Abstract
Silicon nanowire (Si NW) sensors have attracted great attention due to their ability to provide fast, low-cost, label-free, real-time detection of chemical and biological species. Usually configured as field effect transistors (FETs), they have already demonstrated remarkable sensitivity with high selectivity (through appropriate functionalisation) towards a large number of analytes in both liquid and gas phases. Despite these excellent results, Si NW FET sensors have not yet been successfully employed to detect single molecules of either a chemical or biological target species. Here we show that sensors based on silicon junctionless nanowire transistors (JNTs), the simplest possible transistors, are capable of detecting the protein streptavidin at a concentration as low as 580 zM closely approaching the single molecule level. This ultrahigh detection sensitivity is due to the intrinsic advantages of junctionless devices over conventional FETs. Apart from their superior functionality, JNTs are much easier to fabricate by standard microelectronic processes than transistors containing p–n junctions. The ability of JNT sensors to detect ultra-low concentrations (in the zeptomolar range) of target species, and their potential for low-cost mass production, will permit their deployment in numerous environments, including life sciences, biotechnology, medicine, pharmacology, product safety, environmental monitoring and security.
Export citation and abstract BibTeX RIS
1. Introduction
Since their inception in 2001 [1], silicon nanowire (Si NW)-based field effect transistors (FETs) have been extensively studied as chemical and biosensors due to their superb electrical and mechanical properties and their large surface-to-volume ratios. The sensing mechanism has largely been based on the interaction of electrically charged particles (molecules) of the target analyte with a nanowire surface (see figure 1(a)). These charged molecules effectively play the role of a top gate, changing the electrical conductance of the nanowire (transistor channel). In such a way, the interactions of molecules with the nanowires are directly converted into easily detectable electrical signals. Because of the very small cross-section of the nanowires (and the large surface area to volume ratio), even a few charged molecules attached to the nanowire surface are able to effectively modulate the FET current (channel conductance) and generate a sensor signal. Indeed, Si NW-based sensors have already demonstrated excellent sensitivity in both liquid [2] and gas [3] phases. Using FETs built from grown Si NWs, Patolsky et al [4] have shown detection of single influenza type A viruses. Stern et al [5] managed to detect the protein streptavidin at concentrations as low as 10 fM using top-down fabricated Si NW sensors. In our recent paper [6] we have reported on sensing the same protein at even lower concentration, 17 aM, implementing 3D vertically stacked Si NW FETs. To the best of our knowledge, this is the lowest protein concentration detected to date. Although these results are really impressive, there is yet no report on the detection of any species at the single molecule level using Si NW FET-based sensors. The most probable reason limiting the detection sensitivity of these sensors is the inherent low-frequency noise of semiconductor FETs, which is caused by resistance fluctuations and is known as flicker noise or 1/f noise. The ratio of the sensor signal to this noise (signal to noise ratio, SNR) is suggested to determine the detection limit of the sensors [7].
Figure 1. Junctionless nanowire transistors (JNTs) as chemo-/biosensors. (a) Schematic presentation of a JNT sensor fabricated on an SOI wafer. (b) Optical microscopy image of an SOI chip with nine groups of different JNT sensors together with an SEM top views and a TEM cross-sectional view of individual devices having 1, 3, or 20 nanowires of different lengths (0.5 and 1 μm) and widths (10, 20, 30, and 50 nm).
Download figure:
Standard image High-resolution imageRecent studies [8, 9] have shown that in the case of Si NW-FET sensors the 1/f noise does not originate in the nanowire itself but is mainly generated in the oxide on top of the Si NW (the gate oxide) via trapping and releasing of charge carriers at trap states near the semiconductor-oxide interface. Pud et al [9] demonstrated that the noise can be reduced by moving the conducting channel away from the top dielectric layer to the bulk of the Si NW, with the help of an appropriate back-gate potential. They used this effect for tuning the FET operation mode to increase the sensitivity of the fabricated biosensor structure by 50% and suggested that their results reflected a novel way to improve the sensitivity of biosensors by designing devices with controlled channel positioning.
Due to their specific architecture and conduction mechanism, junctionless nanowire transistors (JNTs) [10] naturally meet the above mentioned requirements for ultimate sensor sensitivity: even in the subthreshold mode, their conduction channel is positioned not at the surface close to the semiconductor-oxide interface as in the standard FETs but near the centre of the nanowire. JNTs have been proposed as a disruptive alternative of conventional metal-oxide-semiconductor field-effect transistors (MOSFETs) [10]. A JNT is essentially a gated resistor where the source, channel and drain have the same type of doping without any dopant concentration gradient (figure 1(a)). In the absence of the p–n junctions commonly found in MOSFETs, the current flow in a JNT is entirely determined by the gate potential, which controls the carrier density in the channel. Therefore, there are two important characteristic features of properly functioning JNTs: (i) the cross-section of the transistor channel is small enough to achieve full depletion of charge carriers in the off-state and (ii) heavy doping (up to 1–5 × 1019 cm−3) of the whole device (source, channel and drain) for high on-state currents. The JNT is the simplest transistor structure possible and probably the most scalable of all FET structures. Such a device is easier to fabricate than standard MOSFETs, since it does not require separate doping of source and drain regions and the very challenging formation of abrupt ultra-shallow p–n junctions. This fact, together with the number of performance advantages over MOSFETs [10, 11–13], has quickly attracted a vast interest in JNTs. However, their application as sensors, although very appealing, has not yet been extensively studied.
Currently, there are two main concepts for achieving the ultimate sensitivity (detection limit) with Si NW FET-type sensors: operating them (i) in the subthreshold mode [14] or (ii) in the above-threshold mode [7]. The operation mode of the sensors can be set by applying an appropriate back-gate potential, at the expense of increased power consumption. In the case of JNTs, however, their performance depends strongly on the geometry (height, width, and length) of the channel (NW) as well as on its doping level. Therefore, these parameters can be used to finely tune the operation point of devices into the appropriate mode without having to use an additional power supply, which makes them very energy efficient [15].
We have already implemented JNT-based devices as chemical and biological sensors. A series of experiments for sensing the ionic strength and the pH value of buffer solutions have proven the excellent sensitivity of these sensors [13, 16, 17]. In the present work we demonstrate sensing of the protein streptavidin at a concentration as low as 580 zM, which is by far the lowest concentration of this protein that has been detected to date and corresponds to detection in the range of only few molecules.
2. Experimental
The sensors were fabricated on silicon-on-insulator (SOI) wafers. Device layout and images of sample devices are shown in figure 1(b). To render the fabricated sensors sensitive exclusively to the protein streptavidin, the Si nanowires were functionalised with biotin. Their electrical characterisation was performed using a cascade manual probe station, both under dry and liquid conditions. Fluid delivery to devices was arranged with polydimethylsiloxane (PDMS) stamps (figure 2), polytetra-fluoroethylene (PTFE) tubing and a syringe pump. Details are given in the subsections below.
Figure 2. Microfluidic channels. (a) Schematic presentation of a group of 5 JNT sensors with a PDMS stamp containing a microfluidic channel. (b) Optical microscopy image of an SOI chip with nine groups of different JNT sensors and a PDMS stamp with a microfluidic channel bonded on top of three of the device groups. (c) Ag/AgCl reference electrode incorporated into a flow cell.
Download figure:
Standard image High-resolution image2.1. Fabrication of Junctionless Si NW devices
JNT sensors with various nanowire widths (10, 20, 30, and 50 nm), lengths (0.5, 1, and 10 μm) and numbers (1, 3, and 20 NWs) were fabricated on highly p-doped (1018 cm−3) SOI wafers with top Si device layer of 45 nm and buried oxide (BOX) layer of 145 nm SiO2 (see figure 1(b)). The patterning was done with electron beam lithography (EBL) using a high-contrast and low roughness development process for the negative tone resist hydrogen silsesquioxane (HSQ) [18, 19], followed by a chlorine (Cl2) chemistry based reactive ion etching (RIE). Then an additional 200 nm SiO2 layer was deposited on the whole surface except the device regions, to minimise the leakage current through the BOX. Next, the source/drain metal contacts, interconnects and contact pads were created by deposition of a 100 nm thick nickel (Ni) layer and thermal annealing for 30 min at 400 °C in forming gas (10% H2/90% N2). These two subsequent depositions were done by electron beam evaporation and were respectively combined with two steps of photolitho-graphy and lift-off. A final third step of photolithography was performed with 2 μm thick negative photoresist SU8, which was used to passivate the whole surface of the chips, except the device regions and the metal contact pads. The fabricated devices were inspected by both optical and scanning electron microscopes (SEM). Detailed description of the fabrication process is given elsewhere [17].
2.2. NW surface modification
To make the JNT sensors sensitive only to the protein streptavidin, the Si nanowires were functionalised with biotin. Streptavidin has a high affinity for biotin and they form a very strong non-covalent binding. The functionalisation was done in several steps. First, the SiOx nanowire surface was treated in a piranha solution (3:1 H2SO4:H2O2) to produce a clean hydroxyl-terminated surface. Next (3-Aminopropyl)triethoxysilane (APTES) was covalently attached to the nanowire surface by immersing the samples in a solution of 5 v/v % APTES in anhydrous toluene for 3 h at 50 °C. Then the samples were rinsed with anhydrous toluene, deionised (DI) water and dried under nitrogen (N2). The aminosilanised nanowires (Si-APTES) were subsequently immersed for three hours at room temperature in 2 ml phosphate buffered saline (PBS, pH 7.4) with added 100 μl of E, Z link-NHS-LC-Biotin in dimethylformamide (DMF) (1 mg ml−1). During this time, the NHS-LC-Biotin reacts with the primary amine of the surface-tethered silane to leave a biotinylated surface. Finally, surfaces were rinsed with PBS and DI water and dried under nitrogen. Contact angle measurements changed from 19° (highly hydrophilic) to ∼70° (less hydrophilic), indicating successful surface modification.
2.3. Electrical characterisation and fluid delivery
The electrical characterisation was done at room temperature using a cascade manual probe station and an Agilent B1500 semiconductor parameter analyser.
Efficient fluid delivery to the sensors was enabled by bonding polydimethylsiloxane (PDMS) stamps with a 150 μm width channel to the front surface of the chips (figures 2(a), (b)). Inlet/outlets access holes with diameters ∼400 μm were drilled on the top of the stamp to link the microfluidic channel to external PTFE tubing (figures 2(a), (b)). The tubing was relatively long (∼1.5 m) to enable administration and collection of the fluid from the outside of the probe station. The solutions containing the target analyte were delivered to each sensor at a rate of 100 μl min−1 with a syringe (BD Plastipak, 10 ml) propelled by a pump (Harvard, Pump 11+). From the syringe, the flow passed through PTFE tubing with inner diameter (ID) of 0.4 mm and outer diameter (OD) of 1.0 mm. Approximately 10 cm from exiting the syringe, the flow passed through a T-junction where a small amount of a different solution could be added into the main fluid flow as required. From the T-junction the flow continued along the PTFE tubing (∼1 m) into the probe station. Standard fittings from Upchurch Scientific were used for all connections. Inside the probe station the tubing was downsized to OD 0.4 mm and ID 0.1 mm PTFE tubing using interconnect junctions fabricated in-house from PDMS. The smaller tubing was required to interface to the PDMS stamp but could not be used for the entire length of the fluid supply lines, as excessive backpressure would be generated. The Si NWs were accessible through the small windows on the SU-8 passivation layer.
In some of the measurements under 'liquid conditions', an Ag/AgCl reference electrode (ALS RE-1S, 4 mm barrel diameter) was used to maintain a constant liquid potential. The reference electrode was incorporated into a flow cell and attached to the microfluidic channels by the PTFE tubing. The flow cell consists of a small volume chamber (∼1 μl) at the base of the electrode, which is supplied via a 1 mm diameter channel custom made in PDMS (figure 2(c)).
3. Results and discussion
Figure 1(b) shows an optical microscope image of a single SOI chip containing 45 different nanowire devices as well as a top SEM view of devices having 10, 20 and 30 nm NW width together with a transmission electron microscope (TEM) cross-sectional view of a device with 50 nm NWs. The images clearly demonstrate the excellent results of the patterning process, which delivered very smooth nanowires with dimensions closely matching the designed ones. This is an important prerequisite for the good performance of the sensing devices.
3.1. Electrical characterisation of the fabricated devices
To check the quality of the fabricated and biotinylated sensors, they were first electrically tested under dry conditions. Figure 3(a) shows a sample transfer characteristics of a device constructed from a single nanowire with a diameter of 50 nm and length of 1 μm. The drain current Id was measured as a function of the back-gate potential Vbg by sweeping Vbg forward (circles) and backward (triangles) between −20 and 10 V while keeping the drain potential Vd constant at Vd = 1 V. The two transfer characteristics overlap well, indicating the high quality of the fabricated sensors. The threshold voltage Vth shifts slightly towards the right indicating a small negative charge accumulation. Injected interface charges, which do not dissipate as the gate bias polarity changes, lead to a shift in the threshold voltage [20]. Hysteresis can affect the stability of the sensors causing short- and long-term drift of the sensor response [21]. The little hysteresis observed here (<0.33 V of the back-gate potential) indicates small defect-induced charge trapping at the interface Si/SiO2 [22].
Figure 3. Results of electrical measurements of the sensors. (a) Transfer characteristics (Id–Vbg curves under dry conditions) of a device having a single 50 nm nanowire of 1 μm length. The drain current Id was measured while sweeping the back-gate potential Vbg forward (circles) and backward (triangles) between −20 and 10 V at constant drain potential Vd = 1 V. (b) Transfer characteristics (Id–Vref curves under constant PBS flow) of a device having an array of 20 nanowires of 50 nm width and 10 μm length. The drain current Id was measured while sweeping the reference electrode potential Vref forward (circles) and backward (triangles) between −4 and 2 V at a constant drain potential of Vd = 1 V.
Download figure:
Standard image High-resolution imageAs a next step, a similar measurement was done while continuously flowing (100 μl min−1) PBS through the microfluidic channels aligned over the sensors. The PBS potential was controlled by the reference electrode. Figure 3(b) presents sample transfer characteristics of a device having an array of 20 nanowires of 50 nm width and 10 μm length. The drain current Id was measured as a function of the reference electrode potential Vref by sweeping Vref forward (circles) and backward (triangles) between −4 V and 2 V while maintaining a constant drain potential of Vd = 1 V. Here the two transfer characteristics overlap almost perfectly, which is yet another demonstration of the excellent quality of the fabricated and functionalised sensors.
3.2. Sensing experiments
In this work, streptavidin-biotin has been chosen as a model target-probe pair, with streptavidin in solution being sensed using biotin covalently tethered to the NW surface. This target-probe pair is well known, has one of the strongest non-covalent binding interactions in nature, and very fast association kinetics. It is widely used for evaluation of the sensing performance of various sensors and is, therefore, appropriate for comparison of different sensor concepts. The Si nanowires functionalisation with biotin is described in section 2.2.
In the first series of streptavidin sensing experiments, the drain current Id was measured as a function of the back-gate potential Vbg by sweeping Vbg forward and backward between −20 and 5 V ten times at constant drain potential Vd = 1 V while continuously flowing (100 μl min−1) plain PBS over biotinylated sensors. The measurements of a device with an array of 20 NWs of 0.5 μm length and 50 nm width resulted in on-currents Ion of ∼1.5 μA and off-currents Ioff of ∼0.15 nA (Ion/Ioff ratio of ∼104) as shown in figure 4(a). Then PBS solution with relatively high concentration of streptavidin (0.4 μM) was flowed at the same rate over the sensors instead of the pure PBS and again a series of ten consequent Id–Vbg measurements was done at identical conditions. The obtained Id–Vbg curves for the same device have an obviously much smoother subthreshold slope (SS) and much higher currents, Ion ∼ 10 μA and Ioff ∼ 0.1 μA (Ion/Ioff ratio of ∼102), see figure 4(a). These results indicate successful binding of streptavidin molecules to the biotinylated NW surfaces. Since streptavidin has a weakly acidic isoelectric point (Pi ∼ 5.6) [22], its molecules are negatively charged when dissolved in a PBS solution of pH = 7.4, as in our case. Their binding to the nanowire surfaces acts as a negative gating of the sensor and caused accumulation of majority carriers (holes) in the p-type device channel. Therefore, a significant increase of drain current was observed: up to a factor of 10 for the Ion and a factor of 103 for the Ioff (figure 4(a)).
Figure 4. Results of the sensing experiments. (a) Transfer characteristics (Id–Vbg curves under constant flow of pure PBS or PBS with high concentration of streptavidin) of a biotinylated device having an array of 20 NWs of 0.5 μm length and 50 nm width. The drain current Id was measured while sweeping the back-gate potential Vbg forward and backward ten times between −20 and 5 V at constant drain potential Vd = 1 V. (b) Time dependence of drain current Id for a biotinylated sensor having an array of 20 nanowires of 50 nm width and 10 μm length and biased at Vref = −1.5 V and Vd = 1 V. The drain current Id was measured under a steady stream of pure PBS (100 μl min−1) with periodical injections of small amounts (100 μl) of PBS solutions with increasing streptavidin concentrations (shown in the figure). The dashed line (red online) extrapolates the possible current drift before the first streptavidin injection, while the solid line (light blue online) connects the 'valley' current values after each streptavidin injection.
Download figure:
Standard image High-resolution imageAs a final step, solutions of different streptavidin concentrations in PBS (pH = 7.4) were prepared from a solution with an initial molarity of ∼380 nM by consecutively diluting it in PBS by a factor of 30 eight times until an extremely low molarity of ∼580 zM (580 × 10−21 M) was achieved. During the sensing experiment, a steady stream of pure PBS was flowed through the microfluidic channel at a rate of 100 μl min−1 and periodically small amounts (100 μl) of nine different solutions with increasing streptavidin concentrations (580 zM, 17 aM, 520 aM, 16 fM, 370 fM, 14 pM, 420 pM, 13 nM and 380 nM) were administered one by one into this steady stream, while continuously measuring the drain current Id of the sensing device. The reference electrode was used to maintain constant potential of the solutions. In figure 4(b), Id of a biotinylated sensors having an array of 20 nanowires of 50 nm width and 10 μm length and biased at Vref = −1.5 V and Vd = 1 V is shown as a function of time. First, as a control experiment, 100 μl of plain PBS were injected separately into the main channel solution at the time mark of ∼900 s (figure 4(b)), by the use of a T-junction. From the T-junction, the flow continued along the PE tubing (∼1 m) into the probe station. As expected, this injection caused no significant change in the device current, see figure 4(b). This is a clear proof that all the subsequent signals obtained by the sensor are caused exclusively by streptavidin attachment to the nanowire surface.
After the initial plain PBS injection, 100 μl PBS solution with the lowest streptavidin concentration of 580 zM was injected into the main channel solution at the time mark of ∼1100 s (figure 4(b)). A remarkable response of the sensor to this extremely low streptavidin concentration can be clearly seen in figure 4(b). Following this first streptavidin injection, the microfluidic channel and the device were rinsed by plain PBS for ∼3–4 min. Then, the PBS solutions with increasing streptavidin concentrations were introduced step by step within the continuous stream of pure PBS buffer. After each streptavidin injection, the microfluidic channel and the sensor were consequently rinsed from potentially unbound streptavidin molecules by plain PBS for the same time of ∼3–4 min. With each increasing streptavidin concentration, an increasing response of the sensor is clearly observed in figure 4(b) up to the highest concentration of 380 nM.
As mentioned above, the remarkable responses of the sensor to the streptavidin injections are reflective of the protein attached to the nanowire surface. The possible sensor response to the charged streptavidin molecules passing close to the surface of the nanowires might be ruled out for the following reason. In our experiments we used a solution of PBS with pH = 7.4 and medium range concentration of 10 mM, where the Debye screening length is λD ≈ 3.1 nm. This means that the unbound streptavidin molecules passing by the surface of the nanowires at a distance >3.1 nm will not have any influence on the sensor current. The ones that come closer then 3.1 nm will either bind to the biotinylated surface of the nanowires because of the extraordinarily high affinity of streptavidin for biotin or non-covalently attach to the nanowire surface. Such a non-covalent attachment might be indicative of incomplete biotinylation of the APTES layer and may be facilitated by the expected positive charge of this layer at pH = 7.4 due to the partial protonation of its amine groups [23]. The low positive potential (in the range of few millivolts) of the APTES layer can electrostatically attract and capture the negatively charged (at pH = 7.4) streptavidin molecules. These molecules, together with the covalently bound ones, most probably cause the high peaks in the measured current observed in figure 4(b). However, the non-covalent attachment of streptavidin is weaker than the covalent binding and streptavidin molecules that are not bound to biotin can be washed off by the constantly flowing PBS. This may be the reason for the current drops after the streptavidin injections have passed over the sensor (figure 4(b)). In such a case, the 'peak' current values are indicative of streptavidin molecules both covalently and non- covalently attached to the nanowire surface, whereas the 'valley' current values should be indicative of streptavidin molecules covalently bound to biotin.
The dashed line (red online) in figure 4(b) extrapolates the possible current drift before the first streptavidin injection, while the solid line (light blue online) connects the 'valley' current values after each streptavidin injection. When one compares the two lines, it becomes obvious that even at the level of those valleys there is a significant jump in the measured current already after the first injection with the extremely low streptavidin concentration of 580 zM, which cannot be attributed to a continuous current drift. Therefore, one could convincingly conclude that the sensor response in our case is indeed reflective of streptavidin molecules binding to the biotinylated nanowire surface.
In one of our previous works [6], we performed similar experiment using another type of sensors, namely 3D vertically stacked Si nanowire FETs. In spite of the fact that the sensing devices were quite different from the current ones and had the opposite doping (n-type instead of p-type), the results were comparable with the ones we present here and we were able to detect streptavidin at a concentration as low as 17 aM. This might be considered as an indirect proof that streptavidin can indeed be reproducibly and reliably detected at these low concentrations.
Plots of the peak current values and valley current values for each streptavidin injection as functions of the respective streptavidin concentrations are presented in figure 5. Logistic fits of the two dependences were suggested with the function
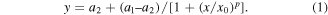
The logistic function is widely used, among others, in pharmacy and chemistry to model the concentration of reactants and products in autocatalytic reaction.
Figure 5. Plots of the dependencies of peak current values and valley current values for each streptavidin injection on the respective streptavidin concentrations.
Download figure:
Standard image High-resolution imageThe plots in figure 5 show the distinct increase of drain current with the increase in the streptavidin concentration. There is, however, some decrease in the increment of the sensing signal current for each subsequent injection from about 25% between the first two injections to about 15% between the injections 7 and 8. This might be caused by a partial saturation of the binding sites of the self-assembled biotin monolayer. There is also a slight decrease in the peak current for the last injection of streptavidin with the highest concentration of 380 nM. It is difficult to speculate what could be the reason for this but it is definitely not a result of breaking streptavidin bonds to biotin. A possible explanation might be either oversaturation of the biotin monolayer or a partial desorption of this layer.
In figure 5 it is clearly seen that the slopes of the two curves are different: the dependence of the peak currents on streptavidin concentration is steeper than the one of valley currents. This indicates that different phenomena determine the values of the peak currents and the valley currents. As discussed above, the peak current values are most probably reflective of streptavidin molecules attached both covalently and non-covalently to the nanowire surface, whereas the values of the valley currents are reflective of streptavidin molecules covalently bound to biotin. If they were a result just of a current drift with time, they would have the same slope. This is yet another proof that the sensor response in our case is indeed reflective of streptavidin molecules binding to the biotinylated nanowire surface.
To the best of our knowledge, the concentration of 580 zM is the lowest streptavidin concentration ever detected by any kind of sensor. At this concentration, the number of streptavidin molecules in the injected volume of the PBS solution (100 μl) was ∼35. The upper limit estimation of the microfluidic channel volume is (W × H × L): 200 μm × 200 μm × 1 cm = 0.4 μl. This means that we had in average only one molecule (statistically less than one molecule) in the microfluidic channel at any time. In addition, the functionalisation approach used in this work implies that surface modification with APTES/biotin happens not only at the nanowires but also at the area around them, which is much larger than the NW surface itself. This fact leads to the existence of a vast number of competing sites outside the nanowires, which are capable of binding the target molecules. Statistically, the number of the binding sites outside the nanowires is greater than the number of the binding sites at the nanowires with a ratio much higher than 35:1. Moreover, due to the constant flow of the PBS solution, streptavidin has only a limited time to bind to the nanowires before the unbound molecules are carried away by the flowing PBS. Taking into account all these factors, it is possible to state with a great certainty that at the concentration of 580 zM we most probably detected only few streptavidin molecules closly approaching detection at the single molecule level.
The observed superior performance of JNT sensors might be due to at least two advantages of JNTs over the classical MOSFETs [10–13], which are especially important for their application as sensors:
- (i)The current flow in JNTs is not controlled by a reverse biased p–n junction as in standard MOSFETs but entirely by the gate potential, which modulates the carrier density in the channel. Therefore, they are more sensitive to any change in the electrostatic potential on the channel surface acting as a gate potential.
- (ii)JNTs demonstrate bulk conductance near the centre of the channel (NW), in contrast to the conductance in a thin surface inversion or accumulation layer near the gate in the inversion mode (IM) or accumulation mode (AM) transistors, which leads to higher drive currents. Moreover, this fact makes the modulation of depletion and the conduction in JNTs less affected by the noise-inducing parasitic surface states than in the case of conventional MOSFETs, which is very important for achieving high signal-to-noise ratio and hence the ultimate detection limit of a single molecule [7–9].
4. Conclusions
Detection of the protein streptavidin at concentration as low as 580 zM is demonstrated for the first time using junctionless nanowire transistors operated as FET-type Si NW sensors. At the particular experimental conditions of the present work, this concentration corresponds to detection of only few molecules, nearly approaching the fundamental limit of a chemo-/biosensor of detection at a single molecule level. As indicated above, the excellent sensing capabilities of JNTs are due to the fact that their performance is, on one hand, more sensitive to any change in the electrostatic potential on the channel surface and, on the other hand, is less susceptible to the noise-inducing parasitic surface states than the performance of standard MOSFETs. This is very important for achieving high signal-to-noise ratio towards the ultimate detection limit of a single molecule. Thus the performance advantages of JNT sensors over the standard FET-type Si NW sensors, together with their very simple structure and relaxed fabrication process make them excellent candidates for low-cost mass production by the conventional semiconductor technology. Therefore, the results presented here can open the way to numerous applications of JNT sensors in various fields where fast, low-cost, label-free, low-volume and real-time detection of chemical and biological species at the fundamental limit of a single molecule is required.
Acknowledgments
The authors would like to acknowledge funding from the EU 7th Framework Programme under the SiNAPS project (no. 257856) and the Science Foundation Ireland (SFI) under the grant no. 09/IN.1/I2602.






