Abstract
The development of effective and stable cathode electrocatalysts is highly desired for fuel cells. Controlling the composition and morphology of Pd-based materials can provide a great opportunity to improve their oxygen reduction reaction (ORR) performance. Here, we report the synthesis of hexagonal close-packed (hcp) Pd2B nanosheet assemblies (Pd2B NAs) via the boronation reaction between as-synthesized Pd NAs and N,N-dimethylformamide. The hcp Pd2B NAs with uniform pore distribution can provide sufficient active sites for ORRs. The insertion of B atoms can induce the phase transition from face-centered cubic structure to hcp structure, as the most thermodynamically stable phase in the Pd-B alloy, which is beneficial for enhancing the ORR stability and toxicity resistance. Therefore, the hcp Pd2B NAs exhibit superior mass activity, specific activity and excellent stability for ORR. The present strategy of boron-intercalation-triggered crystalline transition of Pd-based nanomaterials is valuable for the design of metal–nonmetal catalysts with enhanced performance.
Export citation and abstract BibTeX RIS
1. Introduction
Due to the ever-increasing demand for green energy supplies, the development of energy storage and conversion technologies has intensified in recent years [1–3]. Fuel cells can convert the chemical energy of fuels into electrical energy [4–6]. The development of highly active catalysts for oxygen reduction reactions (ORRs) is the core of fuel cell technology [7–12]. Pd-based materials have been widely reported for ORRs in alkaline media due to their fast ORR kinetics and excellent anti-corrosion [13–20]. However, the activity of Pd-based catalysts is usually lower than that of the state-of-the-art Pt-based catalysts due to their slow electron transfer rate and high oxygen binding energy [21–23]. As such, the development of advanced Pd-based catalysts is essential to enhance their ORR performance.
The ORR activity of Pd-based materials is strongly related to their morphology, composition and crystal structure [24–28]. Nanomaterials with unique morphology (such as tripods, nanowires and network structures) show superior electrochemical activity due to their rich active sites [29–33]. Among various morphologies, the nanosheet structure has appealed to the extensive attention of researchers because of excellent catalytic performance [34–37]. In addition, the combination of Pd with nonmetal elements has received considerable attention because it can tailor the electronic structure of Pd [38–41]. As previously reported, Pd-B can form stable alloys, and the most thermodynamically stable phase is the hexagonal close-packed (hcp) Pd2B phase [42–46]. The incorporation of B into the Pd lattice can induce the lattice expansion of Pd, leading to the decreased bond strength of Pd-O, which enhances the ORR activity.
Inspired by the above considerations, we report a facile synthetic approach to produce hcp Pd2B nanosheet assemblies (Pd2B NAs) via the boronation reaction between as-synthesized Pd NAs and N,N-dimethylformamide (DMF). The nanoflower-like Pd2B NAs can provide sufficient active sites and prohibit the catalyst aggregation. Furthermore, the intercalation of B into Pd can produce lattice expansion, electronic effects and phase transition, which can decrease the oxygen adsorption on Pd, which is favorable for oxygen reduction. Thus, the hcp Pd2B NAs show excellent mass activity of 1.48 mA μg−1 and specific activity of 2.36 mA cm−2 for ORR, which are 9.86 and 9.07 times those of Pt/C. Furthermore, hcp Pd2B NAs possess significantly better durability than Pd nanosheet assemblies (Pd NAs) and Pt/C due to the ultrahigh stability of catalysts.
2. Experimental
2.1. Chemicals and materials
N,N-dimethylformamide (DMF), acetic acid, tetrahydrofuran (THF), dimethylamine borane (DMAB) and isopropanol were purchased from Aladdin. Sodium tetrachloropalladate (Na2PdCl4) was obtained from Sigma-Aldrich. Commercial Pd/C (10 wt%) and Pt/C (20 wt%) were ordered from Alfa Aesar.
2.2. Preparation of hcp Pd2B NAs
The Pd NAs were first synthesized as follows. In brief, 10 mg of Na2PdCl4 was first dissolved in 8 ml of DMF by sonication for 20 min, followed by the addition of 2 ml acetic acid. Then, CO was bubbled into the mixture solution for 6 min and then maintained at room temperature for 12 h. After the reaction, the Pd NAs were collected by centrifugation and washed with ethanol for three cycles. For the synthesis of hcp Pd2B NAs, 295 mg of DMAB and 10 mg of Pd NAs were dispersed in 5 ml of THF for 10 min under sonication, which was transferred to a 20 ml Teflon-lined stainless autoclave and kept at 160 °C for 5 h. Finally, the hcp Pd2B NAs were obtained after centrifugation and three washing cycles.
2.3. Characterizations
The morphology of the samples was characterized using field emission scanning electron microscopy (ZEISS Gemini SEM 500) at 10 kV. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were taken on a JEOL JEM-2100F apparatus at an acceleration voltage of 300 kV. X-ray photoelectron spectroscopy (XPS) was conducted on an ESCALAB MK II spectrometer (VG Scientific) with Al Kα x-ray excitation. The x-ray diffraction (XRD) study was carried out in a PANalytical X'Pert PRO diffractometer with Cu Kα radiation.
2.4. Electrochemical measurements
Electrochemical measurements were conducted by a CHI 760E electrochemical analyzer using a modified glassy carbon rotating disk electrode (RDE) with a geometric area of 0.071 cm2, Ag/AgCl electrode (1 M KCl) and Pt wire as the working electrode, reference electrode and counter electrode, respectively. To prepare the working electrode, 2 mg of the catalyst was dispersed in a 1.0 ml solution containing 0.05 ml of Nafion solution (0.5 wt%), 0.1 ml of isopropanol and 0.85 ml of H2O, followed by coating of 1 μl of catalyst ink onto the RDE surface for drying at room temperature. The electrochemical active surface area (ECSA) of catalysts was determined via the CO-stripping method [47, 48]. The ECSACO of the catalyst was calculated based on the charge associated with the oxidation of the pre-absorbed CO monolayer in the absence of CO (purged with N2) in a 0.1 M HClO4 solution with a linear change in potential (CO stripping). The ECSA values based on QCO were calculated using the equation:
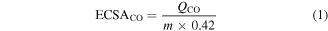
where QCO is obtained from the integrated area of the CO-stripping peak, m (2 μg) is the metal loading on the working electrode and 0.42 mC cm−2 is the charge associated with the monolayer of linearly bound adsorbed CO on the Pd surface.
The ORR activities were investigated using line-sweep voltammetry curves measured at 20 mV s−1 in 0.1 M O2-saturated KOH electrolyte. The kinetic current was obtained from the Koutecky–Levich (K-L) equation for the calculation [49]
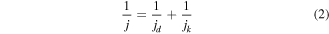
where j is the current density, jk is the dynamic current density and jd is the limiting diffusion current density.
For the accelerated durability test (ADT), cyclic voltammetry (CV) was conducted for 5000 cycles between 0.7 and 1.0 V. A chronoamperometry (CA) test was carried out at 0.7 V for 6 h at 1600 rpm in an O2-saturated 0.1 M KOH solution. All potentials in this article were converted to reversible hydrogen electrodes (RHE) according to the equation
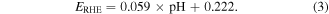
3. Results and discussion
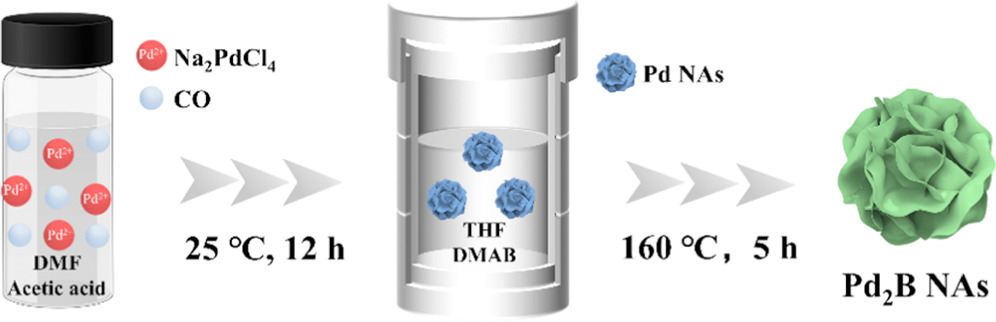
Figure 1 illustrates the synthesis of the hcp Pd2B NAs via a two-step strategy. Pd NAs were first synthesized in DMF using acetic acid as a reductant and CO as a structure-directing agent. During the reaction, the strong binding of CO to the (111) crystalline structure can induce the formation of nanosheet structures [50], followed by the self-assembly of nanosheets into a 3D structure at high temperature. The nanoflower-like Pd nanostructure with assembled nanosheets can be clearly observed in large-scale SEM and TEM images (Figure S1). Then, DMAB serves as an effective boron source to react with Pd NAs, and then boron atoms are gradually incorporated into the Pd lattice to form hcp Pd2B NAs under high-temperature solvothermal conditions [42].
Figure 1. A schematic illustration of the synthesis of the hcp Pd2B NAs.
Download figure:
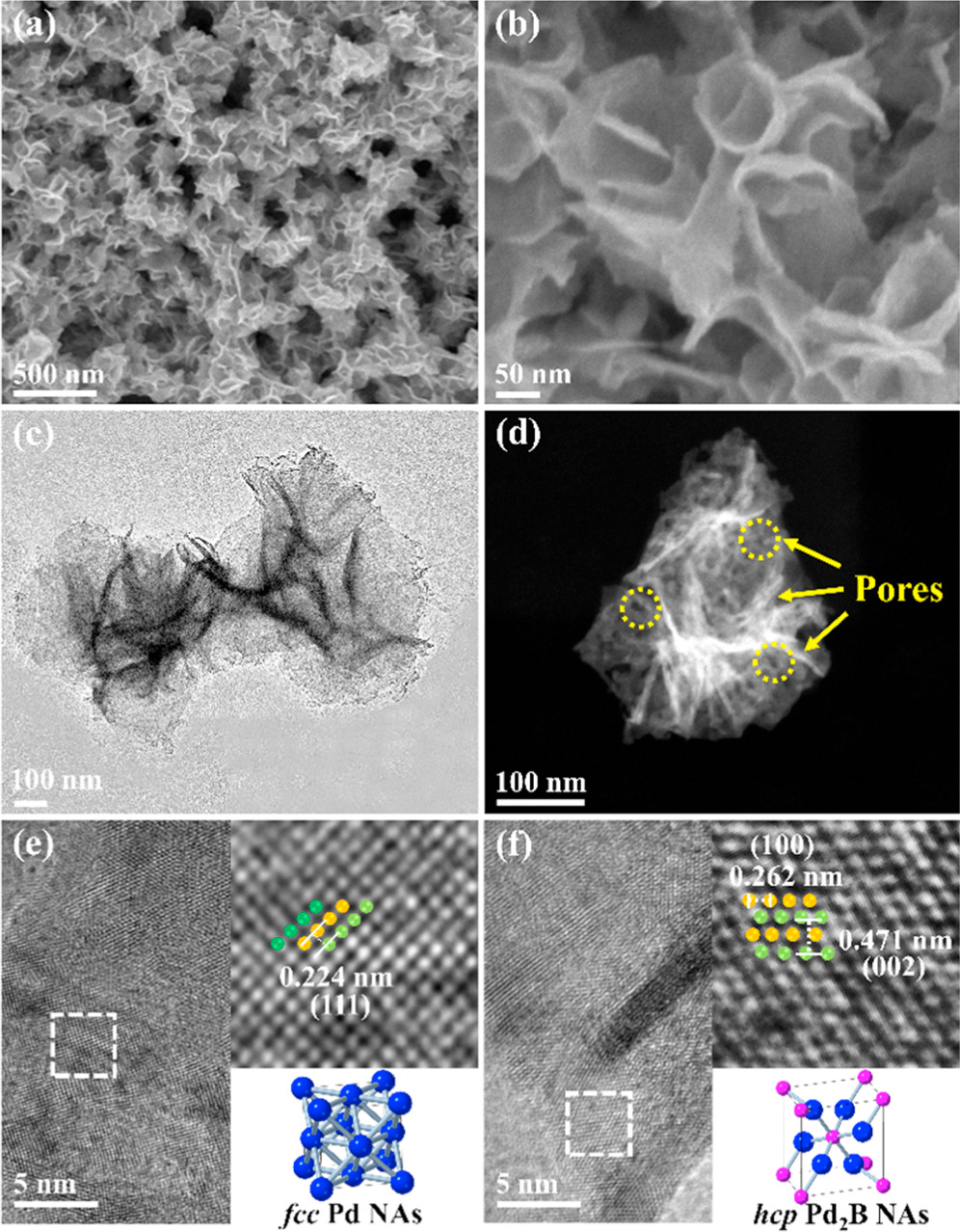
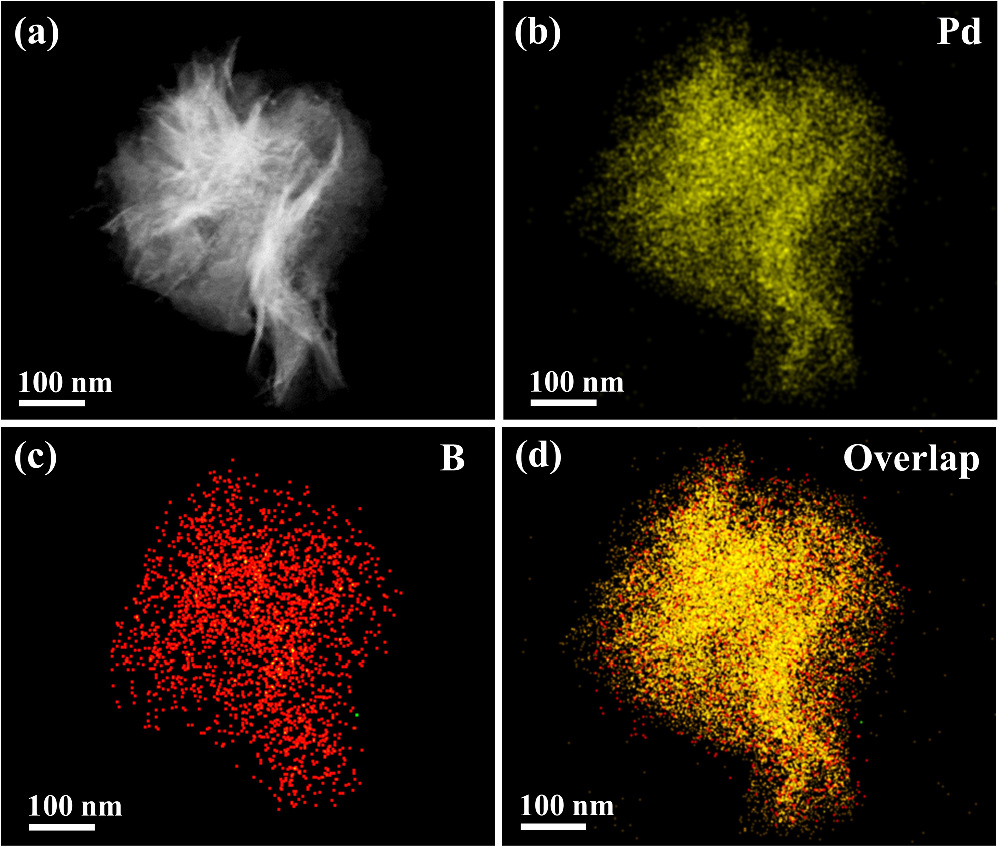
Standard image High-resolution imageThe SEM images show that the hcp Pd2B NAs preserve the initial structure of Pd nanosheets after the boronation process (figures 2(a) and (b)). The TEM image demonstrates that hcp Pd2B NAs are composed of several ultrathin nanosheets stacked together (figure 2(c)). The high-angle annular dark-field scanning TEM (HAADF-STEM) image further shows that the folded Pd2B nanosheets have rich pores induced by CO etching (figure 2(d)) [50]. Figure 2(e) shows the HRTEM images of Pd NAs and the corresponding crystal model, which exhibit a typical face-centered cubic (fcc) crystal structure with ABC stacking with lattice spacing of 0.224 nm, in accordance with the (111) crystal plane of fcc Pd. Figure 2(f) shows that the HRTEM images of hcp Pd2B NAs exhibit a hexagonal dense crystal structure with ABAB stacking [40], indicating the formation of the hcp structure, in which the lattice distances of 0.262 and 0.471 nm can be ascribed to the characteristic (100) and (002) planes of typical hcp Pd2B crystals, respectively. The elemental distribution of the hcp Pd2B NAs was characterized using STEM energy dispersive X-ray spectroscopy (STEM-EDX) mapping images (figure 3), where Pd and B elements distribute uniformly across the whole nanosheet. Additionally, the atomic ratio of Pd:B is estimated to be approximately 68:32 (figure S2), which is indeed close to the 2:1 ratio.
Figure 2. (a), (b) SEM images, (c) the TEM image and (d) the HAADF-STEM image of hcp Pd2B NAs. HRTEM images and the corresponding structural models of (e) fcc Pd NAs and (f) hcp Pd2B NAs.
Download figure:
Standard image High-resolution imageFigure 3. (a) The HAADF-STEM image and the corresponding elemental mapping images of Pd (b) and B (c) elements as well as element overlap (d) of hcp Pd2B NAs.
Download figure:
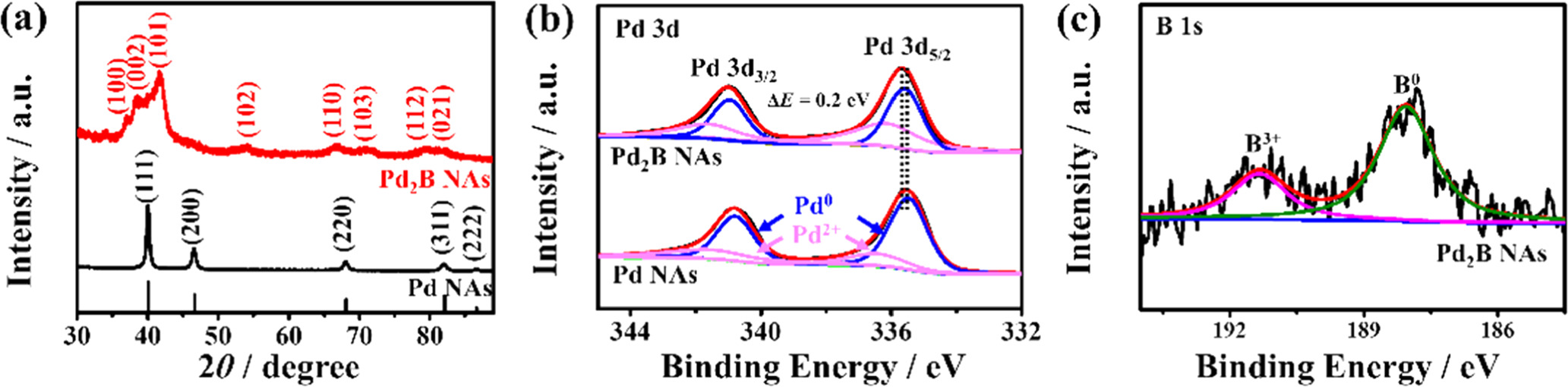
Standard image High-resolution imageXRD was conducted to investigate the crystal structures of the samples (figure 4(a)). The characteristic peaks of Pd NAs are located at 40.03°, 46.55°, 68.02°, 81.99° and 86.44°, corresponding to the (111), (200), (220), (311) and (222) planes of typical fcc Pd crystals, respectively. After the boronation reaction, the hcp Pd2B NAs have different diffraction peaks at 36.9°, 38.28°, 41.6°, 54.08°, 66.54°, 71.14°, 79.38° and 81.66°, corresponding to their (100), (002), (101), (102), (110), (103), (112) and (021) crystal planes of hcp Pd2B crystals [42, 46], respectively. In addition, selected area electron diffraction (SAED) patterns further confirm the fcc and hcp crystal structures of Pd NAs and Pd2B NAs, respectively (figure S3). To explore the crystalline transition from fcc Pd to hcp Pd2B, samples were prepared at different temperatures (90, 120 and 160 °C). From the XRD patterns, it can be found that there are no obvious diffraction peaks of the hcp structure at 90 °C, and the diffraction peaks of the hcp structure start to appear at 120 °C. Moreover, the XRD peaks of the (111) plane for samples prepared at 90 °C (39.82°) and 120 °C (39.33°) have a negative shift compared with that of Pd NAs, indicating the gradual lattice expansion of Pd as the reaction temperature increases due to the slow insertion of boron atoms into Pd lattices (figure S4). When the operational temperature is 160 °C, the sample shows complete transition of the fcc structure to the hcp structure, indicating the successful synthesis of the Pd2B crystals. XPS measurements were conducted to better understand the valence state of the samples. The XPS Pd 3d spectrum presents two different valence states of Pd 3d3/2 and Pd 3d5/2 core levels (figure 4(b)), in which the peaks at 335.65 and 341.00 eV can be attributed to Pd°, while the other two peaks at 336.30 and 341.75 eV can be ascribed to the Pd2+ species caused by surface adsorption of O species in air [51, 52]. Compared with Pd NAs, the hcp Pd2B NAs possess a positive shift of Pd 3d peaks by about 0.2 eV, indicating the presence of the strong electronic structure. The increased binding energy reveals the electron loss of Pd, which induces the d-band center of Pd to move away from the Fermi level, leading to the downshift of the d-band center of Pd. The lower d-band center induces more filled electrons in the anti-bonding band, thus weakening the stability and strength of adsorption bonding between oxygen species and Pd, [53, 54] which is favorable for enhancement of ORR performance. Figure 4(c) is the XPS spectrum of the B 1s, in which the peaks located at 188.08 and 191.38 eV could be assigned to B0 and B3+ [55]. The presence of B3+ is possibly mainly due to surface oxidation of catalysts. From the above results, it is obvious that the B atoms have been successfully incorporated into Pd to form the hcp Pd2B alloy structure [40].
Figure 4. (a) XRD patterns of the hcp Pd2B NAs and Pd NAs. (b) XPS spectra of Pd 3d for the hcp Pd2B NAs and Pd NAs. (c) The XPS spectrum of B 1s for the hcp Pd2B NAs.
Download figure:
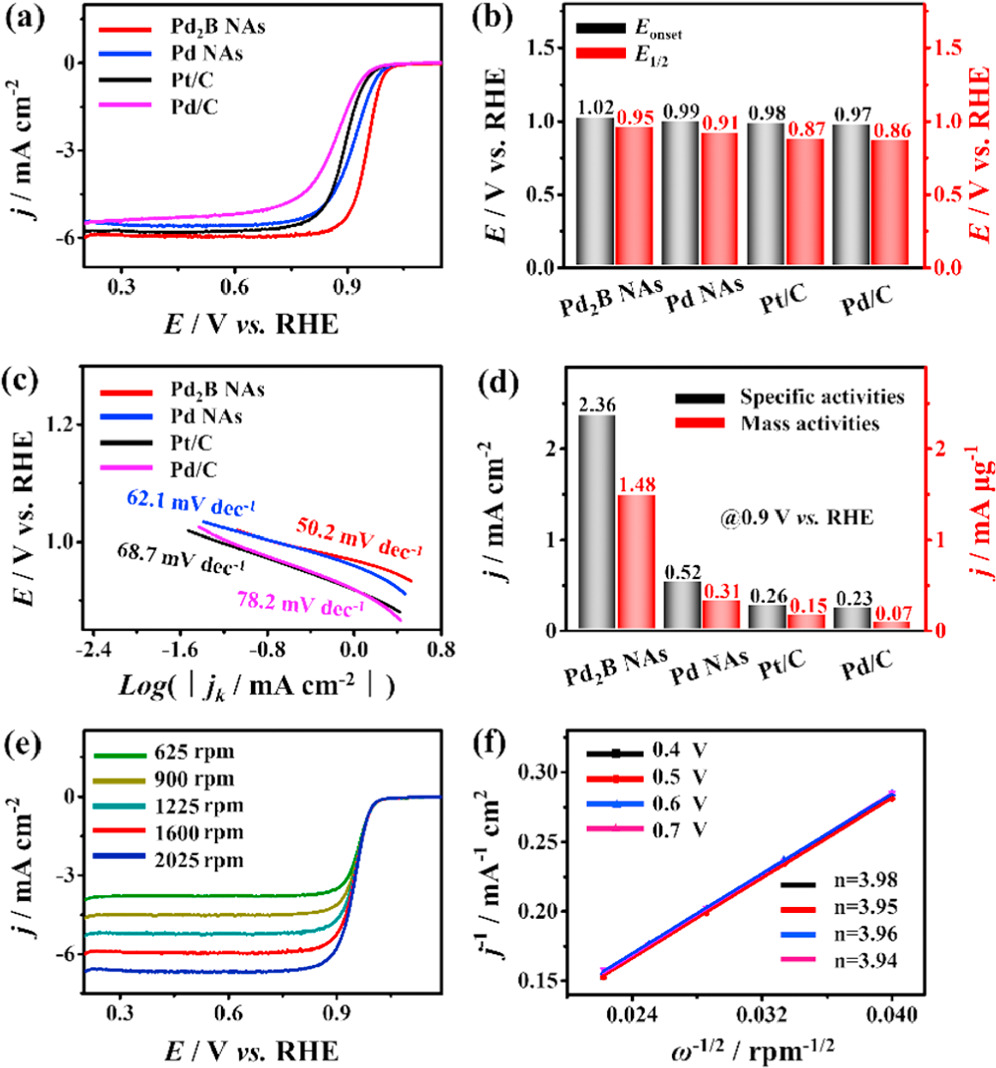
Standard image High-resolution imageThe ORR performance of the hcp Pd2B NAs was investigated and compared with that of Pd NAs, Pt/C and Pd/C. The ECSA was first calculated using the integrated area of CO stripping (figure S5). The ECSA value of the hcp Pd2B NAs is 59.52 m2 g−1, higher than those of the Pd NAs (58.15 m2 g−1), Pt/C (56.9 m2 g−1) and Pd/C (31.42 m2 g−1), revealing the more active sites of hcp Pd2B NAs due to the formation of porous structures. According to the polarization curves of these catalysts, the hcp Pd2B NAs show a higher onset potential (Eonset) of 1.02 V and half-wave potential (E1/2) of 0.95 V (figure 5(a)). The Eonset of the Pd NAs, Pt/C and Pd/C is 0.99, 0.98 and 0.97 V, respectively, and the E1/2 of the Pd NAs, Pt/C and Pd/C is 0.91, 0.87 and 0.86 V, respectively (figure 5(b)). These results indicate that the hcp Pd2B NAs have excellent electrochemically catalyzed ORR activity because the boron intercalation can optimize the oxygen adsorption on Pd. As seen in figure 5(c), Tafel plots were measured to estimate the ORR kinetics. The Tafel slope of the hcp Pd2B NAs is 50.2 mV dec−1, lower than that of Pd NAs (62.1 mV dec−1), Pt/C (68.7 mV dec−1) and Pd/C (78.2 mV dec−1), showing faster kinetics of hcp Pd2B NAs due to the large specific surface area and fast electron transfer rate provided by the porous nanosheet morphology. The specific activity (the kinetic current normalized to the ECSA) and the mass activity (the kinetic current normalized to the Pd mass) of samples were calculated from polarization curves at the potential of 0.9 V. The hcp Pd2B NAs exhibit a mass activity of 1.48 mA μg−1, 4.77, 9.86 and 21.14 times that of Pd NAs (0.31 mA μg−1), Pt/C (0.15 mA μg−1) and Pd/C (0.07 mA μg−1), respectively (figure 5(d)). Meanwhile, the hcp Pd2B NAs exhibit a specific activity of 2.36 mA cm−2, 4.53, 9.07 and 10.26 times that of Pd NAs (0.52 mA cm−2), Pt/C (0.26 mA cm−2) and Pd/C (0.23 mA cm−2), respectively. The high mass and specific activity of the hcp Pd2B NAs principally originated from the high active sites and reduced oxygen adsorption energy on Pd.
Figure 5. (a) ORR polarization curves of catalysts, and their corresponding (b) onset potentials and half-wave potentials, (c) Tafel plots and (d) specific and mass activities. (e) Rotation-rate-dependent current–potential curves of the hcp Pd2B NAs, and (f) the calculated electron transfer number at different potentials.
Download figure:
Standard image High-resolution imageTo further investigate the ORR reaction pathway, a series of polarization curves were recorded at different rotation rates from 625 to 2025 rpm (figure 5(e)). As the rotational speed increases, the limiting current density rises gradually. According to the current density at different speeds, the ORR kinetics was analyzed using the K-L equation. The corresponding K-L points at different potentials (0.4, 0.5, 0.6 and 0.7 V) show good linearity, indicating that the ORR kinetics of hcp Pd2B NAs is a first-order process. Based on the slopes, the electron transfer number (n) at different potentials is calculated to be 3.98, 3.95, 3.96 and 3.94 (figure 5(f)). These results indicate the four-electron reduction of O2 to OH− on the surface of the hcp Pd2B NAs. Similarly, the polarization curves of Pd NAs, Pt/C and Pd/C at different rotating speeds are also analysed, which show similar results to the hcp Pd2B NAs of the four-electron ORR process (figure S6). The rotating ring disk electrode (RRDE) test showed that the hydrogen peroxide production rate of the hcp Pd2B NAs was very low, almost approaching 0, and the number of transferred electrons was four. The hydrogen peroxide production rate of the other three was also tested, and it was found that they were all higher than the hcp Pd2B NAs, and the number of transferred electrons was close to four (figure S7).
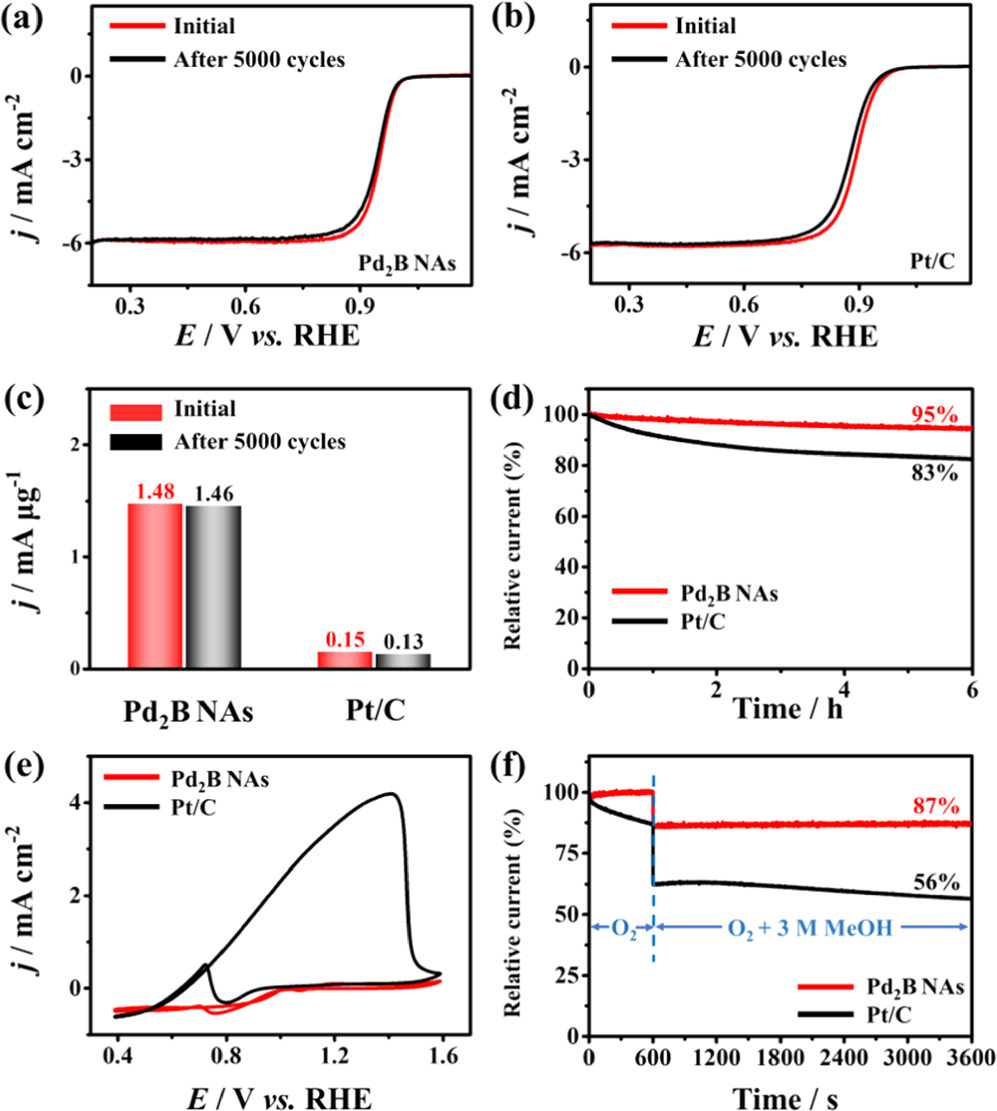
ADT and chronoamperometric measurements were investigated to evaluate the stability of catalysts. ADTs were performed by applying potential cycling between 0.8 and 1.0 V for 5000 cycles in 0.1 M O2-saturated KOH electrolyte. ORR polarization curves of hcp Pd2B NAs before and after the stability test are presented in figure 6(a), in which the E1/2 of hcp Pd2B NAs shifts negatively by 0.01 V compared with the initial curve. In contrast, the E1/2 of Pt/C shifts negatively by 0.017 V (figure 6(b)), and the E1/2 of Pd NAs shifts negatively by 0.19 V (figure S8a). The mass activities of the hcp Pd2B NAs and Pt/C drop by 1.4% and 13.4%, respectively (figure 6(c)). The durability of the hcp Pd2B NAs and Pt/C was further assessed using CA at a fixed potential of 0.75 V in O2-saturated 0.1 M KOH. As shown in figure 6(d) and figure S8b, the hcp Pd2B NAs cause only slight degradation (5%) of current density after continuous ORRs for 6 h, while Pt/C and Pd NAs have high decay of 17% and 27%, respectively. Moreover, the morphology of the hcp Pd2B NAs shows no obviously change after the ADT (figure S9), indicating the excellent material stability due to the highly thermodynamically stable Pd2B alloy structure and nanoflower-like structure [42, 43]. In addition, the methanol tolerance of hcp Pd2B NAs was evaluated in O2-saturated 0.1 M KOH with 1 M CH3OH (figure 6(e)). In the presence of methanol, Pt/C exhibits a distinct methanol oxidation peak, while the hcp Pd2B NAs have no methanol oxidation peak, indicating that hcp Pd2B NAs have excellent methanol tolerance. The chronoamperometric tests, after the addition of 3 M methanol to O2-saturated electrolyte, show that the current density of hcp Pd2B NAs only decays by 13%, while the current density of Pt/C greatly degrades by 44% due to the occurrence of an intensive methanol oxidation reaction (figure 6(f)). The above studies prove the outstanding anti-methanol ability of hcp Pd2B NAs because boron insertion can induce the downshift of the d-band center of Pd, leading to the easy desorption of CO-like intermediates [56, 57].
Figure 6. ORR polarization curves of (a) hcp Pd2B NAs (b) and Pt/C before and after ADTs, and (c) the corresponding mass activities. (d) Chronoamperometric curves of hcp Pd2B NAs and Pt/C in O2-saturated 0.1 M KOH solution with a rotation rate of 1600 rpm. (e) CV curves of hcp Pd2B NAs and Pt/C in O2-saturated 0.1 M KOH with 1 M CH3OH solution. (f) Chronoamperometric curves of hcp Pd2B NAs and Pt/C in O2-saturated 0.1 M KOH electrolyte with the addition of 3 M CH3OH at 600 s.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, hcp Pd2B nanosheet assemblies are successfully prepared using a two-step method for the ORR. The porous nanosheet assembled structure can provide a large surface area and high electron transfer efficiency. Moreover, the introduction of B atoms can not only induce the crystal transformation from fcc Pd to hcp Pd2B, but can also generate lattice expansion and electronic effects, which can cause the downshift of the d-band center of Pd, leading to the decrease in oxygen adsorption on Pd, which is beneficial for enhancing the ORR performance. Therefore, hcp Pd2B NAs exhibit high mass activity of 1.48 mA μg−1 and specific activity of 2.36 mA cm−2, which are 9.86 and 9.07 times those of Pt/C, respectively. This boronation route is a universal strategy for the facile and controllable preparation of other boron-metal nanomaterials, which would be highly promising for ORRs and beyond.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (Nos. 21972126, 21978264, 21905250, and 22278369), the Natural Science Foundation of Zhejiang Province (Nos. LQ22B030012 and LQ23B030010), and China Postdoctoral Science Foundation (2021M702889).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Supplementary data (2.6 MB DOCX)