Abstract
Due to their intrinsically large surface-to-volume ratio, nanowires and nanofins interact strongly with their environment. We investigate the role of the main air constituents nitrogen, oxygen and water on the efficiency of radiative recombination in GaN nanostructures as a function of different surface treatments and at temperatures up to 200 °C. Oxygen and water exposures exhibit a complex behavior as they can both act quenching and enhancing on the photoluminescence intensity dependent on the temperature. For oxygen, these characteristics are already observed for low concentrations of below 0.5% in nitrogen. While the photoluminescence intensity changes induced by oxygen occur independently of illumination, the influence of water is light-induced: it evolves within tens of seconds under ultraviolet light exposure and is heavily influenced by the nanostructure pre-treatment. In contrast to observations in dry atmospheres, water prevents a recovery of the photoluminescence intensity in the dark. Combined measurements of the electrical current through GaN nanofins and their photoluminescence intensity reveal the environmental influence on the interaction of non-radiative recombination processes and changes in the surface band bending of the nanostructures. Several investigated solvents show an enhancing effect on the PL intensity increase, peaking in c-hexane with a 26-fold increase after 6 min of light exposure. Stabilization of the PL intensity was achieved by a passivation of the GaN surface with GaxOy, and ZnO shells. Surprisingly, Al2O3 coatings resulted in a highly instable PL intensity during the first minutes of illumination. Our findings reveal the high importance of controlled environmental conditions for the investigation of nanostructures, especially when aimed at their applications in the fields of environmental sensing, photo-catalysis and light-emitting diodes.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
The interaction of GaN surfaces with the environment is a topic of current interest as a deeper understanding of this phenomenon could trigger advancements in highly relevant fields like sensing, photo-catalysis or the improvement of nanowire (NW) light-emitting diodes (LEDs). In this regard, GaN NWs have attracted increasing interest in the past years as they can be produced with high crystal quality. The well-established nanostructuring enables large surface-to-volume ratios and the exposure of alternative crystal facets such as a- and m-planes [1–7]. Several groups have reported an influence of reducing and oxidizing gases and other chemicals on the electrical properties of GaN transistor devices. However, the fabrication of these devices is complex and requires a sophisticated technology [8–11]. As GaN NWs exhibit good optical properties like a strong luminescence upon electrical or optical excitation, probing changes of the luminescence under controlled environmental conditions is a promising pathway to understand the underlying mechanisms. Approaches to explain the amount of non-radiative recombination and its dependence on the ambient conditions in GaN NWs generally either focus on changes of the surface band bending (SBB) and the resulting depletion width [12–14] or the creation of surface states through adsorbates which open non-radiative recombination paths [15–17]. While in the existing literature a wide range of experimental conditions and approaches has been covered, the interpretation of the results differs between different groups and a consensus as to which mechanism dominates has not yet emerged. Recently, we have investigated the influence of GaN NW geometry, various gaseous atmospheres, the pH value and electrical fields in aqueous solutions on the intensity of the photoluminescence (PL) response and the NW stability [18, 19]. From this earlier work we have concluded that anions accumulating at the NW surface cause an enhancement of non-radiative recombination and, therefore, a reduction of the PL intensity. In this paper, we aim to extend the understanding of the luminescence behavior and the role of surface-related processes of GaN NWs under different atmospheres with a focus on the main constituents of ambient air, nitrogen (N2), oxygen (O2) and water (H2O) vapor. We combine this with the investigation of the effects of pre-treatments, which can have a significant influence on several surface properties of c- and m-planes of GaN, including the magnitude of the SBB [20, 21]. By complementary measuring the PL intensity and the electrical current through GaN nanofins (NFs) and contact potential difference (CPD) on bulk m-plane GaN, we develop a method to differentiate between the effects of SBB and passivation of surface states. Building on this, we test different oxide shells on their ability to stabilize the PL emission of GaN NWs and elucidate the effect of several common solvents on the PL intensity evolution.
Experimental details
In order to study the interaction of GaN nanostructures (NSs) with the environment under ultraviolet (UV) illumination, a clear distinction between emission lines originating from the substrate is necessary. Therefore, substrates should not exhibit PL emission close to the GaN emission, which is the case for growth on, e.g. AlN, Si or diamond. As demonstrated earlier, such samples can be grown by plasma-assisted molecular beam epitaxy (MBE) [22, 23]. The GaN NSs investigated in this work were grown via MBE in a selective area growth mode. To this end, 7–10 nm of Ti were thermally evaporated onto the substrates. The Ti mask was patterned with arrays of holes and lines via e-beam lithography. These holes and lines exposed the underlying substrate and served as nucleation sites for the GaN NW and NF growth during MBE. Prior to the NS growth, the Ti mask was exposed to a nitrogen plasma with a radio frequency (RF) power of 425 W at a temperature of 400 °C for 10 min, followed by an exposure at 800 °C for another 5 min. A schematic representation of this process can be found in the supporting information (SI) available online at stacks.iop.org/NANO/32/495703/mmedia. The GaN NWs were grown on n-type Si (111) substrates (iron-compensated Ga-polar GaN templates) under N-rich conditions at a substrate temperature of 875 °C (950 °C) with a Ga flux of 5.7 (4.6)· 10−7 mbar beam equivalent pressure (BEP) and a N2 flux of 0.363 sccm with an RF plasma power of 425 W for 90 min at a background pressure of about 10−10 mbar. Figure S5 (SI) displays a low temperature PL spectrum of GaN NWs on Si. The near band edge (NBE) emission is about an order of magnitude more intense as the highest defect related emission, which is a sign of good crystal quality. GaN NFs were grown on Al-polar AlN templates with a similar procedure but at a growth temperature of 960 °C. A detailed description of the NW fabrication process can be found in other publications [22, 24–26]. Note that the given substrate temperatures refer to the thermocouple values of the substrate heater. All NSs exhibit similar general appearance, dimensions and crystal quality as all have been grown in the same system and under comparable growth conditions. Figure 1(a) shows a 45°-tilted view scanning electron microscope (SEM) image of a GaN NW array on Si with NW heights of 500–600 nm and diameters of approx. 160–240 nm. The spacing of 500 nm between NW centers ensures a full exposure of the NW sidewalls, which are primarily formed by the crystallographic m-plane [19, 27], to the applied atmospheres. The NWs have a uniform shape and similar dimensions. All samples investigated in this work are not intentionally doped. Nevertheless, when grown on Si, approx. 50 nm of the NW bottom are known to be n-type doped due to interdiffusion of Si atoms from the substrate, whereas the NW top is less affected and exhibits an average doping concentration of approx. 5·1017 cm−3 [28, 29]. Figure 1(b) shows an SEM picture of GaN NFs in 45°-tilted view grown on an AlN template on sapphire.
Figure 1. (a) SEM image of GaN NWs on a Si substrate and (b) GaN nanofins on an AlN substrate in 45°-tilted view. (c) Sketch of the measurement chamber with gas flow and resistive heater.
Download figure:
Standard image High-resolution imageIn order to investigate the influence of common cleaning treatments of the GaN NW surface on their sensitivity to the environment, three batches of NW arrays were grown on Si. The NWs which are referred to as 'as-grown' were left untreated after the MBE growth. 'Aged' NWs were stored in air at room temperature for more than 12 months. NWs which in the following are labeled 'oxidized' were exposed to an oxygen plasma with an RF power of 400 W and an O2 flow of 0.6 sccm at a temperature of 600 °C in an ultra-high vacuum chamber for 10 min. Note, that in this process no accelerating DC bias was applied. For PL measurements in liquid environments, HCl-etched GaN NWs grown on a GaN template were used with diameters of 160 nm, heights of 520 nm and an inter-NW distance of 1000 nm.
ZnO and Gax Oy shells were grown on GaN NWs on Si with diameters of approx. 150 nm in a second (oxide-) MBE system with a background pressure in the 10−10 mbar regime. Prior to the growth, the samples were out-gassed and oxidized by means of an oxygen plasma with an RF power of 400 W at a temperature of 500 °C for 5 min. The ZnO shell growth was conducted at a temperature of 380 °C and a Zn flux of 1. 8 · 10−7 mbar BEP and Gax Oy growth at 600 °C and a Ga flux of 5.3 · 10−7 mbar BEP. The oxygen supply was fixed to 0.6 sccm with 400 W RF power. Al2O3 shells where grown via thermal atomic layer deposition (ALD) using H2O and trimethylaluminium as precursors at a reactor temperature of 200 °C in a home-made system.
The optical investigation and light exposure of the GaN NW surfaces during interaction with their environments was realized by above-band gap light excitation with a quadrupled Nd:YAG laser with a wavelength of 266 nm in continuous wave operation. The PL signal was spectrally analyzed by a DILOR double spectrometer with a focal length of 80 cm and detected by a Peltier-cooled photo-multiplier tube. To record the PL intensity evolution, the measurement wavelength was fixed to the temperature-dependent maximum of the NBE emission of GaN. The time resolution of the setup is 1 s and the laser excitation and signal detection was parallel to the NW axis, i.e. perpendicular to the substrate surface.
The excitation intensity was measured by placing a power sensor instead of a sample into the optical path. In ambient air, a 40× UV-enhanced High-Power MicroSpot objective with a working distance of 1 mm and a resulting laser spot size of roughly 2 μm was used. Most of the data shown is normalized to its initial Pl intensity. The corresponding normalization factors are given in the supporting information.
For measurements in controlled gaseous atmospheres, a flow chamber was designed (figure 1(c)). The chamber body is made from stainless steal and allows a controlled gas flux through the chamber. For the experiments presented here, the gas flux was fixed to 1.5 l min−1. The chamber volume is approx. 100 cm3. Illumination and PL read-out were conducted through a sapphire window. In this case, a 40× UV-enhanced reflective objective was used due to its larger working distance of 5 mm, resulting again in a laser spot size of approx. 2 μm at the sample surface. A resistive heater allows a heating of the sample up to 200 °C. Electrical current measurements were conducted in the same measurement chamber. To this end, ohmic Ti/Al/Ti/Au (20/80/20/120 nm) contacts were evaporated on an array of 50 NFs. Prior to the contact fabrication, the Ti mask was removed by a 5 min dip in an aqueous 50:50 HF (50%):H2O2 (31%) solution to avoid short circuits.
Prior to each measurement, the chamber was purged for at least 1 h with dry N2 to ensure reproducible experimental conditions. For measurements in wet environments, the respective gases have been bubbled through deionized (DI) H2O until a relative humidity of 100% was reached. The N2 gas was extracted by a phase separator from liquid nitrogen. An impurity analysis yielded an O2 content of below 75 ppm and residual water of less than 30 ppm in the N2. For the measurements carried out in synthetic air (SA), 20% bottled O2 (Linde AG, purity grade 4.6) was mixed with 80% N2 via two mass flow controllers. CPD measurements were carried out in vacuum (≤1 × 10−5 mbar) and in the environments described above at room temperature using a custom-built Kelvin probe setup equipped with a commercial controller (Kelvin Control 07, Besocke DeltaPhi) and a focused UV LED with a wavelength of 340 nm (M340L4, Thorlabs), providing a maximum light output of about 60 mW. A piezoelectrically driven gold grid with a diameter of 3 mm and a work function of 4.9 eV was used as the reference electrode. The time resolution of the CPD measurements was 1 s. CPD measurements were performed with commercially available nominally undoped free-standing bulk m-plane GaN plates (MSE Supplies LLC).
For measurements in liquid environments, the sample was mounted into a Petri dish filled with the respective fluid. The filling level was adjusted to approx. 1 mm above the sample surface.
Due to the good thermal conductivity of the substrates and efficient radiative recombination in GaN, only moderate laser-induced heating of the NWs under illumination is observed despite the high excitation powers of several kW cm−2. From the Varshni formula
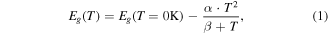
with α = 0.66 meV K−1 and β = 341 K for GaN, the actual temperature of the NWs under illumination can be estimated from the NBE peak-center [30, 31]. At ambient conditions and an excitation of 18 kW cm−2 a peak center at 3.40 eV was observed on the Si substrate, which corresponds to a temperature of 315 K. All temperatures denoted as 'Teff' in temperature-dependent measurements refer to the values determined by the NBE peak position, while 'T' refers to values determined by conventional methods (thermometer, thermo-couple).
Results and discussion
PL Intensity in Air
To demonstrate the complexity of the PL response of GaN NWs in air, the dynamic behavior of the GaN PL NBE of three differently pre-treated samples is depicted in figure 2(a). The samples have been stored under ambient conditions for 14 months (aged), were HCl-etched for 5 min prior to the measurement or oxidized at a high temperature in the MBE chamber. After switching on the laser at 0 min, the intensity drops by 33% for the aged sample and increases slowly afterwards, the HCl-etched NWs lose over 70% of the initial PL intensity and stay at the low level while the oxidation triggers an enhancement by a factor of 2.5 compared to the initial value in the course of several min. For oxidized and HCl-etched NWs the change of PL intensity is reversible in ambient air, i.e. it recovers to its initial value in the dark (figure S3/figure 5(c)). Thus, the respective PL intensity change cannot be caused by a permanent oxidation induced by the UV light or a structural degradation of the material but could be induced by the physical or chemical binding of atoms or molecules from the ambient to the NWs or a slow and persistent charging effect of surface states, which leads to a changed distribution of photo-excited electrons and holes. Analogous to the PL intensity quenching for HCl-etched NWs [18], the enhancement observed for oxidized NWs is dependent on the applied excitation energy. However, while the quenching can be observed already at intensities levels as low as 1 W cm−2, the enhancement is not significant below several hundred W cm−2. To quantify this, we define the enhancement factor (EF), which is the ratio between the PL intensity after a certain time and the initial intensity. Figure 2(b) displays EFs after 10 and 15 min for varying excitation intensities. For excitation power densities lower than about 9 kW cm−2, the EF increases with increasing intensity. This indicates that in this regime the relevant processes are limited by the availability of photo-excited charge carriers. For very high excitation power densities exceeding 10 kW cm−2, a saturation of the EF is observed. As for both 10 and 15 min exposure time the saturation is observed at similar intensities and the EF is higher after 15 min, the absolute PL intensity limit is not reached yet, but the enhancement is still going on. Therefore, in the saturation regime the speed of the PL enhancement has to be limited by the speed of adsorption or desorption of molecules from the environment to or from the NW surface. In the following, we will focus on oxidized and HCl-etched NWs as the "aged" condition is not the result of a reproducible surface treatment.
Figure 2. (a) NBE PL intensity evolution of GaN NWs in ambient air (T = 300 K) normalized to the initial value. (b) Enhancement factor of the PL intensity of oxidized GaN NWs after 10 and 15 min under UV light exposure as a function of the excitation intensity.
Download figure:
Standard image High-resolution imagePL intensity in controlled atmospheres
The experiments discussed above have been conducted in ambient air and at room temperature with freely exposed samples. As ambient air is a mixture of several gases and contains a relatively high amount of humidity, a more precisely controlled experimental environment is needed to understand the interactions at the NW surface. Figure 3 depicts the normalized saturated PL intensity under continuous UV illumination with an excitation intensity of 18 kW cm−2 for HCl-etched and oxidized NWs under varied atmospheres and for different temperatures. The PL intensity measured in dry N2 is used as the reference value for normalization. In this experiment, a fixed spot on the sample was continuously exposed to the laser illumination. The numbers depicted next to the dashed arrows refer to the transition times, i.e. the time it takes until the PL intensity saturates at a constant level after changes of the gas ambient. In dry atmospheres and at low temperature (Teff = 46 °C), HCl-etched and oxidized NWs show qualitatively the same behavior, but the amplitude of the PL intensity reduction is more pronounced for the HCl-etched sample when the atmosphere is changed from N2 to SA. After switching back to N2, the initial intensities are almost recovered, but with a doubled transition time of 2 min for the HCl-etched NWs. When humidity is added to the N2 flow, the intensities are only slightly changed at low temperature with a small increase for the oxidized and a comparable decrease for the HCl-etched NWs. By switching to humid SA, the PL intensity of the oxidized GaN NWs decreases to 60% of the dry N2 level and to 15% for the HCl-etched NWs within 1 min. As these values are lower than in wet N2 and dry SA separately, the quenching properties of oxygen and H2O at low temperatures appear not to be in competition to each other, but the simultaneous presence of both decreases the PL intensity to the lowest of all observed levels. Possible explanations are the adsorption of H2O and oxygen at different sites at the NW surface or the formation of a complex containing more than one molecule, which enables highly efficient non-radiative recombination of charge carriers. This configuration is also the most stable one under continuous illumination. At low temperature, the PL intensity stays constant in N2 for the oxidized NWs as long as the humidity is maintained. For the HCl-etched NWs, the PL intensity recovers only slowly in humid N2. When the flow-chamber is purged with dry N2 as a final step, both samples increase their PL emission back to the initial level within the measurement error, which underlines the reversible nature of the observed effects. The PL response of HCl-etched NWs to atmospheric variations changes drastically when the temperature is increased by external heating of the sample to Teff = 194 °C. Changing from dry N2 to SA, which causes an intensity reduction at low temperature, results in a PL enhancement by a factor of over 5.5 at 194 °C. The intensity change is more persistent than its low temperature counterpart and a full relaxation to the initial value in N2 has not been observed. Adding humidity to the N2 atmosphere results in a doubling of the PL intensity, which is further drastically increased when the oxygen is added. Interestingly, the PL intensity in dry and humid SA are very close to each other at high temperature, while they differ significantly at low temperatures, which indicates that the effect induced by H2O is replaced by the influence of the additional O2 at high temperatures. By a subsequent exposure to first humid and then dry N2, the respective PL levels could not be fully restored but remained at a higher level. Similarly, for oxidized NWs the intensity decrease at low temperature is inverted into an increase by a factor of about 5 within 3 min at high temperature when the atmosphere is changed from dry N2 to SA. A change back to dry N2 reduces the intensity to 1.5 times the initial value, a complete recovery was not observed. Adding humidity to the N2 has only a minor effect on the PL intensity of the oxidized NWs. Both in SA and N2 atmospheres the values obtained for dry and humid environments are similar. Switching back to first wet and than dry N2 reduces the luminescence to about 3 and 1.5 times the initial value, respectively. The time needed to reach the respective saturation is comparable for etched and oxidized NWs at high temperature with the exception of the transition from dry N2 to SA, where the oxidized NWs react more slowly (4 compared to 8 min).
Figure 3. Variation of the stabilized NBE PL intensity of oxidized and HCl-etched GaN NWs in different atmospheres under continuous illumination. The numbers next to the dotted arrows indicate the typical transition time from one steady state to the other. An excitation intensity of 18 kW cm−2 has been used. The given Teff were determined by evaluation of the NBE peak position of GaN.
Download figure:
Standard image High-resolution imageIn their recently published work, Maier et al observe and discuss in detail an ambivalent nature of the influence of H2O adsorbates on the PL intensity of (In)GaN NWs, which can have both, a quenching and an enhancing effect [32]. Our observations confirm these results for HCl-etched GaN NWs as their PL is (slightly) reduced at low temperature by adding humidity to a N2 atmosphere, while at high temperatures, this effect is reversed to an enhancement. We observe a similar ambivalent behavior also for SA. The strong PL intensity decrease observed at low temperature turns into an even more pronounced increase at high temperature. Similar as proposed for H2O adsorbates, [32]. we conclude that also oxygen alone is able to form two fundamentally different interacting states at the NW surface. To date, we lack the tools for a direct observation of the exact nature of these states, however, we can hypothesize that the observed change is related either to (I) the adsorption of different molecular species of oxygen, i.e. O, O2,  (superoxide) or O3, (II) a modification of the m-plane GaN surface due to the high temperature and illumination intensity, which adds new available adsorption sites or (III) the adsorption of the same oxygen species but with altered binding strength, e.g. chemisorption instead of physisorption.
(superoxide) or O3, (II) a modification of the m-plane GaN surface due to the high temperature and illumination intensity, which adds new available adsorption sites or (III) the adsorption of the same oxygen species but with altered binding strength, e.g. chemisorption instead of physisorption.
Decisive for the validation of hypothesis I is that whether or not a specific oxygen species adsorbs should be mainly dependent on the availability of this species in the environment of the NW. The additional thermal energy added through sample heating is below 15 meV, therefore, the purely thermal influence on the relative occurrences of the oxygen species concentration is low. Further, the presence of O3 is most probable limited by the intensity of the incident UV light, as the used wavelength of 266 nm does not create ozone, but efficiently degenerates ozone via photolysis [33]. As the laser power is constant throughout the experiment, it is not likely that the O3 concentration changes significantly. Therefore, we think that the adsorption of O3 from the environment is unlikely to be the major reason for our observations. However, adsorbed  at the NW surface might influence the PL intensity as it is known to catalyze chemical reactions with the surroundings due to its strong reducing character [34]. The availability of
at the NW surface might influence the PL intensity as it is known to catalyze chemical reactions with the surroundings due to its strong reducing character [34]. The availability of  depends on the fact whether/how efficient GaN NWs can reduce molecular O2, which remains a subject of further investigations.
depends on the fact whether/how efficient GaN NWs can reduce molecular O2, which remains a subject of further investigations.
Regarding hypothesis II, to the best of our knowledge, no information on the changes in surface structure or reconstruction in the relevant temperature range are available for m-plane GaN. However, for both polarities of c-plane GaN, phase transitions of the surface reconstruction occur at about 250 °C in UHV [35]. It is possible that for m-planes and under atmospheric pressure, similar phase transitions take place in the temperature range measured. Therefore, hypothesis II can neither be excluded nor proven due to the lack of reliable experimental data.
In favor of hypothesis III is the higher stability of the PL intensity change induced by dry SA at high temperatures: at low temperature, the recovery to the initial PL intensity in dry N2 takes 2 min whereas at high temperature the initial level is not re-gained even after 4 min. As for elevated temperatures the available energy for deconstruction of a surface layer is higher, the more persistent PL intensity change indicates that the high temperature surface modification is significantly more stable, hence, that the binding of the adsorbed species on a molecular level is stronger.
So far, the quenching and enhancing effects of the air constituents have been discussed in terms of relative intensities normalized to the PL intensity observed in dry N2 atmosphere. Further insight can be gained by focusing on absolute intensities. The aforementioned ambivalent role of O2, which can act as both, a quenching and enhancing agent, is again illustrated in figure 4(a), which depicts the absolute PL intensities at three temperatures as a function of the O2 content in a background of dry N2. Already at concentrations of about 0.3%, the O2 content starts to dominate the PL response at low and high temperature, causing a rapid quenching/enhancing, respectively, after which the PL intensity stays roughly at the same level for higher O2 contents. At an intermediate temperature of Teff = 97 °C, the PL intensity is almost unaltered by the O2 content. As already shown in figure 3, the observed PL intensity change is not completely reversible, which indicates a permanent attachment of O2-related adsorbates. Interestingly, the intensity variation in N2 across the temperature range measured is significantly higher than in the O2 atmosphere. Figure 4(b) shows the absolute PL intensities of the same spot of the HCl-etched sample in dry SA and N2 at different temperatures. In dry N2 atmosphere, the PL intensity decrease follows an Arrhenius-like behavior over the measured temperature range. In contrast, the PL intensity in SA is almost constant up to a temperature of about 400 K and decreases at higher temperatures. By assuming that the observed quenching is mainly caused by an increase of non-radiative recombination and that the internal quantum efficiency approaches unity at 0 K, the PL decreasing parts of the experimental data can be modeled by an Arrhenius-type fit. The resulting activation energies (Ea ) are similar, with 240 meV in N2 and 200 meV in SA. This indicates that the thermal quenching of the PL intensity above about 400 K is induced by the same processes in both atmospheres. For GaN, constant PL intensity despite increasing temperature, as observed in SA between 300 and 400 K, is an uncommon behavior and suggests, that an additional, competing effect has to be considered. As discussed later in more detail we suggest that in the case of GaN NWs in SA atmosphere this second effect is the passivation of surface states by oxygen, which leads to a reduction of non-radiative recombination.
Figure 4. (a) PL intensity of HCl-etched GaN NWs in dry atmospheres as a function of O2 concentration in N2 background for several Teff. (b) Arrhenius plot of the temperature dependence of the PL intensity in dry N2 and SA atmospheres. For all measurements, an excitation density of 18 kW cm−2 was used. Dashed lines are Arrhenius fits.
Download figure:
Standard image High-resolution imagePL intensity evolution after illumination onset
Under steady state illumination the environmental changes are decisive for the changes of the PL intensity. In contrast, the PL intensity evolution immediately after the UV excitation onset probes the light-induced changes of the surface properties in a given ambient. The PL response of differently treated GaN NWs in air (figure 2) already demonstrated the impact of surface treatments on the environmental sensitivity. In order to understand the underlying mechanisms, similar measurements in controlled atmospheres have been performed. Figure 5(a) depicts the time-resolved PL intensity of oxidized and HCl-etched GaN NWs immediately after starting the UV excitation in dry and humid N2. The PL intensity of the oxidized GaN NWs in dry N2 increases within 1 min to 140% of the initial value, whereas in humid N2 it reaches only 120%. The HCl-etched GaN NWs show almost no change in the PL intensity in dry N2, but a pronounced quenching of the PL signal to 50% of the initial intensity in humid N2. The equivalent measurements in dry and humid SA (figure 5(b)) show a very similar PL response as in pure N2 with the increasing PL signal of the oxidized NWs and a constant/quenching PL evolution of the HCl-etched NWs. Our results show that the humidity level of the ambient and the GaN NW pre-treatment are the decisive factors for the time-dependent PL intensity during the first minutes of UV illumination and that the O2 content in the ambient only influences the PL evolution to a minor extent. This is particularly interesting as under prolonged illumination (steady state) humidity was only secondary for the change of the PL intensity but rather the O2 content in N2 was the decisive factor. Further, the fact that HCl-etched GaN NWs show almost no PL intensity evolution in dry N2 and SA proves that the adsorption of quenching O2 is not UV-induced but already takes place in the dark.
Figure 5. Normalized PL intensity evolution of oxidized (red) and HCl-etched (blue) GaN NWs after UV laser illumination onset with an excitation intensity of 18 kW cm−2 (a) in N2 (b) in SA. The solid (doubled) lines represent measurements in dry (humid) atmosphere. (c) PL intensity recovery of HCl-etched GaN NWs in different atmospheres. The red symbols show the PL intensity after switching of the laser illumination in humid SA. The black dots (stars) show the recovery behavior in dry SA (N2). The data are normalized to the initial PL intensity in dry SA before the quenching procedure.
Download figure:
Standard image High-resolution imageThe light-induced ad- and desorption respectively the chemical interaction of the water molecules with the GaN surface are influenced by the pre-treatment of the NWs, e.g. oxidized Ga- and N-polar GaN surfaces show a better wetting behavior than as-grown or HCl-etched surfaces [36]. Additionally, the electronic properties of both polar and non-polar surfaces are influenced by pre-treatments like HCl-etching or O2 plasma exposure, since this modifies the surface states and creates distinct surface dipoles due to the presence of adsorbates remaining after the treatments. This behavior is found to be similar for the GaN (0001) and  surfaces, which are the dominant surface facets in the case of GaN NWs [21]. Particularly, each pre-treatment might cause a specific rate of non-radiative recombination due to the different SBB and adsorbates. Upon beginning UV illumination in dry atmospheres, the change of one or several of these properties lower the proportion of non-radiative recombination in the case of oxidized NWs, whereas for HCl-etched NWs the respective properties related to PL intensity are unaffected. In all cases, H2O acts as an additional source of non-radiative recombination, which reduces the PL enhancement for oxidized NWs and leads to a quenching of the PL intensity for HCl-etched NWs.
surfaces, which are the dominant surface facets in the case of GaN NWs [21]. Particularly, each pre-treatment might cause a specific rate of non-radiative recombination due to the different SBB and adsorbates. Upon beginning UV illumination in dry atmospheres, the change of one or several of these properties lower the proportion of non-radiative recombination in the case of oxidized NWs, whereas for HCl-etched NWs the respective properties related to PL intensity are unaffected. In all cases, H2O acts as an additional source of non-radiative recombination, which reduces the PL enhancement for oxidized NWs and leads to a quenching of the PL intensity for HCl-etched NWs.
The reversibility of the PL responses was investigated in a recovery study, the results of which are shown in figure 5(c). The PL signal of HCl-etched GaN NWs was quenched to 30%–40% of the initial PL intensity in dry SA by exposure to the excitation light with an intensity of 21 kW cm−2 in humid SA. Then, the laser was blocked at 0 min. The laser was switched on for 1 s to probe the PL intensity recovery in the dark and immediately switched off again. In humid SA, almost no change in the PL intensity is observed except a slight quenching possibly due to the additional UV illumination of the laser during the short measurements. After 15 min, the measurement chamber was purged with dry N2 and dry SA, respectively. The PL intensity recovers to its initial value (7300 cts) in dry SA within 45–60 min. In dry N2, this recovery to 100% takes longer (100 min), followed by a further PL enhancement to about 130% of the initial PL intensity (10 500 cts) within the subsequent 50 min. This overshoot reflects the higher PL intensity at temperatures close to RT in N2 compared to SA (figure 3). For the oxidized GaN NWs, a recovery from the PL enhancement is observed when measured in a comparable way in ambient air (figure S3, SI). As already mentioned, the complete recovery of the PL intensity shows that no permanent change of the GaN NW surface is responsible for the PL intensity evolution. However, the fact that the PL signal remains quenched in humid SA but a complete recovery of the PL intensity is observable in dry atmospheres shows that H2O from the ambient is not only responsible for the PL quenching after illumination onset but it also hinders the recovery. In the literature, changes in the PL intensity after illumination onset have been attributed to O2 chemisorption on the GaN surfaces, which results in a Fermi level pinning reducing the quantum efficiency of the PL [37–39]. However, our results show that the ad- and desorption of H2O is the decisive factor for the PL intensity evolution after illumination onset, which is also in accordance with the observations by Hetzl et al [18].
Combined PL and electrical measurements
While the PL experiments presented so far clarify the quantitative and qualitative evolution of the NBE PL intensity of GaN NWs in different atmospheres, complementary electrical measurements of GaN NFs can provide further insight into the underlying mechanisms. To this end, the current characteristics of GaN NFs on AlN substrates were investigated in different atmospheres. Figure 6(a) shows the electrical current through GaN NFs under illumination with 266 nm laser light and the simultaneously measured NBE PL in varied dry atmospheres at room temperature. Under illumination, the total current (dark and photocurrent) decreases to 88% and recovers to 95%. At the same time, the PL decreases to about 85% in SA and reaches its initial value 10 min after switching back to N2. The corresponding dark current at room temperature (figure 6(b), blue curve) is reduced only slightly to about 99% of its initial value after replacing N2 with SA. It recovers partially when switching back to N2. The evolution of the PL intensities after illumination onset (figures 5(a)/(b)) showed that the changes of the GaN NW luminescence due to dry oxygen is not light-induced but mainly happens already in the dark. Consequently, the oxygen-related mechanism which causes a reduction of the dark current will similarly reduce the current under illumination. However, the amplitude of the current reduction under illumination is higher, indicating a stronger sensitivity of the photocurrent. A possible explanation lies in the location of photo-excitation within the GaN NF, which mainly takes place in the surface region, while the majority of the dark current contribution is through the conductive channel in the center of the NF. Thus, an increase of the band bending due to oxygen adsorption has a stronger impact on the photocurrent contribution of the overall current, which makes the current under illumination more sensitive. The simultaneously measured PL decreases in SA, too. As speed and amplitude of the PL decrease and current reduction under illumination correlate strongly, it is likely that both effects have the same origin, i.e. changes of the SBB.
Figure 6. Normalized NBE PL intensity and normalized current density of GaN NFs on an AlN template under illumination with 266 nm light at (a) 300 K and (c) 375 K. The atmosphere was changed from dry N2 to dry SA and back under constant applied voltage (1 V) and excitation intensity (18 kW cm−2). (b) Normalized current density of GaN NFs at 300 and 375 K in the dark. Due to the sake of argument (c) is discussed later in the text.
Download figure:
Standard image High-resolution imageGenerally, changes in PL intensity of NSs under constant excitation originate in changes of the ratio of radiative to non-radiative charge carrier recombination. This is either caused by the creation or passivation of non-radiative recombination channels, e.g. unsaturated surface states, or by changing the amount of radiative recombination through spatial separation of electrons and holes, e.g. due to SBB. In contrast, the electrical current through NFs in the dark is influenced by atmospheric changes only via the SBB, which alters the size of the conductive channel within the NS. In the present case, the conductivity of NFs is not sensitive to passivation effects of surface states as long as the band bending remains unaltered. Similar to PL, the photocurrent is influenced by both SBB and the non-radiative recombination rate. However, not necessarily with the same sensitivity.
For confirmation, we measured the macroscopic surface photovoltage (SPV) and CPD, which is a measure for the position of the Fermi level at the surface, of bulk HCl-etched m-plane GaN (figure 7(a)). The CPD value of the GaN surface in the dark is about 0.1 V higher in SA compared to N2, reflecting an increased SBB in SA. Under illumination with 6 mW cm−2 of 340 nm LED light, the CPD follows the same trend with a more pronounced response to oxygen (0.2 V CPD difference). The recovery to the initial CPD values in N2 is faster under illumination, which might indicate a light-induced process accelerating the desorption of oxygen. The SPV was about 0.3 V in N2 and almost 0.2 V in SA. In both atmospheres, the maximum intensity of the UV-LED used was not sufficient to achieve flat band conditions (figure S2, SI). The changes of the SPV and the current under illumination both show a double-exponential decay with similar time constants of 11 s and 430 s, respectively 4 s and 440 s, which indicates that the same oxygen-induced processes are responsible for the reduction of current under illumination and SPV. Figure 7(b) summarizes the CPD and SPV results in a schematic drawing of the GaN NS SBB in SA and N2 atmosphere. In the dark, the SBB in SA is higher than in N2. This difference increases upon above band gap illumination as the SBB reduction due to the light is more pronounced in SA compared to N2. This matches well with the increased sensitivity of the current to changing the atmosphere under illumination compared to in the dark (figure 6): the higher reduction of the SBB under illumination leads to a stronger shrinking of the conductive channel within the NF, which increases the electrical resistivity to a greater extend. The picture changes, when the NFs are heated to 375 K. The dark current (figure 6(b), red curve) decreases to 90% in SA and recovers only slightly in N2. While the current under illumination decreases to about 80%, the PL increases to about 150% (figure 6(c)). Both effects recover majorly within 10 min after switching back to N2. The qualitatively different behavior of the electrical current and PL prove that beside the increase in SBB a second, contrary effect exists, which reduces non-radiative recombination. We suggest that the adsorption of O2 on the heated GaN NF surface passivates non-radiative recombination paths, which increases radiative recombination. The overshoot of the PL intensity after switching back to N2 indicates that the passivation is temporary and reacts to atmospheric changes more slowly than the change in band bending: in the first minutes after the SA is replaced in the measurement chamber, the band bending relaxes relatively quickly, which increases the PL and current. In combination with the more stable passivation, the already lowered SBB results in a peaking PL intensity.
Figure 7. CPD of an m-plane bulk GaN plate in the dark und under 340 nm illumination with an excitation intensity of 6 mW cm−2. The atmosphere was changed from dry N2 to dry SA and back. (b) Schematic drawing of the band bending at a GaN NS surface (green line) in SA (left) and N2 (right) atmosphere at room temperature. The dashed/solid black lines represent the SBB in the dark/under illumination. The red lines illustrate the CPD differences between SA and N2 atmosphere in the dark (upper part) and under illumination (lower part). The corresponding SPV in SA and N2 are indicated in blue.
Download figure:
Standard image High-resolution imageRecently, several groups have reported that oxygen can bind in different configurations to GaN m-plane [40, 41]. Although the underlying mechanism is not yet completely clarified, density functional theory calculations and experimental observations indicate that dissociative adsorption of oxygen and bridge adsorption of O2 can coexist. Due to the differing binding configuration, the respective binding energy is different, with the dissociative absorption being the more stable variant. From the perspective of our results, we speculate that at room temperature bridge adsorption is dominant, while the share of dissociative adsorption increases when the sample is heated during the exposure to O2. Following this argumentation, bridge adsorption of O2 would mainly increase the SBB, while dissociative adsorption would passivate non-radiative surface recombination paths. However, at present this remains only a speculation, which could motivate future work.
PL response in liquid environments
(Al)GaN-based high-electron-mobility transistors (HEMTs) and field-effect transistors are known to be sensitive to H2O, various organic solvents like alcohols, acetone or propanol and the pH-value in aqueous solutions [42–45]. However, there are only few reports which deal with the optical readout of group III-nitride-based sensors in liquids. E.g. it has been shown that the luminescence of (In)GaN NWs can be used to monitor salt concentrations in H2O or the pH-value [1, 18]. The PL response of HCl-etched GaN NWs in various liquids is shown in figure 8(a) on a logarithmic scale. The already discussed PL quenching in ambient air is shown for comparison. In all liquid environments, a PL enhancement is observed. In DI H2O, the PL intensity doubles after 6min of exposure. When the NWs are surrounded by ethanol or acetonitrile, the PL intensity almost quadruples. In cyclohexane the PL intensity even exceeds 26 times of its initial value. The adsorption of molecules from an electrolyte at a semiconductor surface can lead to a charge transfer between the adsorbed species and the semiconductor, causing formation of a Helmholtz layer at the semiconductor surface. This in turn influences surface properties of the semiconductor like its SBB and, thereby, the PL intensity [46]. Further, alcohols like methanol are known to act as hole-scavengers. These influence the recombination mechanisms in GaN NWs e.g. they prevent them from oxidation and decomposition in H2O, [19] which might influence the PL signal as well. Candidates for the significant deviation in the magnitude of PL enhancement in the liquids are (I) their molecular dipole moment, (II) the molecule size, (III) differences in the potential for several redox-reactions and (IV) the oxygen solubility. (I) It is known from (Al)GaN-based HEMTs that the source-drain current is sensitive to liquids with different molecular polarization strengths in the vicinity of the polar GaN-gate as they compensate polarization-induced charges in the heterostructure [43, 44]. Even though GaN NWs expose mainly the non-polar m-plane facets, comparable processes might influence the surface properties of the NWs and their PL intensity. Cyclohexane is an unpolar molecule, whereas ethanol and H2O have comparable dipole moments of 1.69 D and 1.85 D, respectively, and acetonitrile has the highest moment of 3.92 D. However, the amplitude of the PL enhancement for the different liquids does not reflect this ordering as a similar evolution of the PL intensity is observed for ethanol and acetonitrile. Thus, although a secondary role of the dipole moment is possible, it cannot be the main reason for the observed PL intensity evolution. (II) The molecule size might influence the built-up speed and capacitance of the emerging Helmholtz layer manipulating the SBB. H2O is the smallest molecule, ethanol and acetonitrile are approx. twice as large and cyclohexane is significantly larger. This matches the observed ordering of the PL enhancement. We would expect that the built-up time for Helmholtz layers is significantly shorter than the seconds to minutes of the here observed effects. However, further investigations are needed to clarify the role of the Helmholtz layer on the recombination mechanisms of the NWs. (III) The redox energy levels for illumination induced reactions, e.g. water oxidation or superoxide formation are not necessarily the same in different electrolytes. Some of the emerging products of these reactions may alter the GaN surface, leading to passivation of surface states. For us, a definite conclusion over this possible explanation for the observed effects is difficult as no controlled potential was applied to the NWs in the here discussed experiments. Therefore, this approach has to remain open for further investigations. (IV) The mole fraction solubility in units of 10−4 of O2 in the respective liquids is approx. 0.2 for H2O, 6 for ethanol, 8 for acetonitrile and 12 for cyclohexane at room temperature and ambient pressure [47, 48]. As this reflects the overall PL intensity enhancement trend, dissolved oxygen may play a role in the PL response of GaN NWs in liquids, too. Adsorbed oxygen at the NW surface, which acts as a PL quencher at room temperature in gaseous surroundings (see above), dissolves more easily in e.g. cyclohexane compared to H2O, which might cause a much more pronounced PL enhancement.
Figure 8. (a) Normalized PL intensity evolution of HCl-etched GaN NWs after UV laser illumination onset with an excitation intensity of 9.5 kW cm−2 in air (orange), DI H2O (blue), acetonitrile (black), ethanol (red) and cyclohexane (green). The PL intensity axis is shown with a logarithmic scale. (b) Normalized PL intensity of GaN NWs with several shells at an excitation intensity of 9.5 kW cm−2 in ambient conditions. For comparison, oxidized NWs are also shown. ZnO and Gax Oy shells are MBE-grown, the 50 nm Al2O3 was deposited by ALD. SEM images in 45° tilted view of exemplary NWs with different shells are depicted at the right of the graph.
Download figure:
Standard image High-resolution imageThe ambivalent role of H2O around room temperature (PL quenching in gaseous atmospheres and enhancement in a liquid environment) of the HCl-etched GaN NWs might be explained by the work of Shen et al [49]. They found that a full dissociation of H2O molecules occurs at the GaN m-planes, leading to a localized and high concentrations of disordered H3O+ and OH− molecules at the GaN surface, which separate and align at the GaN NW surface under UV illumination. In contrast, when the NWs are in a H2O bath this effect vanishes as the ion concentration is thermodynamically determined by the auto-protolysis of the ambient reservoir. As the ion concentration in the vicinity of the NWs is a decisive factor for the PL intensity evolution this has a major impact [18]. The different magnitude of the PL response in various liquid environments opens a possibility to use GaN NWs to optically detect impurity molecules for, e.g. H2O quality control applications.
GaN-metal oxide core–shell NWs
In figure 8(b), the PL intensity evolution of GaN NWs with several oxide shells after the onset of illumination under ambient conditions in air is depicted. All MBE-coated samples (ZnO, Gax Oy ) show an intensity increase, however, the amplitude differs significantly. While the PL signal of the GaN NBE of the oxidized NWs more than doubled within 2 min, for the thickest Gax Oy shell it increases only by 6%. As a general trend, the enhancing effect inflicted by the atmospheric exposure is smaller the thicker the surrounding shell is. A confirmation that the observed PL intensity increase results from an increased emission related to GaN and not to ZnO is given in figure S4(a) in the supporting information. For the ALD-grown Al2O3 shell, a quenching to 24% of the initial intensity is observed. Although the comparison of absolute PL intensities of different samples has to be treated with care, some clear trends are observed: the absolute PL intensities (table S5, SI) of the GaN-ZnO core–shell NWs decrease by a factor of almost 25 from the thinnest to the thickest shell due to absorption of the excitation laser in the ZnO. Further, the sample with Al2O3 shell exhibits the highest PL signal and is at the same time the only one exhibiting a decreasing PL intensity over illumination time. We speculate that in the case of an Al2O3 shell the electronic interface configuration is already in a beneficial state with high radiative recombination efficiency and cannot be further improved upon UV laser exposure. For the other types of oxide coatings, this may initially be not the case leaving room for UV-induced improvement. The qualitatively different behavior of MBE- and ALD-grown core–shell NWs can be possibly explained by differences in the growth procedure. Prior to the shell growth via MBE, the GaN NWs were oxidized in the MBE chamber due to process requirements. During ALD growth of amorphous Al2O3 no comparable oxidation of the GaN surface took place on the previously HCl-etched NWs. Additionally, an accumulation of positive charges on the Al2O3 side of the heterointerface to GaN has been reported, although their origin remains disputed [50]. These charges can annihilate the band bending of the GaN m-plane [51]. Under illumination, the positive interface charge could be slowly compensated by photo-excited electrons resulting in an upward band bending. From an electronic point of view, this would resemble the adsorption of anions on bare GaN NWs, which leads to a quenching of the PL intensity and has been discussed in detail by Hetzl et al [18]. Further, the spotty appearance of the MBE-grown oxide shells suggests that the shells could be not completely tight and allow some atmospheric molecules to still reach the GaN NW surface. For the tight 50 nm Al2O3 shell, a diffusion of molecules/ions from the environment to the GaN-Al2O3 interface can be excluded [52]. However, as ALD on GaN for passivation can be challenging, it cannot be excluded that the recipe could influence the PL evolution as factors like the GaN pre-treatment, deposition temperature and post-deposition annealing treatments influence the GaN-Al2O3 interface as well as the Al2O3 itself [53–56]. Note that the illumination-induced PL intensity changes for the core–shell NWs were reversible similar to the uncoated NWs described above (figure S4(b), SI), which excludes permanent changes in the GaN-oxide interface like changed oxidation states as possible explanation of the observed effects. In sum, thick ZnO and especially Ga2O3 shells are well-suited to serve as an efficient passivation the GaN NW surface and stabilisator of the PL intensity, which could be exploited in NW-based (opto-)electronic devices.
Conclusion
Understanding the influence of the environment on the optical and electrical properties of nanostructures (NSs) is a subject of high technological importance and scientific interest. In this paper, we performed an extensive and systematic investigation of some major external contributions manipulating the photoluminescence (PL) intensity and the electrical resistance of GaN nanowires (NWs) and GaN nanofins (NFs) on several substrates. The NBE emission PL intensity of GaN NSs exhibits a complex behavior, which is highly dependent on the environmental conditions and the NS pre-treatment. One of the key findings is that both H2O and oxygen show an ambivalent influence with either quenching or enhancing effect, depending on the sample temperature. While for the PL-influencing adsorption of H2O the ultraviolet (UV) illumination plays a key role, the surface reactions related to oxygen also take place in the dark. We found temperature-dependent PL intensities in nitrogen and oxygen atmospheres with a larger intensity range in nitrogen. By combining PL and measurements of the electrical current through NFs we observe a reduction of both current and PL intensity at room temperature, while only the PL shows the opposite behavior at elevated temperatures. Together with complementary CPD and SPV measurements, this indicates the presence of two independent mechanisms of oxygen adsorption at the GaN surfaces, which lead to changes in SBB and non-radiative recombination. In all tested liquids, the PL intensity enhances during UV exposure with the strongest effect in c-hexane. One way to circumvent the environmental influence on GaN NS and to ensure stable performance is the surface passivation with oxide shells. Surprisingly, the commonly used material for this purpose, ALD-grown Al2O3, leads to a highly unstable PL within the first minutes of UV exposure. Alternatively, Gax Oy and ZnO shells with a thickness of more than 50 nm can be used for an effective passivation of the NWs. Our findings highlight the importance of controlled environmental conditions, surface treatments and passivation technologies in order to ensure reliable experimental results and for boosting device performances. The highly complex interaction of the ambient with the optical and electrical properties of NSs is certainly not limited to GaN, but should be at least considered for the investigation of other semiconductors, especially for the upcoming wave of new generation materials. In summary, the detailed understanding of the role of the most common air constituents and temperature for non-radiative processes at the GaN m-plane gained with our results may help for the development of accurate GaN NS-based sensors and improves the performance of photo-catalytic devices and NW LEDs.
Acknowledgments
Financial support was provided by the cluster of excellence 'e-conversion', funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence StrategyEXC 2089/1390 776 260, supported by TUM International Graduate School of Science and Engineering (IGSSE), GSC 81 and funded by TUM.solar in the frame of the Bavarian Collaborative Research project 'Solar Technologies Go Hybrid' (SolTech). We thank Johannes Bartl for valuable discussions about the oxidation behavior of GaN m- and c-planes.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Contribution statement
M Kraut and F Pantle contributed equally.
Conflicts of interest
There are no conflicts to declare.








