monte Carlo calculated value for  (0.15 %), and is added in quadrature to the reported uncertainties on both the experimental and simulated
(0.15 %), and is added in quadrature to the reported uncertainties on both the experimental and simulated  .
.
Table 6.
 data for orthogonal MRI-linacs with B = 1.5 T fulfilling the selection criteria. Orientations are defined according to section 4.1 and figure 7. The bold-faced values in the column Parallel orientations are values for orientation b, all other values are for orientation a. References with (MC) indicate that the
data for orthogonal MRI-linacs with B = 1.5 T fulfilling the selection criteria. Orientations are defined according to section 4.1 and figure 7. The bold-faced values in the column Parallel orientations are values for orientation b, all other values are for orientation a. References with (MC) indicate that the  has been determined with Monte Carlo simulations.
has been determined with Monte Carlo simulations.
| Chamber type | Reference | Perpendicular orientations | |||||
|---|---|---|---|---|---|---|---|
| Orientation d | Orientation e | Parallel orientations | |||||
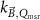
|
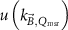
|
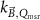
|

|
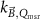
|
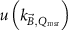
| ||
| IBA FC65-G | Wolthaus et al (2016) | 0.9512 a | 1.00% | ||||
| van Asselen et al (2018) | 0.9520 | 0.21% | 0.9970 | 0.30% | |||
| Malkov and Rogers (2018) (MC) | 0.9917 | 0.10% | |||||
| de Prez et al (2019b) | 0.9560 | 0.34% | 0.9950 | 0.34% | |||
| Billas and Duane (2018) | 0.9570 | 1.00% | 1.003/1.002 | 1.00% | |||
| PTW 30013 | Spindeldreier et al (2017) (MC) | 0.9540 | 0.30% | 0.9590 | 0.30% | 0.9920/0.9930 | 0.30% |
| Pojtinger et al (2019) (MC) | 0.9597 | 0.21% b | 0.9963 | 0.16% | |||
| Pojtinger et al (2019) | 0.9607 | 0.16% b | 0.9575 | 0.05% b | |||
| Malkov and Rogers (2018) (MC) | 0.9881 | 0.10% | |||||
| van Asselen et al (2018) | 0.9630 | 0.21% | 0.9920 | 0.20% | |||
| de Prez et al (2019b) | 0.9630 | 0.34% | 0.9850 | 0.34% | |||
| Billas and Duane (2018) | 0.9660 | 1.00% | 0.9980/0.9980 | 1.00% | |||
| Shipley et al (2019) (MC) | 0.9700 | 0.10% | 0.9580 | 0.10% | |||
| Shipley et al (2019) | 0.9600 | 0.60% | 0.9630 | 0.60% | |||
a
Recalculated from the data in the abstract using equation (13).
b
The uncertainty on the calculated  was estimated to be similar to the uncertainty on the Monte Carlo calculated value for
was estimated to be similar to the uncertainty on the Monte Carlo calculated value for  (0.15 %), and is added in quadrature to the reported uncertainties on both the experimental and simulated
(0.15 %), and is added in quadrature to the reported uncertainties on both the experimental and simulated  .
.
The criteria applied for the selection of chamber types (and  values) were:
values) were:
- reference type ionization chamber (criteria according to McEwen et al (2014));
-
 correction factors determined by measurement and Monte Carlo simulation or by two different Monte Carlo codes;
correction factors determined by measurement and Monte Carlo simulation or by two different Monte Carlo codes; - dead volume considered in Monte Carlo simulations;
- no air gap effect in the experimental determination of
 ; and
; and - estimation of uncertainty for
 .
.
In addition, only chamber types with more than one data point are included in tables 5 and 6. For the data shown in tables 5 and 6, the standard deviation of the mean values were calculated per orientation (d, e or parallel) and per chamber type. The maximum standard deviation of the mean was 0.21%. The reported data and their uncertainties is consistent with these mean values on the level of k= 2 for 18/20 data points.
Pojtinger et al (2019) calculated and measured  factors using a linac combined with an electromagnet. Their Monte Carlo model uses a dead volume determined by FEM simulations and is therefore fully independent from measurements. Calculated
factors using a linac combined with an electromagnet. Their Monte Carlo model uses a dead volume determined by FEM simulations and is therefore fully independent from measurements. Calculated  values for the same beam have been published elsewhere (Delfs et al
2018).
values for the same beam have been published elsewhere (Delfs et al
2018).  values in tables 5 and 6 were calculated from the data of both studies using equation (13). For B = 0.35 T only experimental
values in tables 5 and 6 were calculated from the data of both studies using equation (13). For B = 0.35 T only experimental  data is reported by Pojtinger et al (2019).
data is reported by Pojtinger et al (2019).
O'Brien et al (2016) calculated magnetic field correction factors for different types of Farmer chambers based on Monte Carlo simulations using Geant4. The observed discrepancies of >1% between their values for the PTW 30013 chamber with orientation d (0.976) and other more recent values given in tables 5 and 6 might be explained by not considering the dead volume. Monte Carlo calculations of magnetic field correction factors which take into account the dead volume (see section 5.3 and table 4) have been done by Spindeldreier et al (2017), Malkov and Rogers (2017, 2018) and Pojtinger et al (2019).
6.3.2.
Data for

Two studies discuss the determination of  factors for MRI-linac beams (Malkov and Rogers 2018, de Prez et al
2019b). The study by Malkov and Rogers (2018) reports Monte Carlo calculated
factors for MRI-linac beams (Malkov and Rogers 2018, de Prez et al
2019b). The study by Malkov and Rogers (2018) reports Monte Carlo calculated  values for the same set of chambers used in the TG-51 update. Simulations are based on 7 MV spectrum similar to the one used in an Elekta Unity™ MRI-linac. They show that the calculated values agree well with the
values for the same set of chambers used in the TG-51 update. Simulations are based on 7 MV spectrum similar to the one used in an Elekta Unity™ MRI-linac. They show that the calculated values agree well with the  fits of the TG-51 update. de Prez et al (2019b) published
fits of the TG-51 update. de Prez et al (2019b) published  values based on primary standard measurements in an Elekta Unity™ MRI-linac without a magnetic field present for a PTW 30013 and IBA FC65-G chamber. The agreement for these two chambers between both studies was within 0.2%.
values based on primary standard measurements in an Elekta Unity™ MRI-linac without a magnetic field present for a PTW 30013 and IBA FC65-G chamber. The agreement for these two chambers between both studies was within 0.2%.
Both studies differ in the treatment of the volume correction, kvol. The study of de Prez et al (2019b) excluded kvol from the  factor, while Malkov and Rogers (2018) did explicitely correct for kvol. However, since the simulated beam in the latter study was based on a spectrum from Ahmad et al (2016) the flatness might be different than in an actual MRI-linac beam. In addition, the ND,
w is calculated as the ratio between the dose to the cavity and the dose to a disk of 1 cm radius, which have similar dimensions. Therefore the impact of kvol on the
factor, while Malkov and Rogers (2018) did explicitely correct for kvol. However, since the simulated beam in the latter study was based on a spectrum from Ahmad et al (2016) the flatness might be different than in an actual MRI-linac beam. In addition, the ND,
w is calculated as the ratio between the dose to the cavity and the dose to a disk of 1 cm radius, which have similar dimensions. Therefore the impact of kvol on the  values from Malkov and Rogers (2018) can be considered to be small.
values from Malkov and Rogers (2018) can be considered to be small.
Since kvol is not included in  in these studies there is a small deviation from the TRS-483 formalism in which it is included in
in these studies there is a small deviation from the TRS-483 formalism in which it is included in  . The close agreement between
. The close agreement between  (from TG-51) and
(from TG-51) and  also suggests that
also suggests that  (equation (9)) for MRI-linacs without a magnetic field present is close to unity, which is in line with other facilities for which the concept of machine specific reference fields is applicable.
(equation (9)) for MRI-linacs without a magnetic field present is close to unity, which is in line with other facilities for which the concept of machine specific reference fields is applicable.
7. Discussion and conclusion
7.1. Compatibility with existing Codes of Practice for reference dosimetry
7.1.1. Formalism and reference conditions
Several formalisms for reference dosimetry in MRI-linacs have been proposed and investigated. As shown in section 4 these formalisms, although differently formulated, can be mutually harmonised and can be used to extend formalisms in existing codes of practice (Almond et al 1999, Andreo et al 2000, Aalbers et al 2008, DIN 2008, McEwen et al 2014, Palmans et al 2017). These extensions to existing formalisms require the measurement of beam quality specifier to be magnetic field independent. It was demonstrated that TPR20,10 is independent from the magnetic field. Therefore, the extension of TPR20,10 based CoPs seems to be rather straightforward for current clinical MRI-linac facilities.
%dd(10)x as a beam specifier is not independent from the magnetic field. In addition, in Elekta Unity™ MRI-linacs, the restricted range of SSD for the measurement of %dd(10)x cannot be realized and measurement of %dd(10)x in the absence of the magnetic field is not possible. Therefore, compatibility with TG-51 (Almond et al 1999, McEwen et al 2014) is only feasible when a solid conversion to %dd(10)x, in the definition of TG-51 (i.e. for an SSD of 100 cm), can be made. Both O'Brien et al (2016) and Malkov and Rogers (2018) suggest to base this conversion on a measured TPR20,10 in the presence of a magnetic field following the conversion method of Kalach and Rogers (2003). Malkov and Rogers (2018) demonstrates that the conversion is valid for filtered beams and for a beam based on the spectrum of an MRI-linac. Their calculated kQ, Q0 data corresponds within 0.1% with the kQ, Q0 data from the TG-51 fit (McEwen et al 2014) for four types of ionisation chambers. Comparison of the calculated kQ, Q0 data with the measured data of de Prez et al (2019b) shows a good agreement (<0.2%) for nearly the same TPR20,10 (0.700 vs. 0.695 respectively) for two types of ionisation chambers. This suggests that the proposed conversion procedure seems feasible. A consequence of the proposed conversion procedure is that users of TG-51 will have to measure TPR20,10, which is commonly not part of their procedures for reference dosimetry. A possible alternative method, which avoids this, could be based on a conversion from a measured %dd(10) in the presence of the MRI-linac magnetic field to %dd(10)x, in the definition of TG-51. However, currently no literature is available on which this conversion could be based.
Reference conditions in Codes of Practices are described by a set of values of influence quantities for which a calibration coefficient is valid without further correction factors (Palmans et al 2017), i.e. field size, depth in water, SSD, etc. These include the conditions that dosimeters need to be calibrated in, to be traceable at a level of a national standard. Table 7 shows the reference conditions recommended by TRS-398 (Andreo et al 2000) and TRS-483 (Palmans et al 2017) for high energy photon beams. Based on the evaluation of the existing literature on reference dosimetry for MRI-linacs, the table is complemented with additional influence quantities that are part of the reference conditions for reference dosimetry in MRI-linacs.
Table 7. Reference conditions for the determination of the absorbed dose to water in the MRI-linac systems compared with the recommendation from TRS-398 (for MV photon beams) and TRS-483 for high energy photon beams. Influence quantities not used in existing Codes of Practices are printed in bold italics.
| Influence quantity | Reference value | ||
|---|---|---|---|
| TRS-398 | TRS-483 | MRI-linac | |
| Phantom material | Water | Water | Water |
| Chamber type | Cylindrical | Cylindrical | Cylindrical |
| Chamber orientation w.r.t. B-field | N.A. | N.A. | Parallel (orientation a or b, figure 7) |
| Chamber orientation w.r.t. photon beam | Perpendicular | N.A. | Perpendicular |
| Magnetic flux density | N.A. | N.A. | 0.35 T, 1.5 T |
| Measurement depth (zref) | For TPR20,10 < 0.7, 10 g cm−2 (or 5 g cm−2) For TPR20,10 ⩾ 0.7, 10 g cm−2 | 10 g cm−2 | 10 g cm−2 |
| Reference point of the chamber | On the central axis at the centre of the cavity volume | On the central axis at the centre of the cavity volume | On the central axis at the centre of the cavity volume |
| Position of the chamber reference point | At zref | At zref | At zref |
| SDD | 100 cm | Isoc | Isoc |
| Field size at SDD | 10 × 10 cm2 | 10 × 10 cm2 or size of the msr field | a 10 × 10 cm2 or size of the msr field |
| Gantry angle | 90° | ||
a Field size defined in the absence of magnetic field, i.e. by the photon fluence or kerma distribution.
An additional influence quantity is the orientation of the ionization chamber with respect to the magnetic field. The evaluation of the chamber response change with magnetic field and the overview of  correction factors, indicates that for the parallel orientation the correction is minimized for cylindrical chambers and that its
correction factors, indicates that for the parallel orientation the correction is minimized for cylindrical chambers and that its  value is independent from whether the chamber tip points in the direction of, or in the opposite direction of, the magnetic field vector. Therefore, this is considered as the safest orientation of the ionization chamber for reference dosimetry in MRI-linacs.
value is independent from whether the chamber tip points in the direction of, or in the opposite direction of, the magnetic field vector. Therefore, this is considered as the safest orientation of the ionization chamber for reference dosimetry in MRI-linacs.
7.1.2. Chamber characteristics and correction factors
In the last ten years, a large amount of studies investigated ionization chamber characteristics for measurements in the presence of magnetic fields. These investigations have shown that several chamber characteristics have gained importance when used for reference dosimetry in MRI-linacs. The most important characteristics are the influence of small air gaps around the chamber and the impact of the dead volume of ionisation chambers on response change.
Parallel to these investigations, adequate measurements and Mont Carlo based methods to measure magnetic field correction factors,  , have been developed. Comparison of these methods shows that an adequate consistency between methods has been realized. The evaluation of published data of magnetic field correction factors, using these methods, demonstrates that, based on the specified selection criteria of section 6.3, a consistent data set with an uncertainty of 0.2% for a limited set of ionisation chamber types can be composed. Since intra-type variability of magnetic field correction factors was shown to be reasonably low, the presented data can be used to establish type specific magnetic field correction factors. Most Codes of Practices contain type specific
, have been developed. Comparison of these methods shows that an adequate consistency between methods has been realized. The evaluation of published data of magnetic field correction factors, using these methods, demonstrates that, based on the specified selection criteria of section 6.3, a consistent data set with an uncertainty of 0.2% for a limited set of ionisation chamber types can be composed. Since intra-type variability of magnetic field correction factors was shown to be reasonably low, the presented data can be used to establish type specific magnetic field correction factors. Most Codes of Practices contain type specific  or
or  correction factors for a much larger set of ionisation chambers. Extension of these Codes of Practices would require the determination of
correction factors for a much larger set of ionisation chambers. Extension of these Codes of Practices would require the determination of  for other chambers as well or would require limiting the number of recommended chamber types for reference dosimetry in MRI-linacs. The agreement of
for other chambers as well or would require limiting the number of recommended chamber types for reference dosimetry in MRI-linacs. The agreement of  for MRI-linacs with
for MRI-linacs with  curves from Codes of Practices indicates that this part of the formalism (i.e. for the msr field without a magnetic field present) is also compatible with existing Codes of Practices.
curves from Codes of Practices indicates that this part of the formalism (i.e. for the msr field without a magnetic field present) is also compatible with existing Codes of Practices.
7.2. Inline MRI-linacs
Even though the literature on dosimetry for MRI-linacs contains less references for inline systems than for orthogonal systems, it mainly covers similar topics. The main differences between the two type of facilities observed from the literature is discussed in the remainder of this section.
Because of the different configuration of the magnetic field and radiation field (section 2.3), radiation field characteristics are considerably different for inline MRI-linacs (Liney et al 2016, Begg et al 2019). This is mainly driven by the focussing effect as a result of the parallel direction of the beam with the magnetic field. This becomes mainly apparent in the build-up region of the depth dose profile where a dose increase is observed. From the perspective of reference dosimetry, it can be expected that beam quality specification based on %dd(10)x is considerably affected by this. However, as shown by Begg et al (2019), TPR20,10 remains largely unaffected by the magnetic field.
Several papers have investigated response changes of ionization chambers as a function of magnetic field for inline configurations (Reynolds et al 2013, 2015, 2017, Shukla et al 2017, Malkov and Rogers 2018). An overview is provided in table 8. Since conventional electromagnets do not allow for an inline beam configuration to a high field strength, because of the metal pole caps, very little experimental studies on detector response changes have been carried out and are limited to magnetic field strengths below 0.2 T. The Monte Carlo studies of table 8 show that the increase in response change is not higher than 2.5% for the investigated configuration.
Table 8. Overview of literature data on chamber response as a function of magnetic field strength for a parallel orientation of magnetic field direction with respect to (w.r.t.) beam direction from measurements (exp) and simulations (MC). The data is categorized by chamber type and by different orientations of chamber w.r.t. to beam and magnetic field direction. The values given for the maximum response change for positive magnetic fields (Extremum + and Extremum − respectively) and the magnetic field strength at which these extrema occur have been estimated from the plotted data.
| Chamber type | Paper | Orientation chamber w.r.t. beam | MC/exp | Extremum + (%) | B field (T) extremum + | Extremum −(%) | B field (T) extremum − |
|---|---|---|---|---|---|---|---|
| NE2571 | Reynolds et al (2013) | ∥ | MC | +2 | 1.5 | ||
| Reynolds et al (2013) | ┴ | MC | +2 | 1.5 | |||
| PR06 C | Reynolds et al (2013) | ∥ | MC | +2.5 | 1.5 | ||
| Reynolds et al (2013) | ┴ | MC | +1 | 1.5 | |||
| Reynolds et al (2015) | ∥ | Exp | <0.3% | 0.2 | |||
| Reynolds et al (2015) | ┴ | Exp | <0.3% | 0.2 | |||
| PTW 30013 | Spindeldreier et al (2017) | ┴ | MC | +1.7 | 3.0 | +1.6 | −3.0 |
| A1SLMR | Shukla et al (2017) | ┴ | MC | +0.42 | 1.1 | +0.45 | 1.1 |
For inline MRI-linacs the number of chamber orientations reduces to two, i.e. perpendicular or parallel to the beam. Although only the former configuration is compatible with existing CoPs, the data in table 8 shows that the differences between the two configurations are small (<1.5%). Note that for inline facilities negative magnetic field strengths are symmetrically equivalent to positive magnetic field strengths, which is also supported by the overview in table 8. The study of Spindeldreier et al (2017) investigated the impact of dead volumes for an inline configuration of radiation field and magnetic field. Their data suggests that the impact of dead volumes on Monte Carlo detector response simulations for inline facilities is negligible which is in line with the observations of Malkov and Rogers (2018).
The only set of  data that can be compared is that for a PTW 30013 chamber from the Monte Carlo studies of Malkov and Rogers (2018) and Spindeldreier et al (2017). This data shows differences in the order of 0.4% for magnetic field strengths. No comparison with measured data
data that can be compared is that for a PTW 30013 chamber from the Monte Carlo studies of Malkov and Rogers (2018) and Spindeldreier et al (2017). This data shows differences in the order of 0.4% for magnetic field strengths. No comparison with measured data  is possible currently.
is possible currently.
With future progress to clinical inline MRI-linacs it can be expected that the literature for these facilities will further develop. For now, there is no indication that reference dosimetry for inline MRI-linacs requires techniques or knowledge on chamber characteristics, which are not already available from orthogonal facilities. With further development in this field and comparisons between new data sets from experiments and simulations, the level of consistency of methods and data for MRI-linacs will become apparent.
8. Conclusion
Reference dosimetry for MRI-linacs requires new aspects in the behaviour of ionization chambers to be considered compared to reference dosimetry for conventional linacs. An overview and evaluation of the existing literature is given in this review. Although the physical background of these aspects is not completely understood yet, overall it can be concluded that adequate methods, yielding consistent results and data sets of correction factors, are available for the further development and safe application of CoPs for reference dosimetry for MRI-linacs that are currently in clinical operation. Initially this will be possible for a limited set of ionisation chambers. More data for other chamber types will be needed.
Despite the fact that all relevant ingredients for a Code of Practice for reference dosimetry in MRI-linacs are available, further research is needed. First, there is a need to further improve the understanding of ionization chambers characteristics focussing, for example, on the determination of the shape and size of dead volumes for various types of ionisation chambers. In particular, ionisation chambers with a smaller cavity volume probably exhibit larger effects and might be of interest. Second, the demand for more  data will lead to new data sets, for example based on new primary standards, for other type of ionization chambers and for inline MRI-linacs. Finally, the literature shows that perturbation effects of ionisation chambers in the presence of a magnetic field are much larger than without a magnetic field. Conceptual models of these perturbation effects are lacking but would be helpful in reducing these effects, for example in the design of ionisation chambers.
data will lead to new data sets, for example based on new primary standards, for other type of ionization chambers and for inline MRI-linacs. Finally, the literature shows that perturbation effects of ionisation chambers in the presence of a magnetic field are much larger than without a magnetic field. Conceptual models of these perturbation effects are lacking but would be helpful in reducing these effects, for example in the design of ionisation chambers.
Acknowledgments
The authors would like to thank Stefan Pojtinger (PTB) for providing figures 5 and 10. This project has received funding from the EMPIR programme, grant 15HLT08 MRgRT and grant 19NRM01 MRgRT-DOS, co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme.
Footnotes
- 1
In the remainder all uncertainties are expressed a standard uncertainty with (k = 1).