Abstract
L-3,4-dihydroxy-phenylalanine (L-dopa) is the most widely used drug in Parkinson's disease treatment. However, development of cost-effective and high-throughput sensors to accurate enantioselective discrimination of L-dopa and D-dopa remains challenging to date. Herein, on the basis of the peroxidase-mimic activity of chiral FexCuySe nanoparticles, we demonstrated a novel colorimetric sensor for determination of chiral dopa. The surface chiral ligand, L/D-histidine (L/D-His), endowed the nanozymes with enantioselectivity in catalyzing the oxidation of dopa enantiomers. According to the values of kcat/Km, the efficiency of L-His modified nanoparticles (L-FexCuySe NPs) towards L-dopa was 1.56 times higher than that of D-dopa. While, D-His can facilely reverse the preference of the nanozyme to D-dopa. On the basis of high catalytic activity and enantioselectivity of L-FexCuySe NPs in oxidation of L-dopa, the L-FexCuySe NPs-based system can be utilized for detection of L-dopa. The linear ranges for L-dopa determination were 5 μM–0.125 mM and 0.125 mM–1 mM with a detection limit of 1.02 μM. Critically, the developed sensor has been successfully applied in the quality control of clinical used L-dopa tablets. Our work sheds light on developing simple and sensitive chiral nanomaterials-based sensors for drug analysis.
Export citation and abstract BibTeX RIS
1. Introduction
Neurodegenerative disorders including Alzheimer's disease and Parkinson's disease are recognized as a major causes of mortality worldwide [1, 2]. It has been reported that more than fifty percent of drugs currently in clinical use for neurodegenerative disorders are chiral drugs [3, 4]. The enantiomers of chiral drugs always display different biological activity, toxicity and transport mechanism. Single enantiomer drugs are often pharmaceutically active while the other may be inactive or even exert severe side effects [5, 6]. In particular, L-3,4-dihydroxy-phenylalanine (L-dopa), the dopamine (DA) precursor molecule, is the most effective and widely used drug in Parkinson's disease treatment. However, its enantiomer D-3,4-dihydroxy-phenylalanine (D-dopa) has no such pharmacological activity and even exhibits toxicity to humans [7]. Therefore, accurate enantioselective recognition and discrimination of L-dopa and D-dopa are greatly significant for drug safety and pharmaceutical quality control.
To this end, various approaches to determinate enantiomers have been developed, such as high-performance liquid chromatography [8], nuclear magnetic resonance [9], circular dichroism (CD) [10] and electrochemical detection [11, 12]. Despite these progresses and advances, the complicated procedure and requirement of sophisticated instruments restricted their applicability for high-throughput assay. Developing simple and cost-effective approaches for high-throughput determination of chiral drugs are desirable. Currently, due to the advantage of simplicity, low-cost, practicality and direct readout by bare eyes, colorimetric assay has caused great interest in the field of analysis and point-of-care diagnosis.
Taking the advantages of nanozymes including low-cost, excellent stability and adjustable catalytic activity [13, 14], utilizing enzyme-mimic activities of nanomaterials (nanozymes) to establish novel colorimetric biosensors has been developed since the first peroxidase-like activity of Fe3O4 nanoparticles was reported [15]. On the basis of this concept, numerous nanozymes fabricated from carbon and metal ion have been rationally designed and shown great potential applications in drug analysis [16–18]. Among these nanomaterials, with high copper deficiencies, the nonstoichiometric Cu2–x Se nanoparticles (Cu2–x Se NPs) can induce the generation of hydroxyl radicals in catalytic processes, endowing them with the intrinsic peroxidase-like activity [19]. Although promising, the shortage of active sites leads to the relative low catalytic activity, which obviously hinders their further bioanalytical application. Doping with heteroatom has been regarded as an effective strategy to enhance the catalytic activity of nanozymes due to the synergistic effect of multi-metal mixing [20, 21]. Fortunately, the vacancies in the nonstoichiometric nanocrystals provide spaces to incorporate desired metal ions via chemical doping [22], which offers a broad spectrum for tuning their catalytic activity. Therefore, it is highly desirable to develop novel multicomponent nonstoichiometric nanocrystals with excellent enzyme mimetic activity to expand the application of the nanozyme in drug analysis.
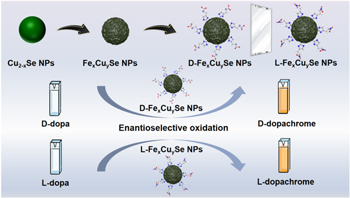
In this work, a simple and rapid colorimetric sensor was proposed for high-throughput determination of chiral dopa on the basis of the peroxidase-like activity of chiral Fex
Cuy
Se nanoparticles (L/D-Fex
Cuy
Se NPs) (scheme
Scheme 1. Schematic illustration of colorimetric sensor for determination of dopa enantiomers using peroxidase-like activity of chiral Fex Cuy Se NPs.
Download figure:
Standard image High-resolution image2. Experimental
2.1. Chemicals and materials
Poly(N-vinylpyrrolidone) (PVP), L/D-Histidine (L/D-His), L-3,4-dihydroxy-phenylalanine (L-dopa) and D-3,4-dihydroxyphenylalanine (D-dopa), L/D-Phenylalanine (L/D-Phe), L/D-Cysteine (L/D-Cys) were obtained from Aladdin (Shanghai, China). L-ascorbic acid (AA) was purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd. CuSO4·5H2O was ordered from Tianjin Yongda Chemical Reagent Co., Ltd. FeCl3·6H2O was obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Levodopa tablets were purchased from Beijing Shuguang Pharmaceutical Co., Ltd. H2O2 was bought from Laiyang Kangde Chemical Co., Ltd. 3,3',5,5'-Tetramethylbenzidine (TMB) was brought from damas-beta. All reagents and solvents were used as received from commercial suppliers without further purification. Deionized water (D.I. water, 18.2 MΩ cm) was utilized to prepare all the solutions.
2.2. Instruments
Transmission electron microscopy (TEM) images were acquired using a JEM-2100 (JEOL) operating at 120 kV. Fourier translation infrared (FTIR) spectra were collected with a Shimadzu FT-IR-8400S. The UV–vis absorption spectra were monitored by a Shimadzu UV-2700 spectrophotometer (Japan). The x-ray diffraction (XRD) patterns were recorded using a SmartLab Focus diffractometer (Rigaku) with Cu Kα source (λ = 1.541862 Å). CD measurement was carried out on a J-1500 CD spectrophotometer (JASCO) with the scanning speed of 10 nm min−1 and data pitch of 0.2 nm. A FEI Super-X microscope was used to obtain TEM elemental-mapping images. X-ray photoelectron spectroscopy (XPS) analysis was carried on ESCALAB 250xi photoelectron spectrometer equipped with an Al Kα monochromated x-ray source (Thermo Scientific, USA). N2 adsorption–desorption isotherms were recorded on a Micromeritics ASAP 2460 gas sorption analyzer. The element content was analysed by Agilent 7800 inductively coupled plasma massspectrometry (ICP-MS). The molar concentration of the nanomaterials was measured by nanoparticle-tracking analysis system using Nanosight NS300.
2.3. Synthesis of Fex Cuy Se NPs
Fex Cuy Se NPs were prepared according to the previously reported procedure with some modification [26]. In brief, 10 ml of 80 mg ml−1 PVP solution was firstly added into 60 ml water, which was followed by addition of Na2SeO3 solution (1 ml, 0.2 M) and AA solution (3 ml, 0.4 M) in sequence. After stirring for 15 min, CuSO4·5H2O (1 ml, 0.4 M) and AA solution (4 ml, 0.4 M) were added into the above red Se solution. The mixed system was allowed to react at room temperature under vigorous stirring until it turned dark green. Finally, FeCl3·6H2O (2 ml, 0.1 M) and L/D-His solution (3.5 ml, 0.229 M) were added into 50 ml Cu2–x Se NPs. The L/D-Fex Cuy Se NPs was obtained after reacting for 1 h with stirring.
The method of preparing L/D-Cys-Fex Cuy Se NPs and L/D-Phe-Fex Cuy Se NPs was same with the synthesis corresponding L/D-Fex Cuy Se NPs while the L/D-His was replaced by L/D-Cys and L/D-Phe, respectively.
2.4. Peroxidase-like activity evaluation
To characterize the peroxidase activity of Fex Cuy Se NPs, H2O2 (10 mM) and TMB (0.42 mM) were added to 400 μl phosphate buffer saline (PBS) (different pH values) containing the Fex Cuy Se NPs (25 μg ml−1) and mixed immediately. After incubating for 15 min, the UV–vis absorption spectroscopy was used to measure the absorbance at 652 nm. All reactions were performed at room temperature, except for special instructions.
2.5. Steady-state kinetic assays of Cu2–x Se NPs and Fex Cuy Se NPs
The kinetic measurements were carried out in time course mode by monitoring the absorbance change at 652 nm. Briefly, Cu2–x Se NPs or Fex Cuy Se NPs (15 μg ml−1) was added to PBS (pH 4.0). The kinetic analysis was performed with H2O2 (10 mM) or TMB (0.42 mM) as the substrate and the total reaction volume was 400 μl. The Michaelis–Menten constant was calculated from the Lineweaver–Burk plot:
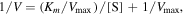
where Km is the Michaelis constant, V is the rate of conversion, Vmax is the maximum rate of conversion, and [S] is the substrate concentration.
2.6. Equilibrium dialysis
1 ml of L- or D-Fex Cuy Se NPs (2 mg ml−1) was dialyzed against a 10 ml solution containing a 1:1 mixture of L-dopa and D-dopa (5 mM). After 24 h dialysis, CD spectra was used to characterize the dialysate.
2.7. Bioassay
The kinetic measurements were carried out in time course mode by monitoring the absorbance change at 475 nm. Experiments were carried out using L/D-Fex Cuy Se NPs (15 μg ml−1) in a reaction volume of 400 μl PBS (pH 4.0) with 10 mM H2O2 and L/D-dopa as the substrate.
The Km and Vmax values were calculated from the Lineweaver–Burk plot. While, kcat was calculated according to the equation:
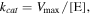
where [E] refers to the concentration of catalyst. The comparison of catalytic activity for different nanozymes was estimated according to their kcat/Km values [27].
2.8. Simulated calculation on optimization molecular interactions
All simulations were performed using the ORCA software [28]. Structure optimizations were carried out with density functional theory utilizing the well-known B3LYP hybrid functional in combination with the def2-SVP basis set. All structures were optimized with default parameters. The interaction energy was calculated according to the follow equation:
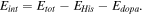
2.9. Optimization of experimental parameters
In order to optimize the experimental conditions for L-dopa oxidation, the pH value and incubation time were optimized, respectively. 4 μl L-Fex Cuy Se NPs (1.5 mg ml−1), 4 μl H2O2 (1 M) and 40 μl freshly prepared L-dopa solution (10 mM) were added into 352 μl PBS (different pH values). The mixture solution was incubated at room temperature for 30 min, followed by the spectral measurements. The absorption intensities at 475 nm were recorded. Furthermore, the same method was used to explore the influence of incubation time on the detection system.
2.10. Selection of chiral ligands
To investigate the effect of chiral ligands on the enantioselectivity of chiral Fex Cuy Se NPs towards dopa enantiomers, Cys and Phe were used as chiral ligands to prepare L/D-Cys-Fex Cuy Se NPs and L/D-Phe-Fex Cuy Se NPs. For the detection of dopa enantiomers, 4 μl H2O2 (1 M) and 40 μl freshly prepared L- or D-dopa solution (10 mM) were added into 356 μl PBS (pH = 4.0) containing different nanoparticles and mixed immediately. The mixture solution was then incubated for 30 min before the spectral measurements.
2.11. Detection of L-dopa
To detect L-dopa, L-Fex Cuy Se NPs (15 μg ml−1), H2O2 (10 mM) and freshly prepared L-dopa solution with different concentrations (0.005, 0.01, 0.025, 0.05, 0.075, 0.1, 0.125, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5 and 2.0 mM) were added to 400 μl PBS solution (pH 4.0). After incubating the suspension for 30 min, UV–vis absorption spectrum was recorded at 475 nm.
2.12. Detection of L-dopa in commercially available levodopa tablets
To determinate L-dopa level in commercially available levodopa tablets, the standard addition method was used. Ten levodopa tablets were accurately weighed and ground into fine powder. The levodopa fine powder (40.39 mg) was then transferred into a 100 ml volumetric flask. PBS (pH 4.0, 100 ml) was added to dissolve the L-dopa. The solution was filtered with microporous membrane and the subsequent filtrate was detected. According to the L-dopa content (250 mg per tablet) provided by the supplier, the concentration of L-dopa in the filtrate was converted into 1.6865 mM after the above treatment.
Typically, L-Fex Cuy Se NPs (15 μg ml−1), H2O2 (10 mM) and 9.5 μl sample solution were mixed in PBS (pH 4.0) with the total volume of 400 μl. After incubating at room temperature for 30 min, the UV–vis absorption intensity at 475 nm was recorded.
Next, different amounts of L-dopa or D-dopa standard solution (0.04, 0.08, 0.36, 0.76 mM) was added to the sample solution respectively. The suspension was incubated for 30 min under the room temperature and then measured by UV–vis spectroscopy at 475 nm.
3. Results and discussion
3.1. Synthesis and characterization of Fex Cuy Se NPs and L/D-Fex Cuy Se NPs
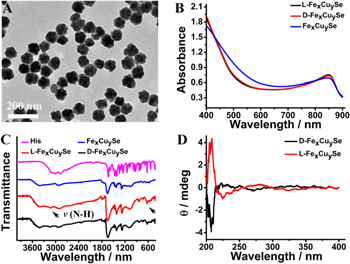
Fex Cuy Se NPs were prepared according to the previously reported procedure with some modification [26]. L-His or D-His as the chiral ligand was conjugated to the surface of Fex Cuy Se NPs through coordination interactions. As shown in TEM image, Fex Cuy Se NPs with porous spherical morphology were monodispersed and had a uniform size of about 80 nm (figure 1(A)), which was quite similar with that of Cu2–x Se NPs (figure S1 (available online at stacks.iop.org/NANO/33/135503/mmedia)). Moreover, Fe doping in Fex Cuy Se NPs was validated by TEM elemental-mapping images and XPS. As shown in figure S2, the TEM elemental-mapping images indicated that Fe, Cu and Se were homogeneously distributed in Fex Cuy Se NPs. Fe 2p, Cu 2p and Se 3d were observed in the XPS spectra of Fex Cuy Se NPs, which further confirmed the successful doping of Fe into Fex Cuy Se NPs (figure S3). In addition, the contents of Fe and Cu in Fex Cuy Se NPs were determined by inductively coupled plasma mass spectrometry (ICP-MS), which corresponded to about 20.9 μg mg−1 Fex Cuy Se NPs and 95.1 μg mg−1 Fex Cuy Se NPs, respectively. The porous structure of Fex Cuy Se NPs and Cu2–x Se NPs was characterized by N2 adsorption–desorption isotherms. As shown in figure S4, both Fex Cuy Se NPs and Cu2–x Se NPs exhibited a typical Type IV isotherm with a hysteresis loop, demonstrating their porous structure.
Figure 1. The characterization of Fex Cuy Se NPs. (A) TEM image of Fex Cuy Se NPs. (B) The UV–vis–NIR spectra of L/D-Fex Cuy Se NPs. (C) The FTIR spectra of His, Fex Cuy Se NPs and L/D-Fex Cuy Se NPs. (D) CD spectra of L/D-Fex Cuy Se NPs.
Download figure:
Standard image High-resolution imageThe characteristic absorbance peak of Fex Cuy Se NPs at approximately 850 nm was also observed in the UV–vis–NIR spectrum (figure 1(B)). The well-defined copper iron selenide (JCPDS no. 81-1959) in Fex Cuy Se NPs was evidenced by x-ray powder diffraction (XRD) patterns, which was quite different from the initial berzelianite (JCPDS no. 06-0680) in Cu2–x Se NPs (figure S5). The successful modification of L-His or D-His on the surface of Fex Cuy Se NPs was validated by the appearance of the broad absorbance band at 3500–3000 cm−1 in FTIR spectra, which belonged to the N–H stretching in the amino groups of His (figure 1(C)). Meanwhile, the typical bands of imidazole stretching at 1800–1400 cm−1 were also observed (figure 1(C)). UV–vis–NIR spectra and XRD patterns indicated that introduction of L-His or D-His could not disturb the structure of Fex Cuy Se NPs. All above characteristics demonstrated that the L-His or D-His was successfully conjugated onto the surface of Fex Cuy Se NPs. CD was used to measure the chirality of L/D-Fex Cuy Se NPs. As shown in figure 1(D), the CD signals of L/D-Fex Cuy Se NPs showed equal in intensity but opposite in the ultraviolet region, further proofed of the grafting of His.
3.2. The peroxidase-like activity of Fex Cuy Se NPs
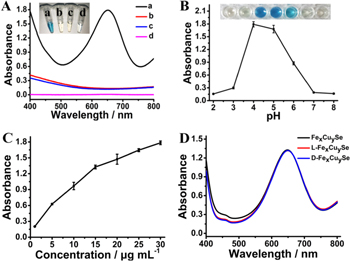
The peroxidase-mimicking activity of the nanozyme was evaluated via the oxidation of TMB by H2O2. As shown in figure 2(A), Fex Cuy Se NPs can catalyze the oxidation of TMB into oxidized TMB (oxTMB) with a color change from colorless to blue in the presence of H2O2. The produced oxTMB exhibited a typical absorption peak at 652 nm. In contrast, the control experiments demonstrated that only H2O2 or Fex Cuy Se NPs can hardly induce the color change, indicating that Fex Cuy Se NPs possessed an intrinsic peroxidase-mimicking activity similar to HRP [15]. Like other peroxidase mimics [29, 30], the catalytic activity of Fex Cuy Se NPs was deeply affected by pH value and the concentration of Fex Cuy Se NPs (figures 2(B) and (C)). Based on the above experimental results and considering the practical application for drug analysis, pH value of 4.0, room temperature and 15 μg ml−1 were chosen as the optimal pH, temperature and concentration of Fex Cuy Se NPs for the subsequent experiments. Critically, for the achiral substrate TMB, L/D-Fex Cuy Se NPs displayed the same catalytic effect as the unmodified Fex Cuy Se NPs under the same conditions (figure 2(D)). This result manifested that the modification of His ligand had little effect on peroxidase-like activity of Fex Cuy Se NPs.
Figure 2. The peroxidase-like properties of Fex Cuy Se NPs. (A) The absorbance spectra of TMB in different reaction systems: (a) Fex Cuy Se NPs + H2O2 + TMB, (b) Fex Cuy Se NPs + H2O2, (c) Fex Cuy Se NPs + TMB and (d) H2O2 + TMB in PBS (pH 4.0) at room temperature after 15 min incubation. Insert was the visual color changes of TMB in the corresponding solutions. (B) The effect of pH value on the peroxidase-like activity of Fex Cuy Se NPs. (C) The effect of the concentration of Fex Cuy Se NPs on their peroxidase-like activity. (D) The absorbance spectra of TMB in the present of different chiral and achiral nanoparticles.
Download figure:
Standard image High-resolution imageThe synergistic effect of multi-metal mixing could enhance the catalytic activity of nanozymes [31, 32]. To demonstrate the critical role of Fe doping in the enhanced catalytic activity of Fex Cuy Se NPs, TMB was employed as the substrate to monitor the catalytic activities of Cu2–x Se NPs and Fex Cuy Se NPs. As shown in figure S6, compared with Cu2–x Se NPs, the catalytic activity of Fex Cuy Se NPs enhanced significantly under the same conditions. The steady state kinetic study was also conducted to further investigate the peroxidase-like property of Fex Cuy Se NPs [27]. As indicated in figure 3, within the certain range of H2O2 and TMB concentrations, typical Michaelis–Menten curves were obtained for both Fex Cuy Se NPs and Cu2–x Se NPs. The apparent Michaelis–Menten constant (Km) and maximum initial velocity (Vmax) were calculated from Lineweaver–Burk plot (table 1). The Km values of Fex Cuy Se NPs for TMB and H2O2 were close to that of HRP [15], indicating that Fex Cuy Se NPs exhibited an excellent catalytic activity. It was worth noting that although the Vmax values of Fex Cuy Se NPs for both TMB and H2O2 were similar to that of Cu2–x Se NPs, the Km values of Fex Cuy Se NPs were much smaller than that of Cu2–x Se NPs, indicating that the porous structure and synergistic effects from the multi-metal mixing enhanced the binding affinity of Fex Cuy Se NPs with H2O2 [29, 33–35]. All these phenomena contributed to the excellent catalytic activity of Fex Cuy Se NPs, endowing them with the great potential to construct nanozyme-based colorimetric sensors.
Figure 3. Steady-state kinetic assay of Fex Cuy Se NPs and Cu2–x Se NPs. Lineweaver–Burk plot of the reciprocals of initial rate versus substrate concentration for the determination of kinetic parameters Km and Vmax of (A) and (B) Fex Cuy Se NPs and (C) and (D) Cu2–x Se NPs with H2O2 (left) or TMB (right) as the substrate.
Download figure:
Standard image High-resolution imageTable 1. Comparison of the apparent Michaelis–Menten constant (Km) and maximum reaction rate (Vmax) between Fex Cuy Se NPs and Cu2–x Se NPs.
| Substrate | Km (mM) | Vmax (10−8 MS−1) | |
|---|---|---|---|
| Fex Cuy Se NPs | H2O2 | 8.525 | 4.738 |
| TMB | 1.837 | 12.750 | |
| Cu2–x Se NPs | H2O2 | 16.305 | 5.095 |
| TMB | 2.455 | 14.401 |
3.3. Enantioselective recognition of L/D-dopa
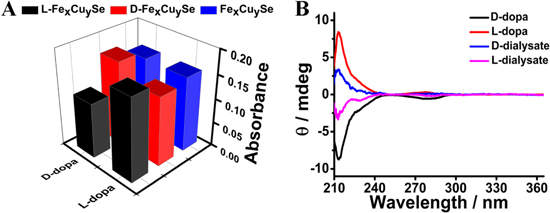
Based on the principles of chiral recognition, we explored the feasibility of detecting L/D-dopa by L/D-Fex Cuy Se NPs-based system. L/D-Fex Cuy Se NPs can catalyze the oxidation of dopa enantiomers with H2O2 to corresponding dopachrome (L/D-dc), which has a characteristic absorption at 475 nm (figure S7(A)). In addition, the experimental conditions including pH value and incubation time for dopa oxidation were optimized (figures S7(B) and (C)). In order to simultaneously obtain a higher catalytic activity and a shorter detection time in practical application, pH value of 4.0 and 30 min were chosen as the optimal pH and incubation time. As illustrated in figure 4(A), achiral Fex Cuy Se NPs exhibited the same catalytic ability towards L/D-dopa. However, after conjugated with L/D-His, the chiral nanozymes displayed enantioselectivity in catalyzing the oxidation of dopa enantiomers. In particular, L-Fex Cuy Se NPs had better catalytic activity for L-dopa than D-dopa, while, D-Fex Cuy Se NPs showed reversed selectivity. All these results demonstrated that the enantioselectivity of L/D-Fex Cuy Se NPs arose from the chiral His units. To further investigate the effect of chiral ligands on the enantioselectivity of chiral Fex Cuy Se NPs towards dopa enantiomers, we also employed Cys and Phe as chiral ligands to prepare chiral L/D-Cys-Fex Cuy Se NPs and L/D-Phe-Fex Cuy Se NPs (figures S8(A) and (B)). As indicated in figures S8(C) and (D), L/D-Cys-Fex Cuy Se NPs and L/D-Phe-Fex Cuy Se NPs showed negligible enantioselectivity for catalyzing the oxidation of dopa enantiomers. Therefore, L/D-His were chosen as the chiral ligands for our subsequent experiments.
Figure 4. (A) The absorbance of dopa enantiomers with Fex Cuy Se NPs (blue), L-Fex Cuy Se NPs (black), D-Fex Cuy Se NPs (red) at 475 nm. (B) CD spectra of L/D-dopa and the dialysate for enrichment in the dopa enantiomer.
Download figure:
Standard image High-resolution imageThe apparent kinetic parameters for dopa enantiomers based on the Michaelis–Menten equation were next measured to elucidate the different catalytic efficiency of the chiral nanozymes towards L-dopa and D-dopa (figure S9). As summarized in table 2, according to the kcat/Km values, the efficiency of L-His modified nanoparticle (L-Fex Cuy Se NPs) towards L-dopa was 1.56 times higher than that of D-dopa. The higher kcat/Km values of L-Fex Cuy Se NPs towards L-dopa arose from the relative lower Km but higher kcat values for L-dopa, indicating that L-Fex Cuy Se NPs had a stronger binding affinity with L-dopa and higher catalytic activity for L-dopa oxidation. In contrast, D-dopa was the preferred oxidation substrate for D-Fex Cuy Se NPs, which showed a selectivity factor of 1.49. The obtained selectivity factors were generally higher than or comparable with the records of other chiral nanozymes reported recently (table 3) [23, 36–38]. The relatively high catalytic activity and enantioselectivity of the L/D-Fex Cuy Se NPs endowed them with great potential for constructing colorimetric sensor for detection of dopa enantiomers.
Table 2. Kinetic parameters for catalytic oxidation of dopa enantiomers by L/D-Fex Cuy Se NPs.
| Nanozyme | Substrate | Km (mM) | Vmax (10-8 MS−1) | kcat (S−1) | kcat/Km (105 M−1 S−1) |
|---|---|---|---|---|---|
| L-Fex Cuy Se NPs | L-dopa | 0.146 | 0.247 | 25.831 | 1.769 |
| L-Fex Cuy Se NPs | D-dopa | 0.196 | 0.212 | 22.171 | 1.131 |
| D-Fex Cuy Se NPs | L-dopa | 0.143 | 0.166 | 17.360 | 1.214 |
| D-Fex Cuy Se NPs | D-dopa | 0.109 | 0.188 | 19.661 | 1.804 |
Concentrations of all nanozymes are 9.56 × 10−11 M.
Table 3. Comparison of the selectivity factors of L/D- Fex Cuy Se NPs with other chiral nanozymes toward dopa enantiomers.
| Nanozyme | Selectivity factor | References |
|---|---|---|
| CeNP@L-Phe | 1.13 | [36] |
| D-Cys@AuNPs-EMSN | 1.47 | [37] |
| L-His75@Fe-COF | 1.64 | [23] |
| D-His@Fe-COF | 1.51 | [23] |
| D-Cys@N-CuO/CoO NPs | 1.36 | [38] |
| L-Fex Cuy Se NPs | 1.56 | This work |
| D-Fex Cuy Se NPs | 1.49 | This work |
To support the enantioselective nature of the catalytic efficiency, dialysis experiments were performed to reveal the binding selectivity of these chiral nanozymes with L/D-dopa [6]. Racemic mixtures of dopa were obtained by mixing equimolar amounts of the pure enantiomers. The mixture was dialyzed against L-Fex Cuy Se NPs or D-Fex Cuy Se NPs, and CD was used to monitor the dialysate which indicated the enrichment of the enantiomer with lower binding affinity to L-Fex Cuy Se NPs and D-Fex Cuy Se NPs in the dialysis tube. As shown in figure 4(B), the dialysate was enriched in D-dopa and L-dopa, respectively. These results were consistent with the Km values, which indicated unambiguously that L-Fex Cuy Se NPs bound preferentially to L-dopa, while D-Fex Cuy Se NPs showed a higher binding ability to D-dopa. All the results demonstrated that conjugation of chiral His units endowed Fex Cuy Se NPs with stereoselectivity towards the oxidation of L/D-dopa and the stereoselectivity was associated with the chirality of His.
For further understanding the role of chiral ligand in the stereoselectivity of nanozymes, the molecular interactions between L/D-His and L/D-dopa were investigated using MD simulations [38]. As shown in figure 5, both L-dopa and D-dopa can bind with His enantiomers via hydrogen bonds. There were three hydrogen bonds formed in the interaction model between L-dopa and L-His. However, L-His formed only two hydrogen bonds with the D-dopa. In addition, the interaction energy obtained by ab initio calculations (Eint) was shown in table S1. The interaction of L-His with L-dopa was stronger than that of L-His with D-dopa. The results were consistent with the stereoselectivity of L-Fex Cuy Se NPs towards L-dopa, which further indicated the important role of the chiral ligand played in enantiomer recognition and chiral selective catalysis.
Figure 5. Energy-minimized average interaction models of (A) L-His with L-dopa, (B) D-His with L-dopa, (C) L-His with D-dopa and (D) D-His with D-dopa.
Download figure:
Standard image High-resolution image3.4. Detection of L-dopa in solution and in real tablets
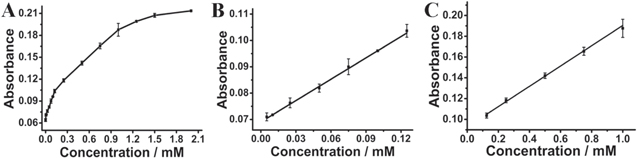
The high catalytic activity of L-Fex Cuy Se NPs for L-dopa oxidation inspired us to explore the possibility of using L-Fex Cuy Se NPs as peroxidase mimics to construct colorimetric sensor for detection of L-dopa. Figure 6(A) showed the response curves of the typical absorbance at 475 nm with various concentration of L-dopa. With the enhancement of the L-dopa concentration from 0 to 1 mM, the absorption intensity of L-dc at 475 nm gradually increased while the solution appeared to brown. There were two linear ranges for L-dopa. One was in the range of 5 μM–0.125 mM with a correlation coefficient of 0.998 (figure 6(B)), the other was from 0.125 mM to 1 mM with a correlation coefficient of 0.996 (figure 6(C)). The linear regression equations were A =0.270[L-dopa] (mM) + 0.069 and A = 0.098[L-dopa] (mM) + 0.093, respectively. When signal to noise was 3 [28], the detection limit (LOD) was calculated to be 1.02 μM. The low LOD and the wide detection range demonstrated the high sensitivity of L-Fex Cuy Se NPs-based system for detecting L-dopa.
Figure 6. The concentration-dependent effects of L-dopa on the peroxidase-like properties of L-Fex Cuy Se NPs. (A) The absorbance intensity at 475 nm versus the concentration of L-dopa. (B) and (C) The liner plot of the absorbance measured at 475 nm as a function of L-dopa concentration.
Download figure:
Standard image High-resolution imageTo assess the feasibility for practical application, the proposed method was then applied to determine the concentrations of L-dopa in tablets. And the detection performance in real pharmaceutical samples was also investigated by using the standard addition method. As displayed in table 4, the concentration of L-dopa was determined to be 0.03999 mM, which was consistent well with the practical value (0.0400 mM) given by manufacturer. In addition, upon addition of certain amounts of L-dopa, the analytical recoveries from medicinal samples ranged within 92.97% to 107.25% and the relative standard deviations (RSD, n = 4) ranged from 2.04% to 9.63%, indicating that the proposed nanozyme-based sensor can be used for qualitative detection of L-dopa in real medicine with good recovery and precision.
Table 4. Determination of L-dopa level in commercial levodopa tablets.
| Sample | Added L-dopa (mM) | Added D-dopa (mM) | Detected (mM) | Calculated (mM) | Recovery (%) | RSD (n = 4, %) |
|---|---|---|---|---|---|---|
| 1 | — | — | 0.03999 | 0.0400 | 99.96 | 9.63 |
| 2 | 0.0400 | — | 0.08188 | 0.0800 | 102.35 | 3.62 |
| 3 | 0.0800 | — | 0.1287 | 0.1200 | 107.25 | 3.76 |
| 4 | 0.3600 | — | 0.4017 | 0.4000 | 100.42 | 2.04 |
| 5 | 0.7600 | — | 0.7438 | 0.8000 | 92.97 | 2.70 |
| 6 | — | 0.0400 | 0.06092 | 0.0800 | 76.15 | 4.87 |
| 7 | — | 0.0800 | 0.09655 | 0.1200 | 80.46 | 8.68 |
| 8 | — | 0.3600 | 0.1524 | 0.4000 | 38.10 | 13.18 |
| 9 | — | 0.7600 | 0.2799 | 0.8000 | 34.99 | 7.17 |
Inspired by the enantioselectivity of the chiral nanozymes in oxidation of dopa enantiomers, we also examined whether the system can be used to detect L-dopa in the presence of D-dopa, which was of great significance for drug quality control since only L-dopa has pharmacological activity. As shown in table 4, when replacing L-dopa with the same amount of standard D-dopa solution, the recoveries of the racemic mixture were significantly lower than that of pure L-dopa samples, which was arose from the lower catalytic ability of L-Fex Cuy Se NPs towards D-dopa. The outcomes exhibited an encouraging conclusion that the proposed chiral nanozyme-based sensor was promising for quality control of the clinical used drug, L-dopa.
4. Conclusions
In summary, a sensitive chiral Fex Cuy Se nanozyme-based colorimetric sensor was successfully constructed for high-throughput determination of chiral dopa. The enantioselectivity of chiral Fex Cuy Se NPs was derived from the better binding affinity between L/D-dopa and L/D-His, the chiral surface ligand of the nanozyme. According to the values of kcat/Km, the efficiency of L-His modified nanoparticle (L-Fex Cuy Se NPs) towards L-dopa was 1.56 times higher than that of D-dopa. While, D-dopa was the preferred oxidation substrate for D-Fex Cuy Se NPs, which showed a selectivity factor of 1.49. Moreover, the incorporation of Fe into the nonstoichiometric Cu2–x Se NPs dramatically improved the catalytic activity of the nanozyme. Based on the high catalytic activity and enantioselectivity of chiral Fex Cuy Se NPs in the oxidation of dopa enantiomers, the proposed sensor has been successfully applied for quality control of the clinical used L-dopa. Our work would shed lights on designing simple and sensitive sensors for chiral drug analysis.
Acknowledgments
Financial support was provided by the National Natural Science Foundation of China (Grant No.21807024), the Youth Top-notch Talents Supporting Plan of Hebei Province (QNBJ19004), the Hundred Persons Plan of Hebei Province (E2018050012), the Natural Science Foundation of Hebei Province (No. H2020206059), Hebei Province High School Science and Technology Research Project (No. ZD2021072) and Science Fund for Creative Research Groups of Natural Science Foundation of Hebei Province (No. H2020206474).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Disclosure
The author reports no conflicts of interest in this work.