Abstract
Great effort has recently been devoted to the preparation of nanoscale surfaces on titanium-based implants to achieve clinically fast osteoinduction and osseointegration, which relies on the unique characteristics of the nanostructure. In this work, we used induction heating treatment (IHT) as a rapid oxidation method to fabricate a porous nanoscale oxide layer on the Ti6Al4V surface for better medical application. Well-distributed vertical nanopillars were yielded by IHT for 20–35 s on the alloy surface. The composition of the oxides contained rutile/anatase TiO2 and a small amount of Al2O3 between the TiO2 grain boundaries (GBs). This technology resulted in a reduction and subsequent increase of surface roughness of 26–32 nm when upregulating the heating time, followed by the successive enhancement of the thickness, wettability and adhesion strength of the oxidation layer to the matrix. The surface hardness also distinctly rose to 554 HV in the IHT-35 s group compared with the 350 HV of bare Ti6Al4V. The massive small-angle GBs in the bare alloy promoted the formation of nanosized oxide crystallites. The grain refinement and deformation texture reduction further improved the mechanical properties of the matrix after IHT. Moreover, in vitro experiments on a mesenchymal stem cell (BMSC) culture derived from human bone marrow for 1–7 days indicated that the nanoscale layers did not cause cytotoxicity, and facilitated cell differentiation in osteoblasts by enhancing the gene and osteogenesis-related protein expressions after 1–3 weeks of culturing. The increase of the IHT time slightly advanced the BMSC proliferation and differentiation, especially during long-term culture. Our findings provide strong evidence that IHT oxidation technology is a novel nanosurface modification technology, which is potentially promising for further clinical development.
Export citation and abstract BibTeX RIS
1. Introduction
Titanium (Ti) and its alloys have wide applications in the medical field of orthopedic and dental surgery due to their excellent mechanical properties and biological performance [1–3]. Surface modification technologies utilizing Ti-based materials to obtain bioactive and biocompatible coatings have attracted particular interest with regards to the intrinsic inertness of Ti-based biomaterials [4–8]. Oxidation technology is one of the surface modification methods and it includes chemical oxidation (CO), anodic oxidation (AO), micro-arc oxidation (MAO) and thermal oxidation (TO). It has been extensively investigated and used in the medical field. The oxide layers after oxidization treatment have various morphologies and properties, and they exhibit great improvement to the bioactivity and osteointegration ability of medical items [9–12]. Among the oxidation methods, although AO and MAO methods are more popular due to their promotion to the formation of porous structures or TiO2 nanotubes on the surface [13], the environmental pollution problem from the waste electrolyte in AO and MAO and long-term stability after implantation would potentially restrict further usage of these two methods. Hence, a more effective, robust, environmentally safe and easily operated method is demanded, which can effectively improve biocompatibility and resistance to the corrosion of the titanium implants based on the specific surface properties of TiO2 bioceramics.
So far, a number of thermal oxidation methods and in vitro/in vivo studies have been developed in order to generate compatible three-dimensional porous surfaces, such as traditional thermal treatment (in a furnace), fiber laser, glow discharge and induction heating oxidation treatment [10, 14–16]. Among them, induction heating treatment (IHT) is a type of rapid thermal oxidation technology, which showed advantages in the surface modification of medical Ti and its alloys, even when it was still in its infancy. IHT has its merits, with an inherently fast oxidation rate, low-energy consumption, its cleanliness and environmental friendliness [17] and it has been extensively utilized in industrial fields—in particular having been used in steel heat treatment for several decades—combining electromagnetic, heat transfer and metallurgical phenomena [18]. Due to the skin effect of the induction current during IHT oxidation, the temperature of the surface part of the sample rises rapidly, while the temperature of the inner part increases at a much slower rate, with less current distributed in the inner part than the surface part [19]. Therefore, oxide coating formation is more rapid on the surface than inside the material in the IHT process, and there is a reduction of the adverse effects of heating on the mechanical properties of the inner material. Fomin et al preliminarily reported the use of IHT technology to yield titanium dioxide coatings with a porous structure and its improved biocompatibility on the surface of medical titanium [20, 21]. However, further studies are required, in terms of optimization conditions, properties and the oxidation mechanism of the oxide layer during IHT heating.
Furthermore, the surface characteristics, such as the morphology, structure, chemical composition, energy, etc, play a significant role in the reaction between the implant and the biological environment. In particular, the surfaces with nanosized morphology and structure and their modification technology have attracted great attention, since the remarkably high surface area and surface energy of the nanoscale structure can result in remarkable biological properties [22–25]. For instance, there have been research reports about biomaterials with nanostructured surfaces that improved the response of osteoblasts [26–30]. Bianchi et al reported the deposition of nanostructured zirconia films on the surface of Ti using the pulsed plasma deposition (PPD) method. The nanostructured surface was suitable for adhesion, proliferation, and the viability of mesenchymal stem cells and osteoblast cells, and significantly decreased the wear rate under dry or NaCl-lubricated conditions [31]. There has also been research about TiO2 nanostructures modified on the surface of a Ti implant, which suggests the better bioactivity and biocompatibility of the nanostructure on the surface and is acquiring much attention. The first study was reported in 1999: TiO2 nanotubes were prepared through an electrochemical anodization process on the Ti surface with optimized parameters, significantly leveling up the formation of hydroxyapatite (HA) in vitro, the adherence and dispersion of cells, and the proliferation and differentiation of stem cells [11, 32, 33]. Furthermore, since stem cells have the unique ability to self-renew and differentiate in specialized tissue types, this unique ability was applied to evaluate the bioactivity and cell response ability of biomaterials in vitro and in clinical trials [34–39].
In this study, we launched a new nanostructured oxide coating with TiO2 nanopillars and an increased thickness on the Ti6Al4V surface fabricated by our IHT protocol. This surface modification protocol possesses the advantages of rapid speed, high cost-effectiveness, environmentally friendly characteristics and easy processing. We also characterized the TiO2 nanopilliars on the surface in terms of composition, morphology, roughness, wettability, adhesion strength and evolution procedure during the IHT period. Furthermore, the TiO2 nanopilliar formation mechanism by IHT oxidation, in view of the microstructure evolution in the Ti6Al4V matrix, was key to the aim of this study in order to prepare a controllable nanoscale oxide layer. For an in vitro study, HA deposition ability in simulated body fluid (SBF) and mesenchymal stem cells (BMSCs) derived from human bone marrow were carried out to evaluate the bioactivity and short-term cell events of adhesion, spreading, proliferation and long-term osteogenic differentiation as the expression of genes and osteogenesis-related proteins on these specific nanoscale surfaces, and compared with untreated Ti6Al4V samples. The results proved that the very fast heating rate of IHT oxidation produces more nucleation points (NPs) for the formation of TiO2 nanopillars on the surface of the oxide. The oxide then possesses the expected surface characteristics, such as high roughness and hardness, enhanced bonding strength, better wettability and HA deposition ability. Furthermore, the nanostructured oxide layer favors the initial adhesion, proliferation and differentiation of BMSCs into osteoblasts without any cytotoxicity. Hence, this method can be considered as novel nanosurface modification technology for further clinical development.
2. Materials and methods
2.1. Specimen preparation
Rolled Ti6Al4V plates with dimensions of 10 × 10 × 2 mm and cylinders with a diameter of 12 mm and a length of 10 mm were successively abraded with #200, #400, #600 and #1000 SiC papers, and then washed with acetone, ethanol and deionized water in an ultrasonic cleaner for 15 min, respectively. The ground specimens were subjected to supersonic frequency induction heating treatment with a frequency of 50 kHz and a power level of 60 kW, meaning it was possible to obtain an induction coil with a heating duration controllable to within 1 s. The IHT period of the Ti6Al4V plates lasted for 20–35 s, and they were then cooled to room temperature (25 °C) in air. However, the IHT times of the cylinders lasted individually for 14, 16, 18 and 20 s with a different induction coil. Finally, the heated specimens were all ultrasonically cleaned in acetone, deionized water and alcohol for 10 min, respectively, and then dried at 50 °C for 24 h in air.
2.2. Morphology characterization of the oxide layer
The surface morphology of the Ti6Al4V specimens before and after IHT oxidation was assessed using a scanning electron microscope (SEM, HATA-CHI SU-70, Japan) at 15 kV. Prior to imaging, the specimens were coated with a gold target for 30 s under vacuum to improve electrical conductivity. Energy dispersive x-ray spectroscopy (EDS) was also performed to investigate the element composition of the oxide layer at specific areas on the surface. In addition, the cross-sectional view of the oxide layers was achieved using other SEM equipment (Helios G3CX, FEI, USA) with a 2 kV, 1.6 nA electron beam. Samples with an inclination angle of 52° was rotated in order to obtain the SEM cross-sectional images. Atomic force microscopy (AFM) was used to evaluate the surface topography and roughness of the oxide coatings, which was performed with a stand-alone instrument (Dimension Icon, Veeco Instruments Inc., USA) operating in tapping mode in air. A SiNi tip with a nominal spring constant of 42 N m−1, was tapped under a scan rate of 1 Hz and at a resolution of 512 samples per line at 5 × 5 μm2 scan area. The values of nanoroughness were calculated by NanoScope Analysis software (version 1.5), included in the AFM. The root mean square (RMS) roughness values were chosen as representative of the average surface roughness.
2.3. Phase composition detection
The surface phase compositions of the IHT-treated plants were examined by x-ray diffraction (XRD, Rigaku, Tokyo, Japan) using Cu Kα radiation with a wavelength of 0.154 nm. The x-rays were generated from a copper source operating at 40 kV and 100 mA. The goniometer was set at a scan rate of 4° min–1 with a 2θ scan from 10–90° using step-scan intervals of 0.02°/2θ. The chemical composition and binding state of the external layer of the specimens oxidized by IHT were analyzed by x-ray photoelectron spectroscopy (XPS, ESCALAB 250, Thermo Fisher Scientific, USA). The XPS spectra were calibrated with respect to the C 1s peak at an energy of 284.8 eV, and recorded using monochromatic Al Kα radiation with a 90° take-off angle. In addition, the Al-Kα energies were 200 eV for the survey and 30 eV for the high resolution scans. High-resolution narrow scanning was also recorded to examine the chemical binding state of each element (e.g. O 1s, Ti 2p, and Al 2p).
2.4. Focused ion beam (FIB) milling and transmission electron microscopy (TEM)
The oxide layer on the Ti6Al4V surface was sliced into a parallel-sided thin foil using a focused ion beam equipped with scanning electron microscopy (Helios G3CX, FEI, USA), both gallium ion (Ga+) beam irradiation and subsequent platinum (Pt) electrode deposition under vacuum. The 2 kV, 1.6 nA electron beam was used for navigating and positioning the interest region of the sample surface. After the selection of a suitable region, the area was covered with electron-beam-deposited Pt (∼300 nm thick). The final oxide lamella with a size of 10 × 6 × 2 μm was formed using a 30 kV, 1 pA ion beam. Then, the thickness of the lamella was thinned to 60 ± 10 nm by the ion beam with decreasing energy (precisely controlling the thickness variations) in order to allow the e-beam to penetrate easily during subsequent tests. TEM analysis was utilized to obtain information about the morphology, crystal structure and lattice spacing of the FIB-thinned sample. The bright-field TEM-images and selected-area electron diffraction (SAED) patterns of the oxide were obtained on a transmission electron microscope (JEOL JEM-2010, Tokyo, Japan) at 120 kV. In addition, high-resolution TEM (HRTEM) images were obtained with a high-resolution transmission electron microscope operating at 200 kV with a maximum sample tilt angle of ±20° and a 1.9 Å point-to-point resolution.
2.5. Electron backscatter diffraction (EBSD) analysis
The microstructure evolution in the Ti6Al4V plates and cylinders before and after IHT oxidation was investigated by EBSD observation using a Hitachi S-3400 N SEM equipped at 20 kV with an HKL-EBSD system. To minimize strain during the sample preparation, the specimens were mechanically polished and finally electropolished in a solution of 35% butarol, 60% carbinol and 6% perchloric acid with a voltage of 30 V at room temperature. The grip and gauge regions of the specimens were chosen for EBSD analysis at a step size of 1.5 μm, respectively. The dimension of the scan area was approximately 480 × 650 μm. The EBSD maps and grain orientations for microstructural characterization were evaluated by Channel 5 software (HKL Technology).
2.6. Other surface property characterization
In order to determine the change of surface wettability of the oxidized Ti6Al4V, the wetting angle (θ) was evaluated according to the sessile drop method. A contact angle instrument (JC2000D3X, Powereach, China) was used to measure the static angle formed by dropping a deionized water droplet on the specimen surface at room temperature. To obtain a stable value for each sample, the measurement was repeated five times for specimens with the same treatment (n = 5). Adhesive strength between the oxide coating and substrate was investigated by scratch test using an automatic scratch tester (WS-2005, Lanzhou Institute of Chemical Physics, CAS, China) with a 200 μm radius diamond tip. The dynamic load increased from 0.2 to 30 N at a spend of 15 N min−1 and was designated as the critical load while the coating was totally peeled off from the Ti6Al4V substrate. Three single scratches were performed for each sample in order to ensure a reliable result. Otherwise, the surface microhardness (HV) test of the Ti6Al4V plants before and after IHT heating was performed using a Micro Vickers Hardness Tester (DHV-1000, China) with a diamond indenter in ambient conditions. A static load of 0.2 kg for 10 s was applied to each sample surface. The averages of five indentations from five different samples were used in the statistical analysis in order to ensure the acquisition of a reasonably representative value.
2.7. Immersion in SBF in vitro
To evaluate the in vitro formation ability of HA, 1.5 times SBF (1.5 × SBF) with various ion concentrations was applied in this work. It was prepared by dissolving appropriate amounts of reagent-grade chemicals of NaCl, NaHCO3, KCl, K2HPO4, MgCl2 · 6H2O, CaCl2 · 2H2O and Na2SO4 in distilled water, buffered at pH 7.40, with (HOCH2)3CNH2 (tris) and 1 M HCl at 37 °C. The mass of every agent in 1.5 × SBF was increased 1.5 times according to Kokubo's recipe [40], which similarly multiplied the ion concentrations in the solution, as shown in table S1 available online at stacks.iop.org/NANO/29/045101/mmedia. The IHT-35 s specimens were randomly chosen to identify the HA deposition ability of the as-prepared nanoscale surface in plastic containers with about 50 ml 1.5 × SBF. After immersion in SBF for 7–14 days, refreshed every two days, the specimens were cleaned and dried in air.
2.8. In vitro stem cell culture
The BMSCs were purchased from ATCC (PCS-500-012, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Invitrogen, USA), 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin (Gibco, USA), 2 mM l-glutamine (Gibco, USA) at 37 °C in a humidified incubator with 5% CO2 in air. The medium was refreshed every two days. The representative specimens oxidized by IHT for 20 and 30 s were selected and prewashed before cell seeding in the following manner: all samples were ultrasonically washed in acetone first for 5 min and then in dilute water for 10 min to remove any contamination; finally, they were dried in a nitrogen stream. After these washing steps, the specimens were sterilized by autoclave and placed in 24-well tissue culture plates. The second passage of the BMSCs was trypsinized from culture flasks and the total cell number was counted using a hemacytometer to calculate the required volume of the medium for resuspension. After centrifugation, the cell pellet was resuspended with DMEM. Next, 500 μl of suspension—about 10 000 cells—was dropped into each well. The cell/sample constructs were incubated for 24 h to allow complete cell attachment, then the medium was transferred to an osteogenic induction medium containing 50 mg ml−1 ascorbic acid and 10 mM β-glycerophosphate sodium (Sigma-Aldrich, USA). The osteogenic induction medium was changed every 2 days during the experimental periods.
2.9. Cell adhesion and proliferation assays
Cell viability was assessed at 24 h post-seeding using live/dead staining (Live/Dead Staining Kit, Invitrogen, USA). The Ti6Al4V specimens were rinsed with phosphate-buffered saline (PBS) and then incubated for 30 min in live/dead stain, calcein AM and ethidium homodimer-1, diluted in PBS according to the manufacturer's instructions. After incubation, the specimens were again washed with PBS and then imaged, and counted using fluorescence microscopy (Olympus CKX41, Olympus DP25 Microscope Camera, Olympus America Inc.). For microscopy, the immunofluorescence staining assays were performed 24 h post-seeding. After 24 h in the culture, the BMSCs were fixed with 4% paraformaldehyde (PFA) for 20 min, treated in 0.1% Triton-X 100 for 10 min, and blocked in 3% bovine serum for 20 min. Mouse monoclonal antihuman F-actin antibody and rat monoclonal antihuman vinculin (VCL) antibody (Abcam, Cambridge, MA, USA) were used at a concentration of 1/200. Sections were inoculated overnight at 4 °C, followed by the application of green fluorescence-labeled donkey antimouse secondary antibody and red fluorescence-labeled goat anti-rat secondary antibody (Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. After culturing for 1, 4 and 7 days, BMSC proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assay. In brief, at the prescribed time points, the specimens were rinsed with PBS and transferred to new 24-well plates. Then, 300 ml of DMEM and 30 μl of the CCK-8 solution (Beyotime, USA) were added to each specimen and incubated at 37 °C for 2 h. The absorbance was measured at 450 nm.
2.10. Cell differentiation assays
The cells were fixed with 4% paraformaldehyde for 20 min, permeabilized in 0.1% Triton-X 100 for 5 min and blocked with 3% bovine serum for 20 min. Mouse monoclonal antihuman runt-related transcription factor 2 (Runx2) primary antibody and rabbit monoclonal antihuman alkaline phosphatase (ALP) antibody (Abcam, Cambridge, MA, USA) were used with 1:200 dilution, as recommended by the manufacturer's datasheet. Sections were inoculated at 4 °C overnight. Red fluorescence-labeled donkey antimouse secondary antibody and green fluorescence-labeled goat antirabbit secondary antibody (Invitrogen, Carlsbad, CA, USA) were used at 1:300 dilution for 2 h. In addition, immunofluorescence stained cells were imaged on an Olympus BX61 inverted microscope system using filters. The expression of several osteogenic genes, the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Runx2, ALP, collagen I (COL-1), osterix (OSX) and osteocalcin (OCN), was analyzed by a reverse-transcriptase polymerase chain reaction (RT-PCR). The extraction of the messenger RNA (mRNA) and the reverse-transcription into complementary DNA (cDNA) were performed according to a previous procedure. Briefly, RNA extraction was performed using an RNeasy mini kit (Qiagen, USA) according to the manufacturer's instructions. The total RNA was reverse-transcribed using SuperScript III transcriptase according to the manufacturer's protocols (Invitrogen, USA). The PCR reactions were performed using Master Mix (Promega, USA). The PCR amplification cycles included denaturation for 30 s at 95 °C, annealing for 90 s and extension for 2 min at 72 °C for 30 cycles. The primer sequences are listed in detail in table 1. The gene expressions were normalized to the internal GAPDH expression, and the relative fold change was expressed by comparing it to that of the control group at each week. A comparative 2-ΔΔCT method was used to quantify the relative mRNA expression.
Table 1. The primary sequences of GAPDH, RunX2, ALP, OPN, BSP and OCN (NCBI reference sequence number).
| Gene | Forward primer sequence (5'-3') | Reverse primer sequence (5'-3') | Sequence number |
|---|---|---|---|
| GAPDH | GTTCCAATATGATTCCACCC | TGAGTCCTTCCACGATACC | 400 M33197 |
| Runx2 | GTTTGTTCTCTGACCGCCTC | CCAGTTCTGAAGCACCTGAAA | 318 L40992 |
| ALP | CCCAAAGGCTTCTTCTTG | CTGGTAGTTGTTGTGAGCAT | 356 NM_000478.4 |
| COL-1 | CTAGGCATCACCTGTGCCATACC | CAGTGACCAGTTCATCAGATTCATC | 371 NM_000582.2 |
| OSX | TCAGCATTTTGGGAATGGCC | GAGGTTGTTGTCTTCGAGGT | 657 NM_004967.3 |
| OCN | CATGAGAGCCCTCACA | AGAGCGACACCCTAGAC | 310 NM_199173.4 |
One hour after seeding, the BMSCs were collected, rinsed and lysed with RIPA buffer (Boston Bioproducts, Worcester, MA, USA). Protein concentrations were determined by a bicinchoninic acid (BCA) kit (Invitrogen, USA). About 20 μl solution for each sample was run. Protein transfer was performed using a semi-dry system (Bio-rad, USA). A polyvinylidene fluoride (PVDF) membrane was used for the transfer after methanol activation and blocked in bovine serum albumin (BSA)-tween solution for 1 h. The ERK, p-ERK and GAPDH antibodies (Abcam, Cambridge, MA, USA) were used for antigen detection (1/1000). The secondary antibody was HRP-conjugated (1/10000). After antibody incubation, the PVDF membrane was thoroughly washed and then scanned using an LI-COR system (LI-COR Biosciences, Lincoln, NE, USA) and the images were analyzed by Quantity One software (Bio-Rad, USA). For the western blot assay of OCN, Runx2 and COL-1 protein, BMSCs were cultured on the Ti6Al4V samples for 1 week, and the same procedure was followed. The tests were repeated three times, and gray levels of the bands were quantified by ImageJ software (WS Rasband, NIH, Bethesda, MD, USA).
2.11. Statistical analysis
Statistical analysis was performed with GraphPad Prism software (La Jolla, USA). All experimental data is expressed as mean ± standard deviation (SD). A two-tailed paired t test for the independent sample was applied to evaluate the differences between the two groups, which were oxidized for different periods, with the statistical significance set at the 5% level (p-value < 0.05).
3. Results
3.1. Phase composition of oxide layer
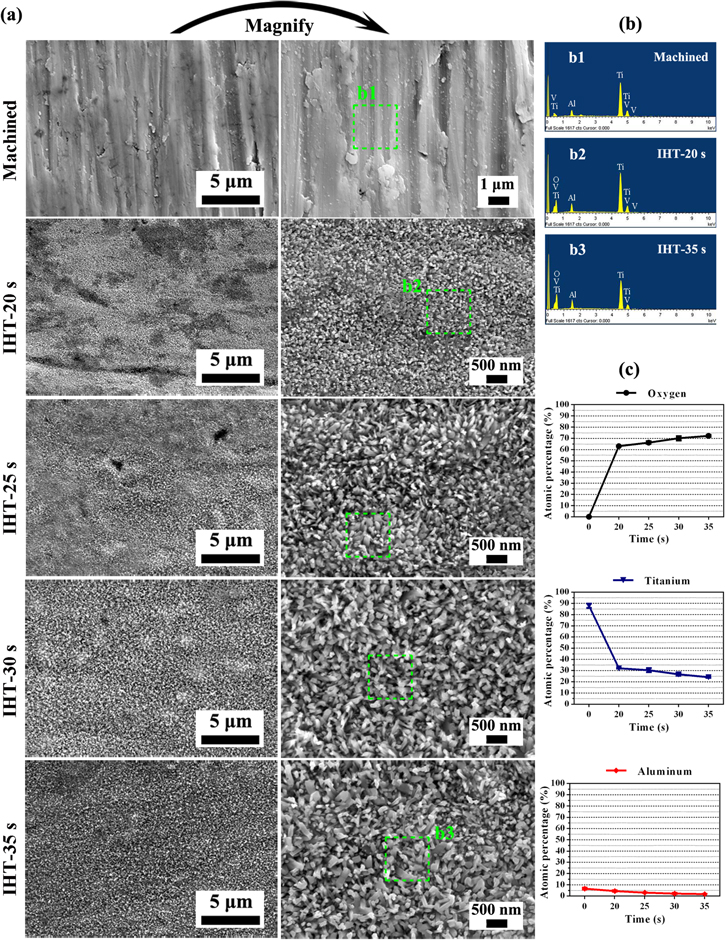
Figure 1 shows the XRD analysis of the Ti6Al4V plates treated by IHT oxidation for 20–35 s. In contrast to the Ti peaks generated by the bare specimen, the position of the diffraction peaks at 2θ with 27.42°, 35.97°, 41.10°, 54.23° and 56.83° detected on the surface revealed the crystalline phase of rutile TiO2. In addition, weak peaks of both Al2O3 at 2θ with 21.26° and anatase at 2θ with 37.88° were obviously observed from the XRD spectra. This showed that the diffraction peaks of TiO2 and Al2O3 were enhanced and the Ti peaks were consequently weakened along with the increasing IHT period from 20–35 s.
Figure 1. The XRD patterns of Ti6Al4V plates before and after IHT oxidation for 20–35 s.
Download figure:
Standard image High-resolution imageTo assess the binding state of the main chemical composition of the oxide layer, the specimens were investigated by x-ray photoelectron spectroscopy. The XPS spectra of the survey, O 1s, Ti 2p and Al 2p of the Ti6Al4V specimen treated by IHT for 25 and 35 s are shown in figure 2. From the XPS survey spectra, the O, Ti, Al and C were all detected (figure 2(a)). In addition, the values of the measured binding energy were calibrated using the C 1s peak (284.84 eV) as the internal standard. Both the O 1s peaks at 529.41, 530.05, 530.91 eV and 529.14, 529.76, 530.80 eV confirmed the presence of TiO2 on the Ti6Al4V surface oxidized by IHT for 25 and 35 s, respectively. Similarly, the presence of Al2O3 in the oxide layer was also proved by the O 1s peaks at 528.90 and 531.97 eV obtained after IHT for 25 s and the peaks at 528.62 and 532.22 eV collected on the sample surface treated by IHT for 35 s. In line with the binding energies of the O 1s peaks, the binding energies of Ti 2p at 458.06, 459.03 eV; 457.85, 458.74 eV and 463.78 eV; 463.61—produced after oxidation for 25 and 35 s—separately corresponded to the Ti 2p3/2 and Ti 2p1/2 of Ti4+, which were ascribed to the Ti-O bond. The Al–O bonds in the oxide layers for 25 and 35 s were also confirmed by the bonding energies of the Al 2p peaks at 74.11 and 73.99 eV, respectively (figures 2(b) and (c)). Table 2 records the bonding energy and atom concentration (%) of the detected elements on the IHT-oxidized surface based on the XPS spectra. This shows that the oxide layers were mainly composed of O, Ti and Al, and the element content increased in increments of IHT time from 25 to 35 s. Otherwise, the value of the Al content was about twice as much as the Ti when heated for both 25 and 35 s, which was in contrast to the XRD results.
Figure 2. The XPS spectra of (a) the survey, the O 1s, Ti 2p and the Al 2p of Ti6Al4V plates treated by IHT for (b) 25 s and (c) 35 s.
Download figure:
Standard image High-resolution imageTable 2. The bonding energy and atomic concentration of the detected elements in the oxide layers of the Ti6Al4V surface after IHT for 25 and 35 s on the basis of high-resolution XPS spectra.
| IHT-25 s | IHT-35 s | |||||||
|---|---|---|---|---|---|---|---|---|
| O 1s | Ti 2p | Al 2p | C 1s | O 1s | Ti 2p | Al 2p | C 1s | |
| Peak BE | 529.48 | 458.06 | 74.06 | 284.84 | 529.49 | 457.87 | 73.96 | 284.84 |
| Atom % | 45.63 | 8.97 | 16.59 | 28.81 | 52.04 | 7.24 | 14.52 | 26.20 |
| FWHM (eV) | 1.73 | 1.19 | 1.68 | 1.57 | 2.14 | 1.71 | 1.24 | 1.38 |
3.2. Morphology of the oxide layer
Figure 3 shows the SEM images of the typical surface morphologies of the oxide layer and element content calculated from the EDS spectra on Ti6Al4V plates such as oxygen, titanium and aluminum, while being machined and then heated by IHT for 20–35 s. A relatively smooth surface texture with distinct abrasive scratches was obviously observed on the machined matrix. However, after IHT heating for 20–35 s, a well-distributed nanoscale pillar-like oxide grew vertically on the Ti6Al4V surfaces, and the nanopillar size successively increased both in width and particularly in length as the heating periods were upregulated (figure 3(a)). To investigate the change in chemical composition of the oxide layer surfaces during IHT, the element content was counted based on EDS measurements. The results showed that the detected O had the highest concentration, followed by Ti, Al and minor amounts of V on the surfaces treated by IHT for both 20 and 35 s, whereas only the substrate elements (Ti, Al and V) were detected on the machined surface (figure 3(b)). When heated for 20 s, it appeared that the oxygen content (at%) increased sharply to more than 60% from the 0 of the bare sample (0 s), and the growth rate slowed down as the IHT period further increased to 35 s. Meanwhile, this resulted in the lowering of the relative content of Ti and Al (figure 3(c)).
Figure 3. (a) FE-SEM images, (b) the EDS spectra of the Ti6Al4V surfaces, and (c) quantitative elemental measurements of oxygen, titanium and aluminum on the Ti6Al4V surfaces after being machined and IHT-treated for 20–35 s. The green dashed squares in (a) mark the EDS detection areas with a size of 1 × 1 μm. The data is shown as means ± SD (n = 5).
Download figure:
Standard image High-resolution imageThe SEM cross-sectional view of the oxide layers was achieved by FIB filling to characterize the thickness of the layers and the growth patterns of the TiO2 nanopillars, and the results are shown in figures 4(a)–(b). In particular, at oxide layers of IHT-30 and 35 s, the oriented TiO2 pillars with increasing thickness (between green parallels) show vertical growth on the Ti6Al4V substrate after IHT oxidation (figure 4(a)). Finer oxide crystallites equivalently show up at the bottom of the oxide layers, revealing that the original fine TiO2 crystallites had combined with large-sized nanopillars during the constant heating process. Otherwise, the surface morphologies of the oxide layers exhibited different levels of damage from the Ga+ ions during FIB milling. Figure 4(b) showed the calculated thickness values of the oxide layers after precise angle correction. The real values were successively increased from 571.2 ± 25.9 nm to 1467 ± 61.9 nm due to upregulation of the IHT period to 35 s.
Figure 4. (a) A cross-sectional view of the FE-SEM images of oriented TiO2 nanopillar layer growth on the Ti6Al4V surfaces after IHT oxidation for 20–35 s; scale bar = 500 nm. (b) The thickness values of the oxide layers in (a); the data is shown as mean ± SD, n = 5. (c) AFM topographical 3D images and (d) quantitative measurements of surface roughness parameters at the nanolevel on the machined Ti6Al4V plants after IHT oxidation for 20–35 s. The data is shown as mean ± SD, n = 5. *p < 0.05, which indicates a statistically significant difference between the arbitrary two experimental groups. **p < 0.01 and ****p < 0.0001 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked.
Download figure:
Standard image High-resolution imageIn order to determine the characteristic topographical features and surface roughness of the oxide layer, AFM was performed within a scanned area of 5 × 5 μm2 and the corresponding results are presented in figures 4(c) and (d). The AFM topographical 3D images demonstrate that the nanomorphological crystallites appeared on the IHT-treated surface and continuously grew as the IHT time length increased (from 20–35 s). In contrast, parallel abrasive scratches with fewer protrusions were only observed on the machined Ti6Al4V surface (figure 4(c)). Moreover, the roughness (Ra) of the IHT-treated surfaces exhibited decreased values compared with that of the untreated surface (42.6 ± 4.5 nm), which was 26.3 ± 2.0 nm after IHT for 20 s, and increased to 31.3 ± 2.7 nm for 35 s, respectively (figure 4(d)).
3.3. Crystal structure of the oxidation layer
The TEM sample was prepared by means of FIB milling technology to investigate the crystal structure of the oxide and the representative results after IHT for 25 s are shown in figure 5. The slender crystallites in the outermost layer grew outwardly, perpendicular to the surface, which was in agreement with the cross-sectional morphologies (figure 4(a)), and the grain boundaries (GBs) of the oxide were obviously indicated in the cross-sectional TEM image (figure 5(a)). Figures 5(b) and (c) showed the HRTEM images and the corresponding SAED patterns taken from the oxidation layer at different regions, illustrating two different crystal structures in the oxide layer. The measured out-of-plane d-spacings were 0.227 and 0.228 nm, and 0.237 nm, respectively. With the inserted SAED patterns, there were good tetragonal (t) and hexagonal close-packed (hcp) crystal arrangements of the diffraction planes, which are attributed to the rutile TiO2 and α-Al2O3 crystallites separately. The hcp arrangement of the α-Al2O3 was located in the narrow region between the grains with different crystallographic orientations, which illustrate that a small amount of α-Al2O3 crystallites had formed at the GBs of the TiO2 (within the dashed line in figure 5(c)).
Figure 5. (a) A cross-sectional bright field TEM image of the oxide layer structure after IHT for 25 s. Black arrows indicate the growth direction perpendicular to the surface of the oxide crystallites. (b) and (c) High-resolution TEM (HRTEM) images taken from the oxide layer. The insets show the corresponding selected area electron diffraction (SAED) patterns.
Download figure:
Standard image High-resolution image3.4. Microstructure evolution of Ti6Al4V after IHT oxidation
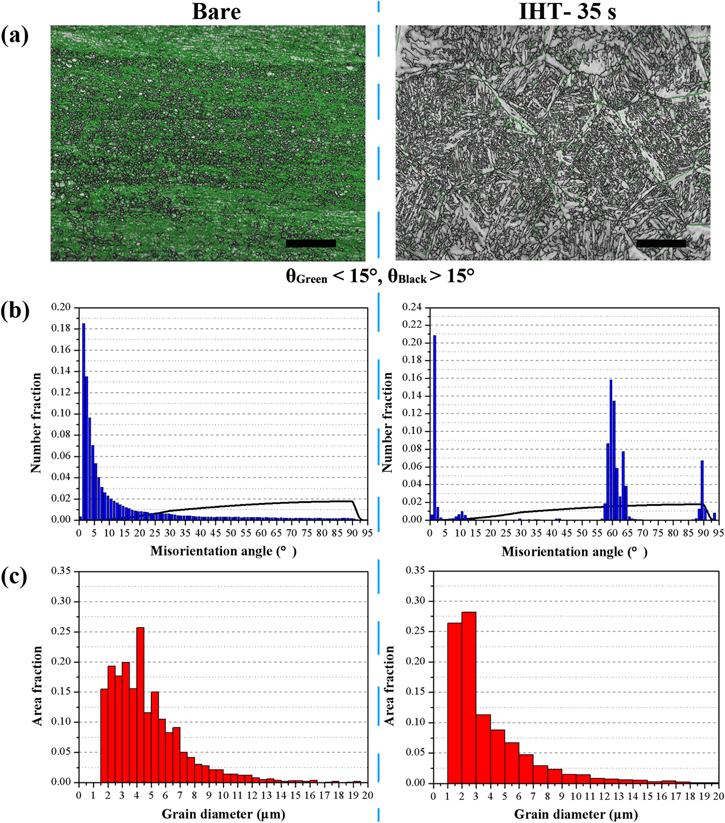
In order to evaluate the microstructure evolution in the Ti6Al4V plates before and after IHT heating, EBSD was applied to characterize the grain size and microrientation angle of the GBs, as shown in figure 6. Phase identification results suggest that the missive α-Ti (more than 99%) and a minor amount of β-Ti phases were found in the untreated Ti6Al4V (α + β Ti alloy), and the α-Ti slightly transformed to β phase after IHT-35 s. According to the grain boundary maps (figure 6(a)), the initial microstructure, which consisted of almost small-angle GBs (θ < 15°, green curves) was translated into large-angle ones (θ > 15°, black curves) when oxidized by IHT for 35 s. The quantitative statistics of the boundary angles shown in figure 6(b) illustrate that the fraction of small-angle GBs in the untreated substrate was estimated to be no less than 73.4%, which mainly resulted from the massive lattice defects existing in the rolled Ti6Al4V plate, such as dislocations. However, the transforming of small-angle GBs into large ones (about 60° and 90°) was caused by twin GBs [41, 42], and the proportion of small-angle ones dropped to 26.5% after IHT for 35 s. Figure 6(c) shows the grain size in the IHT-heated Ti6Al4V compared with that of the bare plate. This suggests that the IHT-treated grain diameters focus on low values (3 ± 2 μm) with a mean size of 4.61 μm, and the initial grains have higher values (4 ± 2 μm) with a mean size of 5.47 μm, contributed by recrystallization during induction heating.
Figure 6. The microstructural field in Ti6Al4V before and after IHT oxidation for 35 s showing (a) an EBSD scan with full grain boundary maps (green curves correspond to small-angle GBs, black curves to large-angle GBs); scale bar = 100 μm. (b) The distribution of the misorientation angles for α-Ti (hexagonal structure) and the random distribution of GBs (black line). (c) The grain size distribution of α-Ti; the domain is limited to 20 μm to emphasize the most common grains.
Download figure:
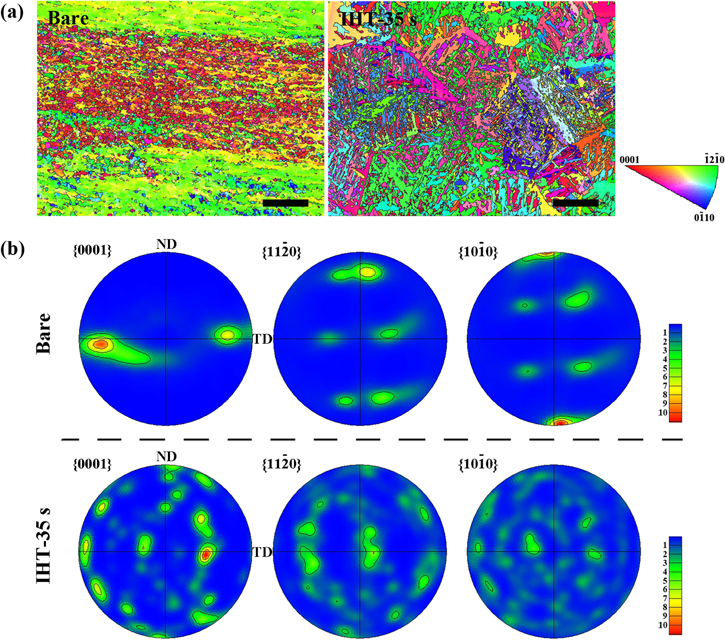
Standard image High-resolution imageFigure 7 shows the EBSD orientation maps and textures with different colors of Ti6Al4V matrices before and after IHT oxidation. In the bare sample, most of the α-Ti grains with special orientations followed  (red color on IPF map)
(red color on IPF map)  (green color on IPF map); in contrast, the grains after IHT oxidation for 35 s possessed their prismatic planes. The martensitic phase with lamellar microstructures formed in the Ti6Al4V matrix due to the high temperature gradients during the rapid IHT process (figure 7(a)) [43]. The
(green color on IPF map); in contrast, the grains after IHT oxidation for 35 s possessed their prismatic planes. The martensitic phase with lamellar microstructures formed in the Ti6Al4V matrix due to the high temperature gradients during the rapid IHT process (figure 7(a)) [43]. The 

 pole figures in figure 7(b) appeared as a symmetrical distribution in the ND/TD section, which suggest the intense preferred orientation of α-Ti grains, and was due to the deformation texture during the rolling process of the Ti6Al4V plate. After IHT oxidation for 35 s, the intensities of the
pole figures in figure 7(b) appeared as a symmetrical distribution in the ND/TD section, which suggest the intense preferred orientation of α-Ti grains, and was due to the deformation texture during the rolling process of the Ti6Al4V plate. After IHT oxidation for 35 s, the intensities of the 

 planes were evenly distributed around the basal pores in the Ti6Al4V alloy, which indicated that the grain orientations varied toward multiple directions and the texture was highly reduced. Furthermore, the grain refinement and deformation texture reduction caused the enhancement of the mechanical properties of the alloy matrix, in terms of strength, plasticity and toughness [44].
planes were evenly distributed around the basal pores in the Ti6Al4V alloy, which indicated that the grain orientations varied toward multiple directions and the texture was highly reduced. Furthermore, the grain refinement and deformation texture reduction caused the enhancement of the mechanical properties of the alloy matrix, in terms of strength, plasticity and toughness [44].
Figure 7. (a) Orientation maps of α-Ti in the Ti6Al4V specimens before (bare) and after IHT-35 s. The maps are colored according to the inverse pole figure (inserted IPF map); scale bar = 100 μm. (b) The 

 pole figures in the ND/TD section of α-Ti in Ti6Al4V specimens and heated by IHT for 35 s.
pole figures in the ND/TD section of α-Ti in Ti6Al4V specimens and heated by IHT for 35 s.
Download figure:
Standard image High-resolution image3.5. Wettability of the modified surface
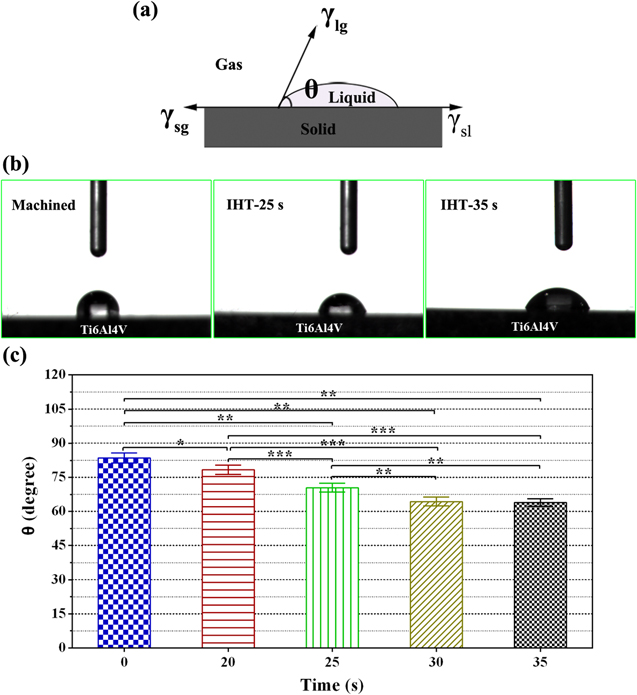
The sessile drop method was applied to measure the contact angles of Ti6Al4V surface oxidized by the IHT, which aimed to identify the evolution of wettability towards water when varying the IHT periods, as shown in figure 8. The θ of a liquid droplet on a solid surface is given by Young's equation [45]:
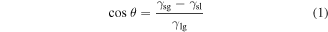
where 
 and
and  are the interfacial free energy per unit area of the solid–gas, solid–liquid and liquid–gas interfaces, respectively. According to equation (1), θ was determined by the included angle between the liquid–solid interface and the liquid–gas interface (figures 8(a) and (b)) [46]. Compared with the value of the machined surface (83.5 ± 2.2°), the θ decreased successively to 63.9 ± 1.7° upon increasing the IHT time to 35 s, which indicates that IHT oxidation plays an important role in both reducing the hydrophobicity and increasing the wettability of the surface of the specimen (figure 8(c)).
are the interfacial free energy per unit area of the solid–gas, solid–liquid and liquid–gas interfaces, respectively. According to equation (1), θ was determined by the included angle between the liquid–solid interface and the liquid–gas interface (figures 8(a) and (b)) [46]. Compared with the value of the machined surface (83.5 ± 2.2°), the θ decreased successively to 63.9 ± 1.7° upon increasing the IHT time to 35 s, which indicates that IHT oxidation plays an important role in both reducing the hydrophobicity and increasing the wettability of the surface of the specimen (figure 8(c)).
Figure 8. (a) A schematic diagram for the contact angle of solids in this study. (b) The water droplet on the Ti6Al4V surfaces before and after being treated by IHT. (c) The contact angles of the surfaces after being machined (0 s) and IHT-treated for 20–35 s. The data is shown as mean ± SD, n = 5. *p < 0.05, which indicates a statistically significant difference between two arbitrary experimental groups. **p < 0.01 and ***p < 0.001 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked.
Download figure:
Standard image High-resolution image3.6. Adhesion and proliferation of BMSCs on IHT-treated specimens
Figure 9 shows the results of the adhesion and proliferation of BMSCs on IHT-treated Ti6Al4V. The effect of specimens treated by IHT for 20 and 30 s on the viability of the BMSCs was assessed at 24 h time points and the LIVE/DEAD assay results are shown in figure 9(a). Green cells were considered live and red cells were considered dead (cleaned out). As evident from the representative LIVE/DEAD images in figure 9(b), the viable cells in the two groups displayed similar staining patterns and there was no significant difference between IHT-20 s and machined group, which indicates that the IHT-20 s treatment had no significant effect on stem cell adhesion for 24 h. However, the cell number on IHT-30 s group was slightly lower than the machined group.
Figure 9. (a) The LIVE/DEAD staining for BMSCs on Ti specimens before and after being treated by IHT for 20 and 30 s after 24 h of incubation; scale bar = 1 mm. (b) The cell counting results of (a). The data is shown as a mean ± SD, n = 5. *p < 0.05, which indicates a statistically significant difference between the two arbitrary experimental groups. ***p < 0.001 and ****p < 0.0001 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked. (c) The immunostaining of BMSCs for F-actin dye phalloidin-alexa fluor-488 (green), vinculin (red) and DAPI nuclear stain (blue) at 24 h; scale bar = 100 μm. (d) Cell proliferation measured by the CCK-8 assay after culturing BMSCs on different specimens for 1, 4 and 7 days. The data is shown as a mean ± SD, n = 3. *p < 0.05 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked.
Download figure:
Standard image High-resolution imageIn order to confirm whether the IHT of Ti6Al4V affected the state of F-actin in the BMSC lineage, we induced F-actin depolymerization in BMSCs over the 24 h time period, as shown in figure 9(c). In line with the LIVE/DEAD assay results, the results of immunofluorescence staining revealed nuclear F-actin and vinculin in the focal adhesion plaques, and there was no significant difference between the machined group and the IHT groups, which indicated that the IHT-treated Ti6Al4V did not make a particularly notable improvement in cell adhesion with the machined surface.
Figure 9(d) shows the results of cell proliferation on the specimens before and after IHT oxidation measured by the CCK-8 assay. At day 1, the BMSC numbers on the IHT-treated Ti6Al4V surface were a little smaller than that on the control group. Moreover, at day 4 and day 7 the cell numbers for the IHT-20 s and IHT-30 s groups increased rapidly. Although the cell numbers on the IHT-20 s group were still no more than that of the machined group for up to 7 days, it possessed the highest values of IHT-30 s specimens after culturing for 4 and 7 days, while there was no statistical significance between the values in each group (p ≥ 0.05).
3.7. BMSC differentiation assays of Ti6Al4V oxidized by IHT
The differentiation of BMSCs and osteoblasts on the IHT-treated specimens was further confirmed by the immunostaining of specific proteins expressed at 7 days, as shown by figure 10(a). Early marker expression was evaluated by ALP expression in 7 days, as per the pattern observed in the above study. Significant expression of the ALP was clearly observed in the cell culture on the IHT groups when compared to those on the machined Ti6Al4V surface. When increasing the IHT period, the expression of ALP in BMSCs was upregulated in the IHT-20 s and IHT-30 s groups. However, the Runx2 expression was also detected in different conditions, with a similar evaluation of the ALP expression pattern in the BMSCs.
Figure 10. (a) Immunocytochemical staining indicating the ALP and Runx2 expression after culturing in BMSCs for 7 days. (ALP: red; Runx2: green; Nuclei: blue); scale bar = 200 μm. (b) Quantitative real-time RT-PCR analysis of the bone-related gene expression in BMSC- loaded Ti6Al4V before and after being treated by IHT. The data is shown as mean ± SD, n = 3. *p < 0.05, which indicates a statistically significant difference between the two arbitrary experimental groups. **p < 0.01 and ***p < 0.001 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked. (c) A western blot analysis for p-ERK, ERK, OCN, Runx2, COL-1 and GAPDH, showing protein levels for 7 days and signaling pathways for 1 h. (d) Quantification of the western blot analysis; the data is shown as a mean ± SD, n = 3. *p < 0.05, which indicates a statistically significant difference between two arbitrary experimental groups. **p < 0.01 (paired t test). A statistically insignificant difference (p ≥ 0.05) was not marked.
Download figure:
Standard image High-resolution imageThe bone-related gene expression profile (e.g. ALP, OCN, Runx2, OSX and COL-1) for the BMSC-seeded specimens is shown in figure 10(b). The expression levels of genes, especially ALP and OSX, increased significantly among the IHT-20 s and IHT-30 s group as compared with the control group in the first week, having higher values of gene expressions than the IHT-20 s group when increasing the IHT period to 30 s, except for CON and OSX. In the second week, the expressions of OCN, Runx2 and COL-1 further increased in the IHT groups and all were higher than the machined group. However, at three weeks the OCN expression dramatically reached its highest value in the IHT-30 s group, compared with the IHT-20 s and control group.
In addition, the western blot for p-ERK 1/2 and ERK 1/2 was performed 1 h after seeding the BMSCs to the IHT and control samples, while that for OCN, Runx2 and COL-1 was performed at 1 week. While the BMSCs were cultured on the IHT and machined samples, osteogenic differentiation was indicated by the expression of osteogenesis-related molecules, as shown in figures 10(c) and (d). The results reveal that IHT treatment obviously increases the p-ERK 1/2 level and further raises the Runx2, OCN and COL-1 levels in the IHT-20 s group. Furthermore, the molecule expression was enhanced a little, accompanied by an increase in the IHT time to 30 s.
4. Discussion
There has been increasing attention on the design and fabrication of the surfaces of porous structures through the functionalization of biomaterials, and the main reason for this is that more economic benefits can be achieved from surface modification than from developing new materials with unique properties. Sequentially, this has motivated research dedicated to the optimization and renovation of nanosurface modification techniques, particularly for orthopedic biomaterials. Herein, we developed and utilized our IHT oxidation technique, a rapid and cost-effective surface modification method within a timescale of tens of seconds, to yield oxide layers with nanotopography as well as rutile TiO2 on Ti6Al4V surfaces. This work demonstrates the well-distributed pillar-like surface texture on the nanoscale, vertically grown on both Ti6Al4V plates and cylinders after IHT heating for 20–35 s and 14–20 s, separately, as well as comparisons with the distinct abrasive scratches of machined treatment (figures 3 and S4). The nanoscale pillars can be manipulated in width and particularly in length by increasing the oxygen content during upregulation of the heating period, which proves that the formation rate of oxides is determined by the ion diffusion process of IHT. From the cross-sectional images of the oxide layers, we can observe the oriented TiO2 pillars vertically growing on the Ti6Al4V substrate after IHT oxidation, and their thickness equally increases with the increment of IHT time length. In addition, the fine oxide crystallites formed at the beginning of oxidation and then combined to produce large nanopillars during the process of heating, which was just like the model of tree roots. The AFM topographical 3D images show that the growth of nanomorphological crystallites on IHT-treated surfaces results in the reduction of surface roughness and its subsequent increase with an increasing IHT period. According to phase analysis (XRD), the oxidation layer was composed of rutile/anatase TiO2 as its main component and a small amount of Al2O3 after quite a long oxidation time. However, the XPS measurement showed that the Al content value was almost twice as much as the Ti of IHT 25 and 35 s. The reason for this could be that it is difficult for the typical detection depth of the XPS to reach a 5 nm depth for a ceramic thin-film with an Al target; amazingly, however, XRD with a Cu target was able to reach 10–20 μm [47]. Consequently, the aluminum oxide was mainly concentrated in the outermost surface of the oxide layer and formed at the initial stage of heating, which was supported by similar results from MacDonald et al [48]. In our other experiment, this was also supported by the HRTEM and SEAD results, in which the tetragonal and hexagonal closely packed crystal structures belonged to rutile TiO2 and α-Al2O3, respectively. In addition, a small amount of α-Al2O3 crystallites formed at the GBs of the TiO2 due to the narrow region among these grains with different crystallographic orientations. Until now, articles have reported that TiO2 phases with various surface morphologies play a vital role in in vitro bioactivity (e.g. apatite deposition), cell response ability (e.g. MG-63, human mesenchymal stem cells) and biocompatibility (e.g. bone contact, bone tissue regeneration) [49–54].
Evaluation of the wettability of the IHT-treated surfaces was performed by measurement of the contact angles towards water via the sessile drop method. In contrast to the surface roughness evolution, increasing the IHT period from 20 s to 35 s resulted in the improvement of wettability (a decrease of the contact angles). The results illustrate the high roughness caused by the low contact angle in line with Wenzel and Cassie's theory [45, 55–57]. Wenzel's theoretical model with a modified Young's equation (equation (1)) was more suitable for interpreting the surface roughness of our case [45, 55]:
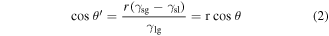
where  is the contact angle at the rough surface, and r is the roughness factor, which is defined as the ratio of the actual area of the rough surface to the geometric projected area. Equation (2) reveals that the surface roughness enhances both the hydrophilicity of the hydrophilic surface and the hydrophobicity of the hydrophobic one (r > 1), which explains the continuous reduction of surface wettability of Ti6Al4V after IHT oxidation. However, compared with the contact angles and surface roughness of IHT-0 s (machined) and IHT-20 s, the high roughness conversely results in a high contact angle (figures 4(d) and 8). Counted all together, the wettability of the Ti6Al4V surface is determined not only by the surface roughness but also the chemical compositions, such as Ti, rutile and anatase TiO2. We conducted a further investigation into the adhesion strength between the oxidation layer and substrate. The results illustrate that the bond strength of the layer to the substrate increases as the IHT times increase (figure S1). The enhanced adhesion ability of the oxidation layer can effectively stabilize the implant in bone healing and reduce surface flaking and loosening after implanting, which are potential factors in the inflammatory response and in clinical failure [58]. If complex stress and wear conditions are considered, the hardness of the IHT-oxidized layers should also be evaluated. After IHT oxidation, the surface hardness had higher values than that of the bare sample, and increased dramatically with the length of the heating time increasing (figure S2). This could be utilized to improve the wear and fatigue resistance of Ti6Al4V implants and effectively reduce the spalling of fragments while implanted in patients [59]. Furthermore, the characterization results by EBSD suggest that IHT heating refines the α-Ti grains due to recrystallization, and reduces their intensity-preferred orientation (figures 6, 7 and S5). Therefore, the mechanical properties of the Ti6Al4V matrix were improved and were better able to withstand stress in the critical clinical application.
is the contact angle at the rough surface, and r is the roughness factor, which is defined as the ratio of the actual area of the rough surface to the geometric projected area. Equation (2) reveals that the surface roughness enhances both the hydrophilicity of the hydrophilic surface and the hydrophobicity of the hydrophobic one (r > 1), which explains the continuous reduction of surface wettability of Ti6Al4V after IHT oxidation. However, compared with the contact angles and surface roughness of IHT-0 s (machined) and IHT-20 s, the high roughness conversely results in a high contact angle (figures 4(d) and 8). Counted all together, the wettability of the Ti6Al4V surface is determined not only by the surface roughness but also the chemical compositions, such as Ti, rutile and anatase TiO2. We conducted a further investigation into the adhesion strength between the oxidation layer and substrate. The results illustrate that the bond strength of the layer to the substrate increases as the IHT times increase (figure S1). The enhanced adhesion ability of the oxidation layer can effectively stabilize the implant in bone healing and reduce surface flaking and loosening after implanting, which are potential factors in the inflammatory response and in clinical failure [58]. If complex stress and wear conditions are considered, the hardness of the IHT-oxidized layers should also be evaluated. After IHT oxidation, the surface hardness had higher values than that of the bare sample, and increased dramatically with the length of the heating time increasing (figure S2). This could be utilized to improve the wear and fatigue resistance of Ti6Al4V implants and effectively reduce the spalling of fragments while implanted in patients [59]. Furthermore, the characterization results by EBSD suggest that IHT heating refines the α-Ti grains due to recrystallization, and reduces their intensity-preferred orientation (figures 6, 7 and S5). Therefore, the mechanical properties of the Ti6Al4V matrix were improved and were better able to withstand stress in the critical clinical application.
As the scheme shows in figure 11, based on the diffusion theory and Wagner oxidation theory [60–62], the atoms in the interface between Ti6Al4V and ambient air are initially ionized at an elevated temperature while heated by IHT (equations (3) and (4)), and interact to form the oxide layer.
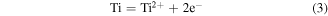
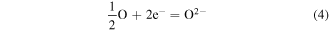
Figure 11. A schematic illustration of the reaction mechanism and formation process of TiO2 nanopillars during the rapid oxidization of IHT.
Download figure:
Standard image High-resolution imageSecondly, the ions (e.g. O2−, Ti4+) and electrons (e−) oppositely diffuse through the formed layer and continue to prepare new ones on the inner and outer sidewalls of the oxide layer. Finally, a thick oxide film was successively produced with ion migration as shown in equation (5),
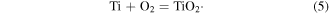
In this work, the Ti6Al4V plates and cylinders were heated at a rapid rate (tens of degrees of Celsius per second), which is much higher than the normal heating rate in a furnace (degrees Celsius per minute) [63]. Inhomogeneous NPs of oxide were preferentially formed at the GBs of the TiAl4V substrate, and the subsequent growth of the crystal nucleus depended on the ions diffusing during IHT heating. Herein, the high IHT heating rate, which was many times the normal heating rate, caused a lower energy barrier for the in situ nucleation of crystallites (nucleation energy) than ion diffusion (diffusion activation energy); hence the ions at the crystal defects preferred to rapidly react inside the α/β-Ti grains under high energy (e.g. vacancy, dislocation, twins) rather than transferring over a long distance. The EBSD results confirmed that massive small-angle GBs resulted from the lattice defects (e.g. dislocations) existing in the α-Ti grains in the originally rolled Ti6Al4V plate (figures 6(a), (b)). On the other hand, the grain refinement (from 5.47 to 4.61 μm) further generated more NPs in the oxide crystallites. The abnormally large number of NPs led to the easy formation and growth of oxide crystallites under such quick oxidation conditions. The fine oxide crystallites that formed at the bottom of the oxide layer also combined to produce large nanopillars. Moreover, the limited space on the specimen surface drove the crystallite to grow in length (perpendicular to the surface) orientation rather than in width, which finally produced vertical nanoscale TiO2 pillars on the surface with respect to the large bulks in the furnace [63, 64]. Based on this manner, the nanostructure on the Ti6Al4V cylinders possessed a larger porosity than that on the plates, which was determined by the higher superficial area of the circular surface on the cylinders than on the plates, as shown in figures 3 and S4. However, according to the reaction process of the oxide, for comparison, the radius of Al3+ at 0.51 Å was less than that of Ti4+ (0.68 Å) [65, 66], which suggested that Al3+ could pass through the oxide layer and interact with the O2− to form a new layer on the outer sidewalls of the oxide layer more easily than the Ti4+ ions could. Hence, there was a small amount of Al in the alloy that finally formed a thin Al2O3 layer on the outermost surface of the oxide, which was confirmed by XPS results. Investigation of the initial nucleation mechanism of the Ti oxide and the growth model of the nanosized TiO2 crystallites was essential to yield the controlled submicro-/nanoscale oxide surface by IHT oxidation for future biomedical application. The mechanism and the model would be sophisticated, and this will also be the main direction of our future work in this field.
In order to assess the biomedical properties of the nanoscale surface yielded by IHT oxidation, the randomly selected samples were immersed in 1.5 × SBF and cultured in stem cells (BMSCs) in vitro. The oxide layers with the nanostructure obtained by IHT-35 s successfully promoted the HA deposition after being soaked in SBF for 7–14 days (figure S3). Meanwhile, cell adhesion was the first step for maintaining the normal functions of the cells on the biomaterials, including proliferation and differentiation [67]. As shown in figures 9(a) and (b), at a 24 h time point, both the IHT-20 s and IHT-30 s groups had nearly the same initial adherent cells in comparison with the machined group, in accordance with the smaller surface area and lower quantity of actin shown in figure 9(c). Our results also demonstrate that cell attachment on rough surfaces decreased at 24 h time points, even though the IHT modifying the surface significantly reduces the water contact angle with a change in surface energy. As known, the surface roughness and wettability plays an important role and has benefits for early cell adhesion, affecting cell behavior in a complicated time-dependent way, rather than a simple linear pattern. It has been reported that BMSCs can detect changes in Ti6Al4V surface roughness of the order of 0.60 μm, and roughness plays a role in cell response per se, independent of the surface texture. However, when the surface roughness drops below a certain level, there is no cell adhesion increase anymore [68]. In our research, the nanoroughness was less than 50 nm in all the groups, causing no notable effect on cell adhesion; thus there was no significant difference in the adhesion results.
We explained that a quick reduction of the surface roughness after IHT heating and a slight decrease in the contact angle were not enough to improve the BMSC attachment. Meanwhile, past literature has suggested that stem cells have a different osteogenic response with weak cell attachment and growth patterns compared to normal tissue cells such as human osteoblasts [69]. In figure 9(d), the cell proliferation was higher, especially in the IHT-30 s group, but there was no statistically significant difference between the control group and the IHT groups on days 4 and 7. When compared with the machined and IHT-20 s specimens, the IHT-30 s group had enhanced cell proliferation, perhaps due to the higher roughness and better wettability of the large-sized TiO2 pillars on the surface. Anyway, the increment of the BMSC proliferation results demonstrate that IHT treatment does not cause any cytotoxicity, and has the ability to improve stem cell proliferation. Since different IHT time lengths generate the subtle promotion of stem cell proliferation, and economic factors were considered, we selected the IHT-20 s and IHT-30 s for the following in vitro experiment.
BMSCs specializing in mineralization were evaluated by measuring the expression of five types of osteogenic gene marker (ALP, OCN, Runx2, OSX and COL-1) in cells adhering to the Ti6Al4V surfaces (figure 10). The ALP and OCN played crucial roles as phenotypic markers for early and later stage bone formation, and increased ALP and OCN expression correlates with an increase in this formation [70]. Owing to the ALP gene guiding protein synthesis, the IHT group had a significantly higher ALP expression compared with the control samples after 1 week, implying that the IHT surface improved protein synthesis and early cell differentiation. In addition, OCN played an important role in preosteoblastic and bone-building processes [71], which was mainly related to the formation of mineralized tissue as an extracellular matrix vesicle in the bone [72]. The results show that the surface of the IHT group stimulates high expression levels of OCN at 2 and 3 weeks, which suggests that IHT oxidation promotes BMSC matrix protein production and osteogenesis. Furthermore, Runx2 was identified as the primary transcription factor for osteoblastic differentiation [73]. In vitro studies show that Runx2 positively controls OCN expression [74]. The BMSC differentiation concludes that Runx2 is highly expressed after 2 and 3 weeks, similar to the OCN expression. The OSX genetic expression from the BMSCs on the surfaces of the machined Ti6Al4V and IHT-treated ones had a different tendency: on the IHT group, the OSX was higher at 1 to 3 weeks than that in the control group. The COL-1 was an osteogenitor marker for the greatest quantity of collagen of the human body, and it was distributed throughout the mineralized extracellular matrix [75]. The IHT group had higher expression levels of COL-1 than the bare Ti6Al4V at all intervals on the IHT samples, which suggested that IHT heating was able to promote extracellular matrix formation. In conclusion, the gene expression results demonstrate that the IHT oxidation of Ti6Al4V can improve the differentiation of BMSCs into osteoblasts, particularly for long-term effects. Recent research shows that osteoblast behavior can be influenced by controlling surface characteristics, such as nanotopography and wettability, and the surface wettability of titanium can affect the differentiation of cells, which indicates that higher wettability has higher osteogenic ability [76]. In our research, the IHT-treated group has the lowest contact angle, which means it has the highest osteogenic marker expression. On the other hand, the gene expressions in BMSCs are somewhat advanced while the IHT time is upregulated from 20–30 s, due to the limited evolution of surface characteristics.
The results of gene expressions were further studied by western blot assay (figure 10(c)). There have been studies reporting that the mitogen-activated protein kinases family (MAPKs, including p38 MAPK, ERK 1/2, and JNK) plays an important role in osteogenesis [77]. Furthermore, the ERK 1/2 in MAPKs was a key downstream factor in the integrin-mediated signal transduction machinery, which controlled the osteogenic differentiation of the stem cells [78]. In this research, we examined the levels of ERK 1/2 and phosphorylated ERK 1/2 (p-ERK 1/2), OCN, Runx2, COL-1 and GAPDH in the BMSCs by western blot test. The results show that IHT treatment obviously increases the expression of osteogenesis-related molecules, especially for p-ERK 1/2 levels. As reported before, ERK 1/2 can prevent the translocation of activated Smad to the nucleus and lead the BMP signaling pathway to potentiate, leading to the upregulation of many osteogenic markers [79]—like OCN, Runx2 and COL-1—further confirming that the IHT oxidation of Ti6Al4V greatly promotes the osteoblastic differentiation of BMSCs.
As a consequence of the above results of BMSC culturing, this novel random nanotopography with well-distributed TiO2 nanopillars results in the indistinctive improve-ment of cellular adhesion, and then influences the cytoskeletal tension, because the adhesion generates the anchor points of the cytoskeleton on the surface of the oxide layer [80]. In addition, the nanostructured surfaces significantly enhance cell proliferation and the expression of osteogenesis-related genes and corresponding proteins; that is, bone-specific stem cell differentiation. These findings can be interpreted and are supported by recent observations of the morphology and structure [81, 82], chemical composition [7, 83] and material stiffness [84]. Our nanomorphologies with a large surface area and good wettability have provided an enormous quantity of anchor points for early cell adhesion and cytoskeletal tension, and thereby promote cell proliferation and osteoblastic differentiation. Kuhlman et al have reported that nanoscale surface variability also significantly affects integrin conformation and clustering, which thereby alter the dynamic organization of signaling proteins in focal adhesion [85]. The osteogenic potential of the nanoscale surface of pure Ti and its alloys is related to their ability to modulate osteogenesis signaling pathways, such as ERK1/2, BMP and integrins [86]. It has been reported that the nanostructured Ti surface can activate the PERK-elF2α-ATF4 pathway to induct osteogenics in rat BMSCs [87]. Relying on stimulations of the expressions of osteogenic genes and related proteins, the nanostructured oxide layer obtained by IHT further promotes the osteoblastic differentiation of BMSCs. Otherwise, the influence of nanoscale surface features on the fate of stem cells is complicated and still a subject of controversy. Hence, additional research and data are necessary in order to be able to totally understand the interaction mechanism.
5. Conclusion
In this work, IHT was applied to the rational modification of the surface of Ti6Al4V as a rapid thermal oxidation method. After that, oxide layers with vertically well-distributed nanopillars and increased thickness were rapidly prepared on the Ti6Al4V surface. The oxide layers mainly contained the rutile/anatase TiO2 and a small amount of Al2O3 between TiO2 grain boundaries. Compared with the machined matrix, the nanomorphology resulted in a reduction of the surface roughness and subsequent increase; if the heating time increased, so did the successive enhancement of wettability and surface hardness. Therefore, the adhesion strength of the oxidation layer to the substrate was distinctly enhanced upon increasing the IHT time from 20–35 s, and was therefore better able to stabilize the implant for bone healing if further used in implanting. The massive small-angle GBs in the bare alloy matrix promoted the formation of nanosized TiO2 crystallites in view of the microstructure. After IHT oxidation, grain refinement and deformation texture reduction occurred and further improved the mechanical properties of the alloy. The nanoscale oxide layers obtained by IHT successfully promoted the HA deposition in SBF in vitro. According to the BMSC culture in vitro, the IHT oxidation of Ti6Al4V shows favorable initial adhesion and proliferation without any cytotoxicity. On the other hand, the obtained nanoscale oxide layers significantly promote the differentiation of BMSCs into osteoblasts by enhancing the expressions of osteogenesis-related genes (ALP, OCN, Runx2, OSX and COL-1) and their corresponding proteins after culturing for 1–3 weeks. Moreover, the limited evolution of the surface characteristics of the oxide layers caused by increasing the IHT period from 20–30 s slightly advanced the BMSC differentiation. Depending on this data, the rapid oxidation method can be considered as novel nanosurface modification technology, which is promising for the further clinical development of Ti-based biomaterials.
Author contributions
The paper was written through contributions from all the authors. All of them have given approval to the final version of the manuscript and declare no competing financial interest.
Acknowledgments
The authors acknowledge the financial support for this research through The Fundamental Research Funds of Shandong University (2015JC018, 2016JC024), Jiangsu Natural Science Funds (BK20161240) and Suzhou Science and Technology Bureau (SYG201615), Shandong Provincial Natural Science Foundation of Shandong Provincial Natural Science Foundation of China (ZR2017MEM014), Project of Shandong Province Higher Educational Science and Technology Program (J17KA004), Fundamental Research Funds of Shandong University (21350073614072), China Postdoctoral Science Foundation (2014M561942), Shandong Provincial Natural Science Foundation, China (ZR2017BH030).