Abstract
We design a facile approach to prepare a bimetallic transition-metal-sulphide-based 3D hierarchically-ordered porous electrode based on bimetallic metal-organic frameworks (Ni–Co–MOFs) by using confinement growth and in-situ sulphurisation techniques. In the novel resulting architectures, Ni–Co–S nanoparticles are confined in bowknot-like and flower-like carbon networks and are mechanically isolated but electronically well-connected, where the carbon networks with a honeycomb-like feature facilitate electron transfer with uninterrupted conductive channels from all sides. Moreover, these hierarchically-ordered porous structures together with internal voids can accommodate the volume expansion of the embedded Ni–Co–S nanoparticles. The pseudocapacitive behaviours displayed in the NCS@CBs and NCS@CFs occupied a significant portion in the redox processes. Because of these merits, both the as-built bowknot and flower networks show excellent electrochemical properties for lithium storage with superior rate capability and robust cycling stability (994 mAh g−1 for NCS@CBs and 888 mAh g−1 for NCS@CFs after 200 cycles). This unique 3D hierarchically-ordered structural design is believed to hold great potential applications in propagable preparation of carbon networks teamed up with sulphide nanocrystals for high energy storage.
Export citation and abstract BibTeX RIS
1. Introduction
Lithium-ion batteries (LIBs) are a critical technology for portable electronic devices, electrical vehicles and hybrid electric vehicles as well as electrical grids [1–4]. This motivates the intensive search for new electrode materials that are able to deliver higher specific capacities, a longer life cycle and improved rate capability. The dominant anode material in commercial batteries, graphite, has a limited theoretical lithium storage capacity (372 mAh g−1) that cannot meet the increasing requirements for high energy storage devices. Other potential anode materials that exploit conversion reaction mechanisms exhibit higher theoretical capacities for lithium storage. Transition metal sulphides are a particularly promising class of these conversion anodes [5–12]. Compared to commercial graphite, transition metal sulphides have attractive properties for high energy storage, including high specific capacity, economic affordability and safety. However, the large volume changes and structural transitions associated with the conversion reaction mechanisms lead to pulverisation followed by rapid capacity degradation [12, 13]. They also suffer from poor intrinsic electrical conductivity, which hampers the potential of fast diffusion at high power densities [7]. Current approaches to improving the electrochemical properties of transition metal sulphides focus on reducing diffusion length, boosting electrical conductivity and the lithium diffusion coefficient, and suppressing the volume variation through nanostructured design and hybridisation with conductive materials. Despite a great deal of work on nano-structuring, the as-reported electrochemical properties of transition metal sulphides remain unsatisfactory with respect to rate capability, reversible capacity and capacity retention. This is due to the agglomeration of active materials and the polysulfide shuttling effect, additional side reactions between sulphides and electrolytes, and poor conductivity compared with bulk materials [14]. An alternative approach, involving hybridisation with carbonaceous materials to synthesise 3D hierarchical ordered architectures built from micro/nanostructures, has been identified as an effective strategy to enhance the electrochemical performance of anode electrodes [5, 8].
Metal-organic frameworks (MOFs) are a burgeoning class of porous materials with tunable structures, composition, and porosity. They are encouraging sacrificial precursors for synthesising various metal-based nanoparticle/carbon hybrid materials, because they can be simultaneously transformed into metal-based nanoparticles and carbon frameworks [15–25], the former dispersed in the latter and avoiding agglomeration with shortened diffusion lengths. The carbon frameworks efficiently buffer mechanical stress of the nanoparticles and facilitate electron transfer, thereby improving cycling performance and boosting rate capability. An impediment to progress is a lack of available bimetallic MOFs with appropriate hierarchical morphology, that can be synthesised in one-step processes. Bimetallic transition metal-rich MOFs with unique structures that effectively template the formation of bimetallic transition metal sulphide/carbon composites would open a feasible route to enhanced electrochemical performance.
Here, we propose a simple and innovative self-templating strategy for the fabrication of Ni–Co–S/C micro/nanostructures. By using confinement growth and in-situ sulphurisation techniques, porous NiCo2S4/NiS/C bowknots and flowers (denoted as NCS@CBs and NCS@CFs, respectively) were obtained. Importantly, the carbon bowknots and flowers have honeycomb-like features, which can provide more active sites for lithium ion storage. In virtue of the unique micro/nano-structures, the bimetallic transition metal sulphide/carbon composites delivered high rate performance and outstanding cycling performance when served as potential anode electrodes for LIBs.
2. Experimental
2.1. Synthetic procedures
The Ni–Co–1, 3, 5-benzenetricarboxylate (referred as Ni–Co–BTC, C18H12O15M3 · 12H2O (M=Ni,Co)) bowknot-like and flower-like structures (denoted as NCBTCBs and NCBTCFs, respectively) were prepared based on a surfactant-assisted coprecipitation method. The synthetic strategy is displayed in scheme
Scheme 1. Schematic illustration of the synthesis process of NCBTCBs and NCBTCFs.
Download figure:
Standard image High-resolution imageThe as-prepared Ni–Co–BTC precursors were initially heated to 500 °C in a tube furnace with a ramp of 2 °C min−1 under a nitrogen flow and maintained for 1 h. After being naturally cooled down to room temperature, the black products with different morphology were collected and denoted as NC@CBs and NC@CFs, respectively. Then 110 mg of the as-prepared black products were placed in a tube furnace with 550 mg sulphur powder and further heated in an argon flow for 2 h at 600 °C with a heating ramp of 5 °C min−1. The corresponding products were denoted as NCS@CBs and NCS@CFs, respectively.
2.2. Materials' characterisation
X-ray powder diffraction (XRD) measurements were recorded on a PANanalytical x-ray diffractometer (Cu Kα source, λ = 1.541 78 Å). The scanning electron microscopy images were recorded by a Carl Zeiss Ultra field-emission scanning electron microscopy (FESEM) unit equipped with energy dispersive spectroscopy (EDS). The morphology and microstructure of the materials were further conducted on a JEOL 2200 TEM with an acceleration voltage of 200 kV. X-ray photoelectron spectrophotometer (XPS) measurements were carried out with a Thermo Fisher ESCALAB 250 Xi XPS instrument to characterise the element composition and valence state. The surface area of the NCS@CBs and NCS@CFs material was investigated by an Autosorb iQ Station 2 instrument at 77 K. Thermogravimetric analyses (TGA, Discovery Instrument) were performed under a nitrogen flow from 50 °C to 700 °C at a heating rate of 5 °C min−1. The Raman spectra were collected with a Renishaw RM2000 Raman spectrometer at room temperature.
2.3. Electrochemical measurements
To obtain the working electrodes, a slurry mixture of the active materials, super P and carboxymethyl cellulose (8:1:1 in mass ratio) was coated on copper foils and then dried at 80 °C for 12 h in a vacuum oven. The loading mass of the electrodes was around 1.6–2.0 mg cm−2. The electrochemical properties of the active materials were carried out using CR 2032 coin-type half cells assembled in an Ar-filled glove box (H2O, O2 < 1 ppm). The metallic lithium foils served as the reference and counter electrodes, where 1 M LiPF6 in a 1:1 (v/v) mixture of ethylene carbonate and dimethyl carbonate with 5 wt% flouroethylene carbonate (FEC) worked as the electrolyte. The galvanostatic measurements were evaluated on a LAND-CT2001A batteries tester at room temperature at different current densities within a 0.01–3.00 V (versus Li/Li+) voltage window. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS, 100 kHz–0.01 Hz) were performed on a CHI660D electrochemical workstation at room temperature.
3. Results and discussion
The fabrication process of NCS@CBs and NCS@CFs involves three main steps. Firstly, we chose bimetallic MOFs (Ni–Co–BTC) with the bowknot-like and flower-like morphology, simultaneously including Ni, Co and C elements, as the precursors to obtain Ni–Co/C hybrids. Figure 1 shows the FESEM images of the bimetallic MOFs with the bowknot-like and flower-like morphology. The uniform Ni–Co–BTC bowknots (denoted as NCBTCBs) with an average diameter of 18 μm were comprised of numerous blade-like nano-sized substructures, while the Ni–Co–BTC flowers (denoted as NCBTCFs) with an average diameter of 32 μm consisted of copious microrods. The corresponding XRD pattern can be well indexed to the simulated XRD patterns of M3(BTC)2 · 12H2O (M=Co, Ni) (CCDC 1274034), as shown in figure S1, which is available online at stacks.iop.org/NANO/30/155701/mmedia.
Figure 1. Typical FESEM images at different magnifications of (a)–(c) NCBTCBs; (d)–(f) NCBTCFs.
Download figure:
Standard image High-resolution imageThen the bimetallic MOFs were directly annealed into the intermediate precursor under an argon atmosphere based on the results of TGA (figure S2). The corresponding FESEM images of the NC@CBs and NC@CFs at different magnifications indicate that the resulting structures have wholly inherited the original morphology, whereby considerable metallic nanoparticles are homogeneously embedded in carbon bowknots or flowers (figure S3). The XRD patterns of these samples confirm that the as-prepared MOFs were totally converted to metallic Ni and Co particles at a high temperature (550 °C) (figure S4). Consequently, these intermediate precursors reacted with a certain amount of sulphur at a high temperature (600 °C) under an argon atmosphere to achieve the desired products (NCS@CBs and NCS@CFs). It therefore appears that this self-template method provides a facile route to novel and complicated bowknot-like and flower-like micro/nanoarchitectures.
The crystalline nature and chemical composition of the NCS@CBs and NCS@CFs were then investigated by XRD and XPS. As shown in figure 2(a), the diffraction peaks correspond to the cubic NiCo2S4 phase (JCPD card no. 20-0782) and hexagonal NiS (JCPD card no. 02-1280), indicating that the Ni–Co/C bowknots and flowers have been entirely transformed into Ni–Co–S/C bowknots and flowers. XPS analysis (figure 2(b)) shows that Ni, Co, S, C and O are present in the NCS@CBs and NCS@CFs, while the O is distributed differently depending on exposure of the samples to air. As shown in figures 2(c) and (d), the Ni 2p and Co 2p high-resolution spectra can be fitted to Gaussian peaks with two spin–orbit doublets and two shakeup satellites (Sat.). The binding energies situated at around 853.3 and 870.5 eV of the Ni 2p spectrum can be assigned to Ni2+, while the binding energies located at 857.0 and 874.9 eV correspond to Ni3+ [26–28]. Likewise, in the case of the Co 2p spectrum, two spin–orbit doublets detectable at 778.7 and 793.8 eV are characteristic of Co3+, whereas those at 780.4 and 798.0 eV are associated with Co2+ [27, 28]. Notably, the coexisting Ni3+/Ni2+ and Co3+/Co2+ cations in the electrode materials' composite supply multiple active sites for lithium storage. The high-resolution S 2p spectrum (figure 2(e)) can be fitted by deconvolution into four peaks and two shake-up satellite peaks, with the binding energies at 162.6 eV and 161.5 eV attributed to S 2p1/2 and S 2p3/2, respectively [6, 27]. The component peak at about 163.6 eV is associated with metal–sulphur bond in the NCS@CBs, while the final component peak at 164.5 eV is due to sulphur ions in low coordination states at the surface [29, 30]. The high-resolution C 1s spectrum displays two prominent peaks at about 284.6 and 285.3 eV, corresponding to sp2 C and sp3 C, respectively (figure 2(f)). The carbon structure in the NCS@CBs and NCS@CFs was also investigated by Raman spectrum, in which two broad peaks located at 1350 and 1570 cm−1 could be assigned to typical D- and G-bands of carbon, respectively (figure S5). The D-band features the breaking symmetry originating from defects and disorders, and the G-band corresponds to the in-plane tangential stretch vibration mode of the graphitic layer [31]. The relative intensity ratio between the D- and G-bands (ID/IG) in the Raman spectra were about 0.80 and 0.61, respectively, implying the partially graphitised nature of the MOF-derived carbon in the NCS@CBs and NCS@CFs [32]. This is conducive to succeeding in better electronic conduction between adjacent metal sulphide nanoparticles [33, 34].
Figure 2. (a) XRD patterns and (b) XPS survey spectra of NCS@CBs and NCS@CFs; high-resolution spectra of (c) Ni 2p, (d) Co 2p, (e) S 2p and (f) C 1s for NCS@CBs.
Download figure:
Standard image High-resolution imageThe morphology and detailed nanostructures of the NCS@CBs and NCS@CFs samples were further elucidated by FESEM, TEM and element analysis through energy-dispersive x-ray spectroscopy. Figures 3(a)–(f) exhibit the SEM images of the NCS@CBs and NCS@CFs at different magnifications. The microsized carbon bowknots and flowers were still observed without collapse at low magnification, demonstrating the robustness of their structure. At high magnification, these microsized carbon bowknots consist of blade-like carbon building blocks and Ni–Co–S nanoparticles, while microsized carbon flowers contain carbon fibres and substantial Ni–Co–S crystals. Notably, these transitional metal sulphide nanoparticles are anchored into porous carbon networks, which can accommodate the volume change on cycling and avoid the aggregation of nanoparticles formed during lithiation/delithiation. The formation of the porous structure and void space is assigned to the volatilisation of CO2 and H2O from the MOFs during the annealing process and the well-designed hierarchy. This architecture is distinct from the previously reported MOF-derived structures.
Figure 3. Typical FESEM images at different magnifications of (a)–(c) NCS@CBs; (d)–(f) NCS@CFs.
Download figure:
Standard image High-resolution imageThe TEM images confirm that the Ni–Co–S nanoparticles are embedded into porous carbon networks (figure 4), and consistent with our SEM results. Notice that the carbon networks have honeycomb-like features, as presented in the insets of figures 4(a) and (d), which should favour lithium ion storage [35–39]. In the high-resolution TEM images, the characteristic lattice fringes with the inter-planar spacing of 0.33 nm and 0.195 nm are consistent with the (220) plane of NiCo2S4 and the (102) plane of NiS, respectively. The amorphous carbon coated on the surface of nanoparticles can also be detected.
Figure 4. (a) TEM image, (b), (c) HRTEM images of NCS@CBs; (d) TEM image, (e), (f) HRTEM images of NCS@CFs.
Download figure:
Standard image High-resolution imageThe elemental mapping analysis by EDS (figure S6) further shows that the Ni–Co–S nanoparticles are uniformly anchored in 3D carbon networks, demonstrating that this architecture integrates features of micro- and nano-structures. Nitrogen adsorption measurements of the NCS@CBs and NCS@CFs show a reversible type-IV isotherm with a typical hysteresis loop indicative of mesopores in the carbon networks (figures S7(a) and (b)). The specific surface area of NCS@CBs is high (estimated ∼26 m2 g−1 according to the Brunauer–Emmett–Teller method), which is beneficial for lithium ion diffusion. In contrast, the NCS@CFs sample shows a smaller surface area of 10 m2 g−1. The Barrett–Joyner–Halenda pore size distribution curves shown in the inset of figure S7(a) indicate that NCS@CBs contains 4 nm mesopores with a narrow size distribution, while NCS@CFs contains 4 and 7 nm mesopores (see the inset of figure S7(b)).
The NCS@CBs and NCS@CFs electrodes were evaluated as anode materials in a half-cell of LIBs. Figure 5(a) displays the cyclic voltammograms of NCS@CBs and NCS@CFs in the potential window between 0.01 and 3.0 V at a scan rate of 0.1 mV s−1 during the first several cycles. The cathodic peaks in the initial cathodic scan located at 1.66, 1.39 and 1.07 V can be ascribed to Li+ insertion into the NiCo2S4 and NiS crystal, to form LixNiCo2S4 and Ni3S2, respectively, and the subsequent conversion reaction to form Ni, Co and a Li2S matrix [13, 14, 28, 30]. The weak reduction peaks located in the range 0.3–0.8 V correspond to the formation of a solid-electrolyte interface film (SEI). The anodic peaks detected at 2.11 and 2.49 V arise from the delithiation reaction and sulphuration of Ni and Co to NiSx and CoSx and the synthesis of polysulfide intermediates, respectively. In the second cycle, the main cathodic peaks shift from 1.66 to 1.70 V and from 1.07 to 1.27 V due to irreversible structural changes to NiCo2S4 after the first cycle. Likewise, cyclic voltammograms for NCS@CFs shows similar features to NCS@CFs, as shown in figure 5(b). In subsequent cycles, the reduction and the oxidation peaks both overlap with those of the second cycle, showing the good reversibility of these electrochemical reactions and the high stability of the NCS@CBs and NCS@CFs electrodes. These 3D architectures yield superior battery performance for the NCS@CBs and NCS@CFs electrodes.
Figure 5. CV curves of NCS@CBs (a) and NCS@CFs (b). Galvanostatic charge–discharge curves of NCS@CBs (c) and NCS@CFs (d).
Download figure:
Standard image High-resolution imageFigures 5(c) and (d) illustrate the discharge/charge profiles of the NCS@CBs and NCS@CFs for the first several cycles at a current density of 0.2 A g−1. As shown in figure 5(c), the NCS@CBs electrode delivers an initial discharge capacity of 1089 mA h g−1 at a current density of 0.2 A g−1, corresponding to a high Coulomb efficiency (CE) of 76%. The Coulombic efficiency reaches 99% after a few cycles. Similar behaviour is seen in the voltage profiles of the NCS@CFs. We ascribe the as-observed initial irreversible capacity loss to the formation of the SEI films on the active materials, in addition to electrolyte decomposition. As for metal oxides, the NCS@CBs and NCS@CFs show large voltage hystereses upon insertion and deinsertion of Li+, due to the multiphase nature of the conversion-type reaction.
Cycle performance—i.e., rate capability at different current densities—is a significant criterion for measuring the electrochemical properties of electrodes in LIBs. Figures 6(a) and (b) show the cycling performance for the NCS@CBs and NCS@CFs electrodes at a constant current density of 0.2 A g−1. From figure 6(a), the Coulombic efficiency rises beyond 94% to 99% after a few cycles. Simultaneously, these electrodes exhibit a stable cycling performance with a high CE of over 99% and exhibit excellent cyclic retention, delivering a reversible discharge capacity of 994 mAh g−1 for the NCS@CBs and 888 mAh g−1 for the NCS@CFs after 200 cycles. As shown in figures 6(c) and (d), the rate capability of the NCS@CBs and NCS@CFs was recorded by organising the charge/discharge measurements at various current densities. The electrodes formed from the NCS@CBs and NCS@CFs were both found to deliver outstanding capacity retention with the increase of the current densities from 0.1 to 4 A g−1. Furthermore, when the current density is reduced back to 0.1 A g−1, the reversible capacity is recovered to the original value and exhibits a high specific capacity of 949 mA h g−1 for the NCS@CBs and 927 mAh g−1 for the NCS@CFs after 60 cycles. These two electrodes can deliver stable capacities of 914 (912) mAh g−1 for NCS@CBs (NCS@CFs) at 0.2 A g−1; 850 (840) mAh g−1 for NCS@CBs (NCS@CFs) at 0.5 A g−1; 784 (769) mAh g−1 for NCS@CBs (NCS@CFs) at 1 A g−1; 708 (695) mAh g−1 for NCS@CBs ( NCS@CFs) at 2 A g−1; 594 (597) mAh g−1 for NCS@CBs (NCS@CFs) at 4 A g−1; and 403 (398) mAh g−1 for NCS@CBs (NCS@CFs) at 8 A g−1. Average discharge capacities of 207 mA h g−1 for the NCS@CBs and 171 mAh g−1 for the NCS@CFs are achieved even at high current densities of 16 A g−1. More importantly, the reversible capacity of the electrode can recover to its initial capacity value of 1007 mAh g−1 for the NCS@CBs and 943 mAh g−1 for the NCS@CFs at 0.1 A g−1 after two successive sequence measurements. These two electrodes also exhibit a remarkable cyclic stability for lithium storage at a higher C rate of 0.5 A g−1 for the subsequent 100 cycles that followed the twice successive sequence measurements. The above results confirm the impact of the 3D hierarchical micro/nanostructure design, which endows these anode materials with highly efficient lithium-ion/electron transport channels that deliver superior rate capacity compared to the previously reported sulphides [14, 28, 30, 40].
Figure 6. Cyclic performance of the NCS@CBs (a) and NCS@CFs (b) at the current density of 0.2 A g−1; typical rate capabilities of the NCS@CBs (c) and NCS@CFs (d) at various current densities.
Download figure:
Standard image High-resolution imageAdditionally, to further understand the electrochemical behaviours of both electrodes, electrochemical impedance spectra (EIS) were employed and the Nyquist plots are shown in figure 7(a), where the semicircle at the high and middle frequency is related to the SEI layer resistance and charge-transfer resistance (Rct). The slope line in the low frequency region is associated with the Warburg impedance of the lithium-ion diffusion. The NCS@CBs electrode represents smaller charge-transfer resistance compared to the NCS@CFs electrode, indicating the enhanced conductivity of the NCS@CBs electrode. The Warburg line of the NCS@CBs, which displays a slope at a larger angle, exhibits superior reversible capacity over the NCS@CFs. The differences in the Warburg line indicates the presence of more capacitive behaviours in the NCS@CBs electrode, which could contribute to enhanced rate performance [41]. The Li+ ion diffusion coefficient for the NCS@CBs and NCS@CFs electrodes can be compared according to the following equation:
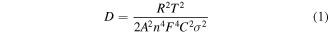
where R is the gas constant, T is the absolute temperature (K), F is the Faraday constant, A is the surface area, n is the number of electrons during the process of Li+ ion transportation, C is the molar concentration of Li+ ion and σ is the Warburg coefficient related to Zre. The Warburg diffusion can be obtained in the low frequency region (0.1 Hz–0.01 Hz) of the EIS result through the following equation:
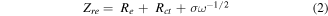
in which Re is the resistance of the electrolyte, Rct is the charge transfer resistance and ω is the angular frequency in the low frequency region (ω = 2πf). Both Re and Rct are kinetic parameters independent of frequency. Therefore, σ is the slope for the plot of Zre versus the reciprocal square root of the angular frequency (ω−1/2), which are shown in the figure 7(b). The Li+ ion diffusion coefficient (D) of NCS@CBs is relatively larger than that of NCS@CFs as the slope σ of NCS@CBs is much smaller, indicating that there is a faster lithium ion diffusion process in NCS@CBs. This result is consistent with the corresponding GITT test, which was used for elucidating the ion diffusion and conductivity properties of the NCS@CBs and NCS@CFs electrodes at the specific lithiation/delithiation states. The diffusion coefficients (Dapp,Li) was calculated based on the following equation:
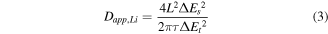
where L stands for lithium ion diffusion length (for a compact electrode, it corresponds to the thickness of electrode), ΔEs indicates the steady-state potential change (V) by the current pulse, τ signifies the relaxation time (s), and ΔEt denotes the potential change (V) during the constant current pulse after eliminating the iR drop (figure S8). Figure 7(c) represents GITT curves for the NCS@CBs and NCS@CFs electrodes, in which the NCS@CBs electrode possesses a higher average diffusion coefficient, which is beneficial for the performance rate of the batteries.
Figure 7. (a) Electrochemical impedance spectra (EIS) of NCS@CBs and NCS@CFs tested at open circuit potential; (b) the variations and fittings of Zre and ω−1/2 in the low-frequency region of NCS@CBs and NCS@CFs; (c) GITT curves and the corresponding Li+ diffusion coefficient at discharge/charge states of NCS@CBs and NCS@CFs.
Download figure:
Standard image High-resolution imageTo investigate the pseudocapacitance effect of NCS@CBs and NCS@CFs, cyclic voltammetry measurements were carried out to further interpret the lithium storage behaviour at different scan rates from 0.1 and 4 mV s−1 (figure 8). We found that the lithium storage exhibits both non-Faradaic and Faradaic behaviours in these samples, based on the observation that the peak current is not proportional to the square root of the scan rates [16, 42–46]. It is widely recognised that there is a power law relationship between the measured peak current (i) and the sweep rate (v) in the sweep voltammetry test.

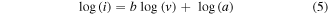
where a and b represent variables parameters, and b determines the type of Li+ insertion/extraction. When b = 0.5, the electrochemical reaction of the electrode is governed by ionic diffusion. The process is wholly governed by pseudocapacitance when b = 1. As shown in figure 8(b), the three redox peaks in NCS@CBs can be fitted to b = 0.79, 0.83 and 0.65 (for NCS@CFs in figure 8(d), b = 0.80, 0.77 and 0.66), indicating pseudocapacitive behaviour in both cases [25].
Figure 8. CV curves at different scan rates after five cycles of the NSC@CBs (a) and NSC@CFs (b) electrodes. The fitted lines and ln (peak current) versus ln (scan rate) plots at different oxidation and reduction states for the NSC@CBs (c) and NSC@CFs (d) electrodes. The pseudocapacitance contribution at various scan rates for the NSC@CBs (e) and NSC@CFs (f) electrodes.
Download figure:
Standard image High-resolution imageThese excellent electrochemical performances can be attributed to several reasons. Firstly, the widespread free spaces provided by the unique structures can effectively alleviate the volume dilatation during the discharging/charging process. Secondly, numerous micropores and mesopores means these electrodes are decorated with a huge number of active sites, facilitating close contact with the electrolyte. Combined with a short lithium ion/electron transport pathway for fast lithium ion diffusion, this gives rise to their excellent electrochemical performance. Thirdly, the robust carbon frameworks provide the speed conductive networks and safeguard the integrity of the electrodes. Lastly, the pseudocapacitive behaviours displayed in NCS@CBs and NCS@CFs occupied a significant portion in the redox processes, leading to high capacity and rate performance.
4. Conclusions
We have presented a facile and self-template strategy for the fabrication of NCS@CBs and NCS@CFs hybrids with unique 3D architectures. The carbon building blocks in NCS@CBs and NCS@CFs were stacked into 3D hierarchical networks and the Ni–Co–S nanoparticles homogeneously distributed in the 3D carbon networks. These architectures possess the features of 3D micro/nanostructures and provide a robust and highly efficient pathway for electron/lithium ion transport. The NCS@CBs and NCS@CFs electrodes show superior electrochemical performance in terms of rate capability, specific capacity and cycle stability. This strategy offers guidance for developing 3D MOFs and studying hierarchical structures for energy storage.
Acknowledgments
This work was supported by the Australian Research Council (DP150100018) and the Fundamental Research Funds for the Central Universities (Grant No. 53200859035) as well as the National Natural Science Foundation of China (Grant No. 51702289), Zhejiang Provincial Natural Science Foundation of China (No. LQ17E020003) and China Postdoctoral Science Foundation (Grant No. 2016M601963). T Yang thanks China Scholarship Council scholarship under the State Scholarship Fund, Engineering Research Center of Non-Metallic Minerals of Zhejiang Province, Key Laboratory of Clay Minerals, Ministry of Land and Resources.










