Abstract
Ferroelectric and magnetic properties are investigated for Bi2Fe4O9 nanoparticles with different shapes (cuboid and sphere-like) synthesized by hydrothermal and sol-gel method. The magnetic study reveals that coercivity, Neel temperature and remanent magnetization strongly depend on shape of the particle. The nanoparticle with sphere-like shape exhibits magnetization curve of antiferromagnetic (AFM) ordering with ferromagnetic (FM) component. As the particle shape changes from sphere-like to cuboid, the AFM component is dominating over the ferromagnetic component. A small exchange bias is also observed at low temperature in both the sphere-like and cuboid nanoparticle. The coercivity, remanent magnetization and Neel temperature of sphere-like nanoparticle is greater than cuboid nanoparticle. Ferroelectric measurement shows the remanent polarization of cuboid is greater than sphere-like nanoparticle but the coercivity is almost same. This Bi2Fe4O9 nanoparticle shows a small change in polarization under magnetic field. The polarization value decreases with magnetic field increases. The magnetoelectric coupling-measured by change of remanent polarization under magnetic field are found to be greater in Bi2Fe4O9 sphere-like nanoparticles. These shape dependent magnetic and ferroelectric properties are coming because of shape anisotropy.
Export citation and abstract BibTeX RIS
1. Introduction
Due to its diverse physical characteristics as well as amazing multifunctional device applications, magnetoelectrically linked multiferroic materials have been the subject of intense academic study in recent decades [1, 2]. The interaction of magnetic and electrical order characteristics in these materials is known as the'magnetoelectric (ME) effect. The coupling of these two ferroic order characteristics adds another level of flexibility to device design by allowing magnetic and electric polarisation to be sensitively controlled with electric and magnetic fields, respectively. Furthermore, the simultaneous existence of both ferroic types at ambient temperature is of fundamental as well as technological significance. Most ME multiferroic systems that have been discovered so far work at temperatures well below room temperature. As a result, the quest for single phase multiferroic materials with the best ME effect at room temperature continues and remains a realistic problem. The size-dependent properties of multiferroic material have been investigated so far [3, 4]. Now it is interesting to control the shape of the nano multiferroics and explore the correlation between material properties with its shape. The shape anisotropy, surface anisotropy, and magnetocrytalline anisotropy play important role in magnetic properties. It has earlier been shown that the blocking temperature of γ-Fe2O3 changes due to the change in the shape of the particles [5, 6]. The coercivity, retentivity, Neel temperature of a magnetic material can be changed due to the change in shape. Therefore, magnetic properties are very responsive to the shape of the material. BiFeO3 is the well-known room temperature multiferroic [7]. In recent days, Bi2Fe4O9 has been reported as a multiferroic material having Neel temperature (TN) around 250 K and ferroelectric Curie temperature around 260 K [8]. This Bi2Fe4O9 belongs to orthorhombic lattice system with Pbnm space group having two formula units per unit cell with two different sites of Fe atom [9]. Bi2Fe4O9 is a very attractive material as a unique pentagon spin frustration comes in it due to the challenging exchange interaction among the different kinds of Fe3+ ions, so it leads to a non-collinear magnetic structure [10]. In the unit cell of Bi2Fe4O9 four Fe1 atoms are situated in Fe1O6 octahedral. This Fe1 on each side of the unit cell interact ferromagnetically, whereas four Fe2 atoms are positioned in the Fe2O4 tetrahedra, and the spins are interacting here antiferromagnetically among themselves, representing the super exchange interaction [11]. Now, this ferromagnetic and antiferromagnetic type exchange interaction between these two types of iron atoms produces a pentagon spin frustration within the system. The antiferromagnetic interactions in the pentagon are different types. Here the interaction in the Fe2–O1–Fe2 bond is strongest antiferromagnetic, whereas Fe2–O3–Fe1 and Fe2–O2–Fe1 is weak and weakest, respectively. Another important property of this material is the ferroelectric property. The origin of ferroelectricity of Bi2Fe4O9 is not clear until now. Tian et al reported that Bi2Fe4O9 is a ferroelectric material, which is generated by the lone pair of Bi atoms [4]. A lone s2 pair of electrons of the Bi3+ ion may hybridize with the 2p orbital of the O2+ ions to form a localized lobe, which leads to the noncentrosymmetric distortion and hence ferroelectricity. So, this is a multiferroic material in nature, and we are trying to investigate its dependency on shape anisotropy, which makes us easy to choose the appropriate shape in material science application [12–14].
In this work, we have synthesized Bi2Fe4O9 nanoparticles with different shapes. The crystallographic structure, magnetic, ferroelectric properties have been examined. We found a small room temperature multiferroic coupling in Bi2Fe4O9 nanoparticles, and this coupling is shape-dependent.
2. Experimental section
2.1. Materials and chemicals
Bismuth nitrate (Bi(NO3)3 × 5H2O) (99%), Iron nitrate (Fe(NO3)3 × 9H2O) (99%), anhydrous citric acid, Nitric acid (HNO3) (69%), Iron chloride (FeCl3 × 6H2O) (98%), Ethylene glycol (EG), Poly Vinyl Pyrrolidone (PVP), Sodium hydroxide were purchased from Sigma Aldrich. These materials were used without any purification.
2.2. Synthesis of sphere-like Bi2Fe4O9
Bi2Fe4O9 sphere-like nanoparticles was synthesized by sol-gel method. 0.004 M of (Bi(NO3)3 × 5H2O), 0.008 M of (Fe(NO3)3 × 9H2O) and 0.008 M of anhydrous citric acid were dissolved in 80 ml de-ionized water with 3 ml HNO3 under magnetic stirring at room temperature. The mixture was heated at 80 °C until the complete evaporation took place, and we would obtain a solid product. Then this solid product was heated at 150 °C for 1 h. Next, the powder was grounded with a mortar and heated at 700 °C for 4 h.
2.3. Synthesis of cuboid Bi2Fe4O9
(Bi(NO3)3 × 5H2O) and (FeCl3 × 6H2O) were dissolved in 50 ml EG with stoichiometric ratio (1:2 molar ratio) under stirring. Next, 500 mg PVP was added to this solution. Then 50 ml distilled water was added and stirred for 30 min. Next concentrated ammonia was added until the pH value of the solution became 10–11. The precipitate amount was centrifuged out several times until the pH value was 6–7. Next, 5 M NaOH solution was added with the centrifuged sediment and stirred for 30 min. Then the solution was transferred to a Teflon sealed stainless steel autoclave and heated at 180 °C for 48 h. The final product was centrifuged by distilled water and ethanol then dried at 80 °C for characterization.
2.4. Characterization
The crystal structure of as prepared sample was measured by Philips X'Pert x-ray diffractometer with Cu-α radiation (λ = 1.54056 A°) in the 2θ range 10–90° at room temperature. The micrograph was investigated by field effect scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). For TEM measurement, sample dissolved in ethanol by sonicating in an ultrasonic vibrator for few hours then drop cast on carbon copper TEM grid and operated by Tecnai G2 30ST electron microscopy operating at 300 kV. High resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), and energy dispersive spectroscopy (EDX) were also carried out using this instrument. The x-ray photoelectron spectra (XPS) of two samples were collected using PHI 5000 Versa Probe II spectrophotometer (Physical Electronics Inc., USA). Magnetic measurements were carried out using a physical property measurement system (PPMS) equipped with a vibrating sample magnetometer (VSM) (Quantum Design). This VSM is calibrated with a standard Ni sphere and the powder sample was filled in a plastic sample holder at the time of measurement. The sample holder was vibrated with a constant frequency to measure the magnetic moment. For electrical property measurement, the sample was casted on Si/SiO2 substrate. The two probe connection on this Si/SiO2 film was done by thermal evaporation of Cu/Ag as conducting material. The remanent ferroelectric polarization was measured by using a ferroelectric loop tracer (Precision LC-II of Radiant Inc.) under different magnetic fields (across 0–5 kOe) at room temperature.
3. Results and discussion
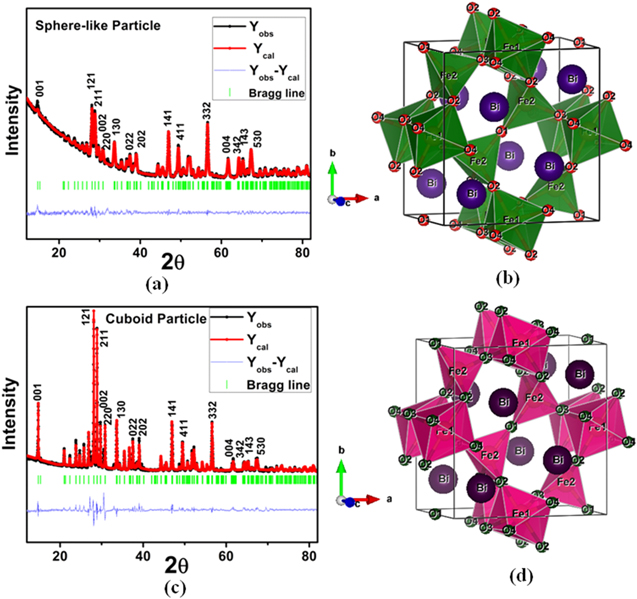
Figures 1(a) and (c) show the refined XRD pattern of Bi2Fe4O9 sphere-like nanoparticles and cuboid, respectively. These diffraction peaks are well refined with Fullprof software. All the experimental peaks are well-matched with theoretical. This refinement result shows that Bi2Fe4O9 cube and sphere-like nanoparticles both are in orthorhombic Pbam space group, and there is no trace of any impure phase. The refined lattice parameter of sphere-like and cuboid Bi2Fe4O9 are a = 7.966 A°, b = 8.450 A°, c = 6.008 A° and a = 7.955 A°, b = 8.463 A°, c = 6.008 A° respectively. It shows that the lattice parameter c in both the unit cell of cuboid and sphere-like nano particle is same, whereas a small difference in the lattice parameter a, b is seen here. But in this case, the unit cell volume of cuboid and sphere-like nanoparticles is almost the same 404.51 A°3 and 404.96 A°3, respectively. We have drawn the crystal structure of these two types of Bi2Fe4O9 nanoparticles using VESTA software. Figures 1(b) and (d) represents the crystal structure of sphere-like Bi2Fe4O9 and cuboid Bi2Fe4O9, respectively. The super exchange interaction between the Fe ions is important for understanding the magnetic characteristics of Bi2Fe4O9.
Figure 1. Room temperature x-ray diffraction data of Bi2Fe4O9 (a) nano quasi-sphere-like nanoparticles (b) nanocuboid and crystallographic structure for Bi2Fe4O9 (b) nano quasi-sphere-like nanoparticles (d) nanocuboid.
Download figure:
Standard image High-resolution imageTo explain the change in magnetic behavior caused by changing the surface to volume ratio of the material, it should be checked the bond angle between the Fe ions. Here we observed that the angle between Fe2–O1–Fe2 and Fe2–O2–Fe1 is unaltered for both the particle. But the bond angle of Fe2–O3–Fe1 of the cuboid is 6° greater than the sphere-like nanoparticles. The value of Fe2–O3–Fe1 is 136° and 130° for cuboid and sphere-like nanoparticles, respectively. A change in the bond angle of Fe1-O3-Fe1 is also noticed here. This feature is expected to obtain a change in the magnetic and ferroelectric behaviour due to the difference in the shape of the Bi2Fe4O9 nanoparticle.
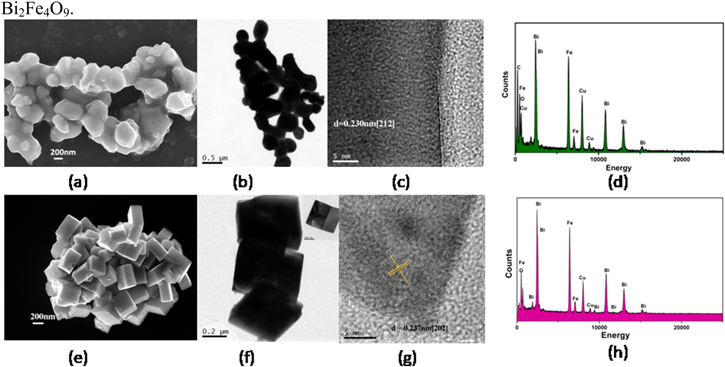
The field emission scanning electron microscopy images are shown in figures 2(a) and (e). Two different but uniform shapes (sphere-like and cuboid) are observed here with the size 200–400 nm. The TEM image of as-prepared Bi2Fe4O9 is represented in figure 2. The bright-field images are consistent with FESEM images of Bi2Fe4O9 sphere-like nanoparticles and cuboid. These sphere-like and cuboid nanoparticles are shown in figures 2(b) and (f), respectively. The HRTEM images are taken here to establish the crystallinity of the particle. Figure 2(c) is the HRTEM image of sphere-like Bi2Fe4O9 nanoparticle where the lattice inter-planar spacing is calculated to be 0.230 nm, corresponding to [212] plane. Similarly figure 2(g) is the HRTEM image of cuboid Bi2Fe4O9 nanoparticle. The lattice inter-planar spacing of Bi2Fe4O9 cuboid is 0.237 nm corresponding to [202] plane. The high resolution transmission electron microscopy images confirm that these sphere-like and cuboid nanoparticles are well-crystalline in nature. The energy dispersive spectroscopy data shows that the elemental compositions of the particles are only Bi, Fe and O [figures 2(d) and (h)]. No impurity is present here.
Figure 2. (a) FESEM image (b) TEM image (c) HRTEM image, and (d) EDX image of Bi2Fe4O9 sphere-like nanoparticles. (e) FESEM image (f) TEM image (g) HRTEM image, and (h) EDX image of Bi2Fe4O9 cuboid nanoparticles.
Download figure:
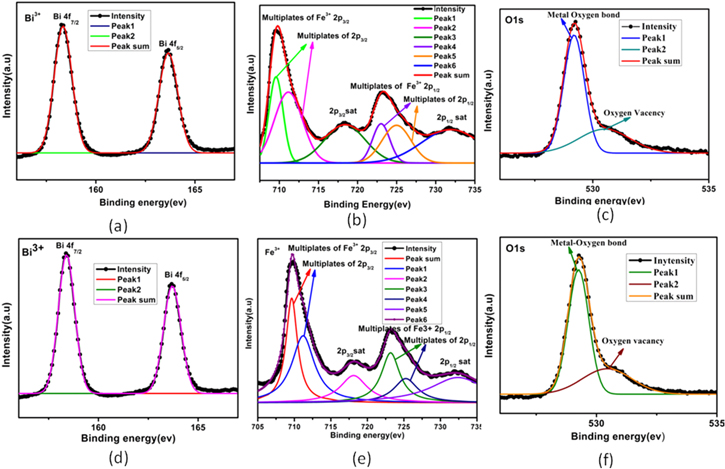
Standard image High-resolution imageTo understand the chemical composition, charge state, and surface defect of sphere-like and cuboid Bi2Fe4O9 nanoparticles, x-ray photoelectron spectroscopy has been carried out. The XPS spectra of Bi2Fe4O9 sphere-like particles are shown in figures 3(a)–(c). Figure 3(a) shows two distinct peaks of Bi. The binding energy 158.4 eV corresponds to Bi 4f7/2 state, and the binding energy 163.6 eV corresponds to Bi 4f5/2 state. These peak positions are pointed to the presence of Bi3+ ions, as expected for Bi2Fe4O9 [15]. The spectra of Fe consists of two peaks at 709.9 eV and 723.6 eV corresponding to Fe 2p3/2 and Fe 2p1/2 state, respectively, in figure 3(b) [16]. Fe 2p3/2 is deconvoluted into two peaks situated at 709.6 eV and 711.2 eV corresponding to Fe3+ and Fe2+ oxidation state respectively. The presence of Fe2+ is pointed to the existence of oxygen vacancy,
Figure 3. The XPS data showing the peaks for Bi3+ in (a) nano sphere-like nanoparticless and (d) nanocuboids; the peaks for Fe3+ in (b) nano sphere-like nanoparticless and (e) nanocuboids; and the peaks for O 1s in (c) nano sphere-like nanoparticless and (f) nanocuboids.
Download figure:
Standard image High-resolution imagewhich will be confirmed by the oxygen 1s spectrum [17, 18]. Similarly, Fe 2p1/2 is also deconvoluted into two peaks. Two satellite peaks are present here at the binding energy 718.1 and 732.1 eV. The oxygen 1s spectrum is deconvoluted into two peaks at 529.2 and 531 eV in figure 3(c). The peak at 529.2 eV is coming from metal-oxygen bond, and the peak at 531 eV is coming due to the presence of oxygen vacancy, [19] which also verifies the presence of Fe2+. This Fe2+ ion and oxygen vacancy is responsible for ferromagnetic ordering in Bi2Fe4O9. On the other hand, for the nanocuboids, we have observed the binding energies are almost the same as the binding energies of nano sphere-like nanoparticless (figures 3(d)–(f)). Bismuth is in Bi3+ state. Iron has a mixture of Fe2+ and Fe3+ states. Oxygen is in 1s states.
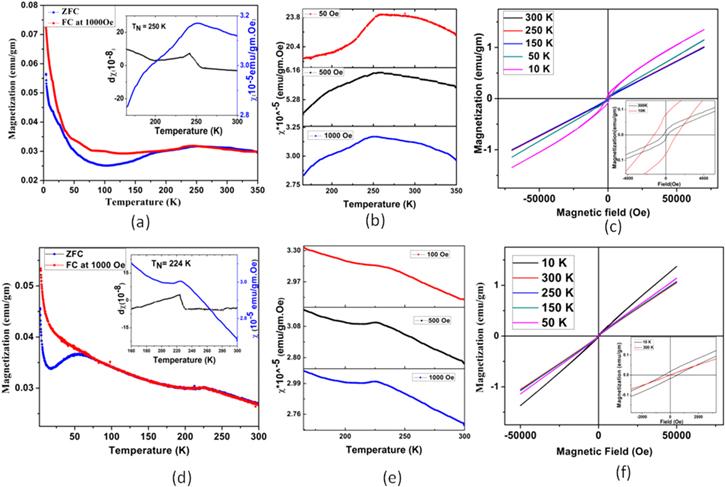
Figure 4(a) shows the temperature-dependent magnetization of Bi2Fe4O9 nano sphere-like nanoparticles under zero field cooling (ZFC) and field cooling (FC) at 1000 Oe magnetic field. It is shown that the FC and ZFC curves increase with decreasing the temperature. A broad cusp is observed at 250 K in the material, which reveals its antiferromagnetic behaviour. The ZFC and FC curve also started to split from this temperature. FC curve increases like before, but ZFC curve decreases up to 100 K. Beyond this temperature, ZFC curve increase with temperature decrease. To evaluate the exact antiferromagnetic transition temperature or Neel temperature (TN ), we have plotted d χ/dT with the temperature at the inset of figure 4(a), which confirms that the antiferromagnetic transition temperature of Bi2Fe4O9 nano sphere-like nanoparticles is 250 K.
Figure 4. (a) Temperature dependent ZFC and FC magnetization and the derivative of magnetization (inset) showing the transition at 250 K. (b) Magnetic susceptibility of ZFC curves for Bi2Fe4O9 under different magnetic field (50 Oe, 500 Oe, 1000 Oe). (c) Field dependent magnetization at different fixed temperature (M-H loop). Inset is the enlarged central part of M-H loop for Bi2Fe4O9 nano sphere-like nanoparticles. (d) Temperature dependent ZFC and FC magnetization for Bi2Fe4O9 nanocuboid and the derivative of magnetization (inset) showing the transition at 224 K. (e) Magnetic susceptibility of ZFC curves at different magnetic field (100 Oe, 500 Oe, 1000 Oe) for nanocuboid. (f) Complete hysteresis loop at different fixed temperature and inset shows the blown up part of near the origin for nano cuboid.
Download figure:
Standard image High-resolution imageWe have measured the zero field cooling magnetization curve under the different value of magnetic field (50 Oe, 500 Oe, 1000 Oe). The broad cusp has been seen at the same temperature (250 K) for all three curves. It is shown in figure 4(b). M-H curve at different temperatures from 10 to 300 K range is shown in figure 4(c). A small ferromagnetic ordering is seen in Bi2Fe4O9 nano sphere-like nanoparticles at room temperature and this ferromagnetic ordering increase with temperature decreases. The inset of figure 4(c) shows remanent magnetization (Mr ) and the coercive field (Hc ) at 300 K are 0.012 emu gm−1 and 236 Oe, respectively, whereas remanent magnetization (Mr ) value and the coercive field (Hc ) at 10 K are 0.078 emu gm−1 and 1194 Oe, respectively of Bi2Fe4O9 sphere-like nanoparticle. We observe an exchange bias of 100 Oe at 10 K. It comes due to the antiferromagnetic ordering at bulk and ferromagnetic ordering at the surface. This ferromagnetic ordering comes from the double exchange interaction between Fe3+ and Fe2+ ion through oxygen ion. The presence of Fe2+ ions is confirmed through XPS analysis. With decreasing the temperature, this ferromagnetic interaction increases. Bi2Fe4O9 is also known as a magnetically frustrated system having a unique pentagon spin frustration. The degree of frustration is defined as f = θ(CW )/TN , where this θ(CW ) is the Curie–Wiess temperature. The frustration index for the Bi2Fe4O9 nano sphere-like nanoparticles is 6. So, a frustrated antiferromagnetic spin is present in the material.
To make a comparison of magnetic properties between Bi2Fe4O9 nano sphere-like nanoparticles and nanocuboid, we have studied the same properties with the same condition. Figure 4(d) shows the temperature-dependent magnetization at zero field cooling condition and field cooling condition at 1000 Oe magnetic field. The zero field cooling curve and field cooling curve both show that the magnetization increases with the temperature decreases. However, a sharp cusp is present at a temperature of 224 K. This is the antiferromagnetic ordering temperature or Neel (TN) temperature of Bi2Fe4O9 nanocuboid. The ZFC and FC curves start to split at 80 K, and a broad peak is also observed at this 55 K; beyond this temperature, the FC curve is increasing with the previous nature, but the ZFC curve is decreasing with temperature. The temperature decreases further than 17 K, ZFC curve again follow an increasing behaviour.
To ensure the position of the antiferromagnetic transition temperature, we have plotted d χ/dT with the temperature at the inset of figure 4(d), which confirms that the antiferromagnetic transition temperature of Bi2Fe4O9 nanocuboid is 224 K. Figure 4(e) shows the zero field cooling magnetization curve under different magnetic field (100 Oe, 500 Oe, 1000 Oe) where a small cusp is present at the same temperature (224 K) for all three curves. Figure 4(f) shows the M-H loop in the temperature range 10–300 K. A very small ferromagnetic ordering is present in the Bi2Fe4O9 nanocuboid, which gradually increases with temperature decreases. The inset of figure 4(f) shows remanent magnetization value and the coercive field at 300 K is 0.0004 emu gm−1 and 11 Oe whereas remanent magnetization value and the coercive field at 10 K 0.0173 emu gm−1 and 512 Oe. A tiny amount of exchange bias (29 Oe) is shown in Bi2Fe4O9 cuboid nanoparticle at 10 K. The frustration index for Bi2Fe4O9 nano cuboid nanoparticles is 2.8. So, the frustration index in Bi2Fe4O9 nanocuboid is lower than the frustration index of Bi2Fe4O9 nano sphere-like nanoparticles.
In comparison, the Neel temperature of Bi2Fe4O9 nano sphere-like nanoparticles is 250 K and Bi2Fe4O9 nano cuboid is 224 K. The calculated frustration index of nano sphere-like nanoparticles is higher than the frustration index of nanocuboid. The DC hysteresis loop measurement shows that Hc and Mr values of nano sphere-like nanoparticles are greater than the Hc and Mr values of nanocuboid. So, ferromagnetic ordering or magnetic behaviour of nano sphere-like nanoparticles is stronger than nanocuboid but the antiferromagnetic behaviour is stronger in the nanocuboid. So, in the case of shape-dependent magnetic behaviour, these magnetic measurements confirm that the magnetic properties of Bi2Fe4O9 nanoparticles are completely shape-dependent. The change in Neel temperature may be appeared due to the modification in the influence of the spin canting in the material with changing the shape [20]. For the hysteresis behaviour, sphere-like particles exhibit slow demagnetization fields at higher magnetic field whereas cuboid particles display high demagnetization at lower magnetic field with high and low coercivity, respectively [20]. This change of coercivity in the magnetic hysteresis loop with different shape has also been theoretically shown in our previous work [21].
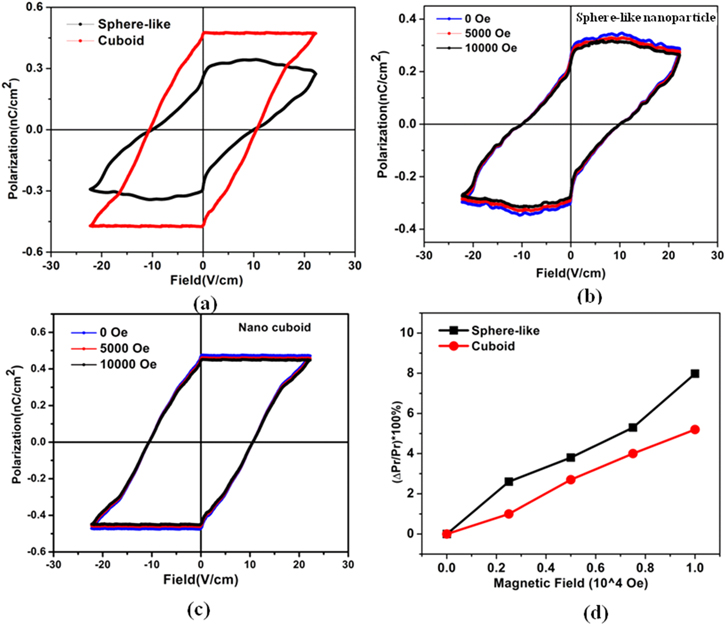
We measured the ferroelectric and magnetoelectric properties of both the shape of material. The remanent ferroelectric hysteresis loops were measured by employing a special protocol within the modified Sawyer-Tower circuit [22]. We have shown the electric field versus room temperature remanent polarization loop for Bi2Fe4O9 nano sphere-like nanoparticles and nano cuboid in figure 5(a). Here the electric coercive field (Pe ) of both the nanoparticles is almost same, but the remanent polarization (Pr ) value of Bi2Fe4O9 nanocuboid is greater than the nano sphere-like nanoparticles. So, the ferroelectric properties are stronger in nanocuboid.
Figure 5. (a) The room temperature remanent ferroelectric hysteresis loops for the nano sphere-like nanoparticless and nanocuboids. The remanent ferroelectric hysteresis loops under different magnetic fields cross 0, 5000 Oe and 10000 Oe (b) nano sphere-like nanoparticless and (c) nanocuboids measured at room temperature; (d) variation of magnetoelectric coupling with magnetic field in both the cases.
Download figure:
Standard image High-resolution imageNext, we measure the electric polarization of Bi2Fe4O9 nanoparticles at room temperature with and without magnetic field. Figure 5(a) represents the room temperature polarization versus electric field (P-E) loop of sphere-like and cuboid Bi2Fe4O9. Figures 5(b) and (c) shows the ferroelectric polarization under 0, 5000 Oe and 10000 Oe magnetic field of Bi2Fe4O9 nano sphere-like nanoparticles and nanocuboid. It appears that the magnetic field suppresses the remanent polarization in both cases. In the present case, it turns out that switching of magnetic domains under magnetic field reorients the ferroelectric domains in such a way so that across the assembly of particles, the overall remanent polarization decreases. Here the dependence of remanent polarization under magnetic field is very trivial. Figure 5(d) shows the magnetoelectric multiferroic coupling, which appears to be greater in the nano sphere-like nanoparticles. This change in behaviour comes from the shape anisotropy of the particle.
4. Conclusion
In conclusion, the Bi2Fe4O9 nano sphere-like nanoparticles and nanocuboid are synthesized by sol-gel auto combustion and hydrothermal method. We observe the change in magnetic, ferroelectric properties. The antiferromagnetic transition temperature for nano sphere-like nanoparticles and nanocuboid are 250 K and 224 K, respectively. The magnetic coercive field and remanent magnetization value of nano sphere-like nanoparticles is greater than nanocuboid, whereas the remanent polarization value of nanocuboid is greater than the nano sphere-like nanoparticles. These Bi2Fe4O9 nanoparticles show room temperature multiferroic coupling, this is strong in sphere-like nanoparticles. The remarkable shape dependence of the properties appears to originate from that of the anisotropy. These results could influence the design of new device architectures for future nanospintronic applications.
Acknowledgments
AS like thank the Department of Science Technology (DST), Govt. of India for the providing INSPIRE fellowship during the execution of the work.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.