Abstract
Synthesis and application of nanostructured molybdenum disulphide particles and complex composites have been studied for several decades. They offer many attractive properties which are linked to the transition character of the base element, i.e. molybdenum, and high chemical activity of sulphur, an element of the oxygen family. Significant progress in our understanding of the processes involved in nucleation, growth, and shaping of molybdenum disulphide nanoparticles was achieved, and the mechanisms underlying their biological properties and catalytic activity were investigated; however, many questions remain. In this topical review, a number of representative examples are used to illustrate recent progress in nucleation and growth of various molybdenum disulphide nanostructures with the aim to provide a snapshot of the spectrum of practically important fabrication methods, from simplest solution-based techniques to the most advanced chemical vapour deposition and plasma-enhanced chemical vapour deposition techniques. We then review the most promising applications of these nanostructures in medicine, focusing on anti-cancer therapy, drug delivery and medical imaging, with the key advantages and opportunities presented by molybdenum disulphide nanoparticles and composites over other similar materials and nano-architectures. The outlook section focuses on present challenges in the synthesis, e.g. sophisticated control over particle structure and chemical activity, as well as advanced biomedical applications of molybdenum disulphide nano-structures, and proposes some strategies to overcome these challenges and problems.
Export citation and abstract BibTeX RIS
1. Introduction
A large number of advanced materials derive their properties from their composite, multicomponent, and hierarchical nature [2–4]. Indeed, even relatively simple one- and two-component nanostructures can deliver superior properties while also being relatively easy to produce and assemble [5–7].
There is little doubt that nanotechnology, a science that began with the discovery of unique properties offered by structures with nanoscale dimensions, such as carbon nanotubes and graphene, has now reached the point of enabling realization of many emerging and advanced applications across all facets of human life [8, 9]. A wide range of 1D nanomaterials, e.g. single- and multi-walled nanotubes of carbon, silicon or boron nitride, have now been shown to offer promising electrical and thermal conductivity, mechanical and chemical stability, finding applications across many fields, from material science to mechanical engineering, chemistry, biology and medicine. Spherical nanostructures, such as fullerenes [10] and other capped and cage-like materials, offer great potential for applications such as medical imaging and drug delivery [11]. A rich diversity of multicomponent nanoarchitectures [12, 13] have also emerged, favourably combining attractive features of individual materials to expand their application even further and give rise to novel features that can help advance and even revolutionise biology, medicine, catalysis and other smart fields [14, 15].
As these structures and architectures become increasingly more complex, they require additional forms of control for the assembly so as to preserve the desired features, increasing the overall complexity of the fabrication process. Indeed, development of methods that enable high-throughput fast and affordable synthesis of complex multicomponent structures with excellent quality and nanoscale resolution remains a challenge [16, 17]. Nevertheless, impressive results have already been achieved in the synthesis of multicomponent structures and their application in cancer therapy [18], targeted delivery of drugs and biomolecules [19, 20]. Structures, the behaviour and movement of which in vivo can be controlled via application of external electric and magnetic fields, are of particular interest for a number of biomedical applications, from diagnostics to treatment and tissue engineering applications [21]. Similar advances can be achieved in other areas, with application of complex multicomponent nanostructured materials enhancing the efficiency and increasing the life span of space technology [22, 23], nanostructure-enabled emission devices and cathodes [24, 25], plasma thrusters [26, 27], energy conversion materials [28, 29] and electronics [30, 31].
Among a plethora of available materials, nanostructured molybdenum disulphide (MoS2) compounds and nano-architectures attract particular attention because of a favourable combination of mechanical stability, photochemical reactivity, tuneable electric properties, which makes these materials promising for applications in catalysis [32, 33], sensing [9, 34], medicine [35, 36] and biology [37]. They are also relatively affordable and easy to fabricate using a number of technologies, ranging from simple water-solution based techniques [38] to more complex but versatile plasma- and discharge-based methods [39, 40]. Depending on the synthesis technology (such as exfoliation, chemical vapour deposition, laser-assisted and others, see figure 1), molybdenum disulphide nanostructures may possess either metallic or semiconducting properties, with both n-type and p-type conductivity types being achievable; such a wide diversity ensures various applications of the nanostructured molybdenum disulphide, including nanoelectroncs, advanced batteries, solar cells, biosensors and many others. Owing to high surface activity and unique properties, the nanostructured MoS2 are particularly promising for biomedical applications, including anti-cancer therapy and diagnostics.
- A general purpose of this review is to present, aiming at the widest readership, a general view on the types, techniques, and anti-cancer applications of nanostructures MoS2 materials, and thus to focus attention on this challenging but highly promising material, and to inspire further studies in this dynamic field of significant importance. A highly representative selection of the most recent (within three years) publications was selected to emphasise the most recent achievements in the field.
Figure 1. How to control the structure and properties of MoS2 nanostructures, and ensure their suitability for desired application? Selection of fabrication technology is the first step that determines key process parameters that can serve as controls for the synthesis and assembly of MoS2 nanostructures. Featuring both metallic and semiconductor properties, molybdenum disulphide could show n- and p-type conductivity, depending on the fabrication technique. The ability to tune the properties enables numerous applications ranging from catalysis and energy transformation to photodetectors, sensors, batteries, as well as many critical medical applications in cancer therapy, targeted and highly controllable drug delivery, and others. Active interaction of MoS2 nanostructures with biologically active substances can be harnessed to control biocompatibility of structures; lower image shows how modified lysozyme can promote exfoliation of bulk MoS2 material via an ultrasonic process. Reproduced from [1]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageThis topical review is organized as follows.
- In section 2 we briefly outline some basic properties and characteristics of molybdenum disulphide and its crystalline structure, including specific energies and barriers, which significantly influence the selection of method for synthesis of MoS2 nanostructures. Without going into excessive detail which may be found in a great number of referenced papers, we emphasize the uniqueness of molybdenum disulphide as a platform for nanofabrication; a question 'Why molybdenum disulphide?' is the key point of this section, and special importance of the nanostructured MoS2 is stressed. We also briefly outline physical mechanisms involved in the synthesis of MoS2 material, stress the importance of an initial nucleation stage, and demonstrate routes for structure control using one of the most important parameters, namely chirality, as an illustration.
- In section 3 we overview several fundamental approaches to the synthesis of MoS2 nanostructures, such as chemical route, vapour–liquid–solid mechanism, and highly efficient mechanisms of structure control via hydrogenation.
- In section 4 we overview various aspects and possibilities of several representative technologies which have proven their efficiency in the synthesis of MoS2 nanostructures, such as heat-enabled methods including chemical vapour deposition, thermal vapour sulphurization, exfoliation/restacking, pulsed laser deposition, solvothermal process, photolithography, and MoS2 growth on epitaxial graphene; several representative plasma-based techniques such as plasma enhanced atomic layer deposition, magnetron sputtering, sulphurization in fluorocarbon plasmas, arc discharge techniques including high pressure arc discharges and arc discharges in liquids are presented. Plasma-based techniques for post-processing are also briefly reviewed, such as nitrogen doping, as well as hydrogen, oxygen, and argon plasma-based techniques, and remote plasma treatment.
- Section 5 outlines a number of the most important and representative examples of MoS2 application in medicine, with the special stress on applications in anti-cancer therapy. Recently achieved results on the application of various MoS2 nanostructures (nanoflakes, nanodots, complex and printed 3D MoS2 nano-architectures) for diagnostics and photothermal oncotherapy, as well as several advanced approaches such as targeted fluorescent imaging, surface-functionalized MoS2 nano-sheets for drug delivery and surface-modified photothermally triggered drug release system are also examined.
- And finally, the summary and outlook section brings some thoughts and ideas on the possible future trends and current challenges facing the use of molybdenum disulphide nanostructures for anti-cancer applications, including very promising yet not well developed fields such MoS2 nanoparticle systems with anti-angiogenic effect, MoS2 nanostructures for simultaneous diagnostic and therapy, and MoS2 nanostructures for synergistic onco-therapy are discussed.
2. General properties of molybdenum disulphide
2.1. Crystalline structure and characteristic energies of molybdenum disulphide
2.1.1. Why MoS2?
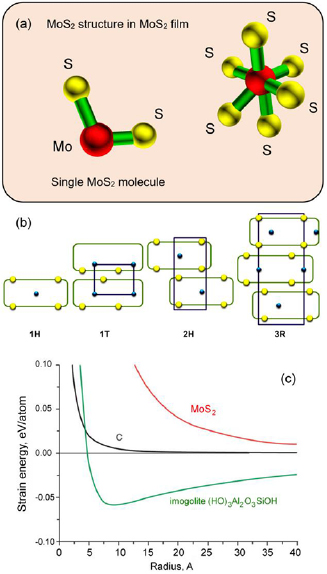
Molybdenum is a transition metal with a partially filled d shell, which predetermines their high chemical activity and ability to form a rich diversity of compounds. Even as pure elements, transition metals exhibit high catalytic activity; this activity could be significantly enhanced by combining them with elements of the oxygen family, such as oxygen and sulphur (figure 2). Among these two-element compounds, molybdenum disulphides attract particular interest due to high chemical activity of sulphur, as well as bioactivity when applied to biological tissues and other biological system due to the role sulphur plays in the formation and activity of iron–sulphur proteins and many important polyatomic molecules. The disulphides feature strong S–S bonds, thus making the metal-disulphide compounds and nano-composites very attractive for numerous mechanical, electrical and other applications [44]. Usually, catalysis using transition metal-based compounds and nanocomposites proceeds by the formation of temporary bonds with the surface of the catalyst, thus weakening the internal bonds in the reactants and promoting desirable chemical reactions. Fundamentally similar mechanisms are involved in the biological catalytic processes.
Figure 2. (a) Structure of a single molybdenum disulphide molecule and an elemental unit of molybdenum disulphide film, with the molybdenum disulphide in bulk featuring the following parameters: space group P63/mmc; a = 0.312 nm; c = 1.198 nm [41]. (b) Schematic of common MoS2 polytypes. Reprinted from [42], copyright 2016, with permission from Elsevier. (c) Why is MoS2 highly active? The strain energy of molybdenum disulphide significantly exceeds the energies of carbon and many other similar compounds. Reproduced from [43] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageMany innovative methods for the fabrication of metal-disulphide materials and molybdenum disulphide (MoS2) in particular have recently been proposed [45, 46] for a variety of advanced and emerging applications [47, 48]. Indeed, the fabrication approach is known to directly influence the properties, structure and therefore potential applications of MoS2 nanostructures, eventually resulting in the synthesising of many various types of nanosystems, including nanotubes, fullerenes and encapsulated hollow nanoparticles [43].
- Molybdenum disulphide features an attractive set of material properties for a wide range of applications
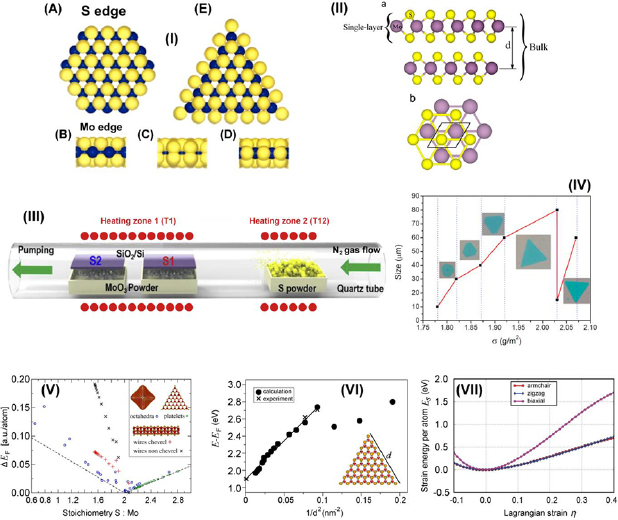
The structural configuration of thin singly layered MoS2 nanoclusters have remained as a critical area of interest to many, as indicated in numerous notable research articles published on that matter [49–51]. The electronic structure of the edge states has direct influence on the chemical properties of these edges. In particular, their reactivity towards hydrogen is facilitated by the ability of hydrogen to form stable chemical bonds with both low-Miller indexed edges of MoS2. It has been demonstrated that the edges of the nanoclusters are associated with 1D metallic edge states and the triangles can thus be regarded as closed, nanometer-sized wires. In general, it is anticipated that these 1D, metallic edge states will also pertain to other geometries. Having, for instance, a Mo edge with adsorbed S dimers, the step edge will be metallic in nature, having two localized, 1D conducting channels [52]. As illustrated in figure 3((I)(a)), determination of which termination edge is more stable is not trivial owing to the fact that edges may not be simple termination points of stoichiometric MoS2 under certain sulfiding conditions employed [51].
Figure 3. Structure and properties of nanoscale molybdenum disulphate. (I) (a) A ball-model (top view) of a bulk truncated MoS2 hexagon with Mo and S edges being exposed. The Mo (blue) atoms at the Mo edges are coordinated to only four S atoms (yellow), whereas the Mo atoms in the bulk are coordinated to six S atoms. (b)–(d) Side view of Mo edges. (b) The naked Mo edge in (a). (c) The Mo edges are terminated with S atoms resulting in S dimers at the edges; i.e. we have two S per Mo atom at the edges. In (d) there is only one S atom per Mo atom at the edges. In this case, the S edge is reconstructed with respect to the S dimer model in (c). The S atoms move vertically to the Mo plane and shift laterally with half a lattice constant. (e) A top view of (d). Models (d) and (e) depict the triangular structure observed in the STM images with the S edge atoms being out of registry with the S atoms in the basal plane of MoS2. Reprinted figure with permission from [51], copyright 2018 by the American Physical Society. (II) Bulk and single-layer MoS2 material. (a) MoS2 bulk and single layer. The interlayer distance is denoted by d (distance between Mo atoms of different layers). (b) Top view of the MoS2 single-layer unit cell. Reprinted from [56], copyright 2015, with permission from Elsevier. (III) Example of experimental furnace-based setup used for synthesis of MoS2 thin films. Reprinted from [57], copyright 2018, with permission from Elsevier. (IV) Correspondence between the surface density of MoO3 vapour and the size and morphology of MoS2 films. Reprinted from [58], copyright 2018, with permission from Elsevier. (V) Formation energy, ΔFE, of prototypical molybdenum sulphide nanostructures as a function of their composition. All formation energies are calculated with respect to the infinitely extended MoS2 sheet, Mo bulk, and the S8 crown (α-sulphur) as reference compounds. The dashed lines informed by a continuum model indicate the energy offset due to the deviation from the ideal MoS2 stoichiometry. Reprinted with permission from [59]. Copyright 2015 American Chemical Society. (VI) Calculated absorptions in the visible-light region of a set of MoS2 nanoflakes as a function of edge length d. Reprinted with permission from [60]. Copyright 2014 American Chemical Society. (VII) Energy-strain responses of graphene-like MoS2 under armchair, zigzag, and biaxial strain. Reproduced from [60] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageThe results from numerical simulations employing density functional theory (DFT) calculations have indicated that Mo terminated edge structures exhibit either one figure 3((I)(b)) or two figure 3((I)(c)) S atoms at each edge where a Mo atom is present at the edge. In both of these instances, the Mo atoms are saturated through coordination to six other S atoms (as is the case for Mo atoms in bulk MoS2). Therefore these two structures exhibit relatively similar stabilities. However, results from DFT simulations have identified that only structures with singular S atoms for each Mo atom at an edge figure 3((I)(d)), result in S atoms at the edges being shifted by half a lattice constant relative to S atoms in the basal plane (as observed through experimental STM imaging). None of the simulated structures (for S terminated edges) appear to be reconstructable, and this is verified with results from STM measurements. Through correlation of calculations with experiment, it can therefore be concluded that the model as illustrated in figure 3((I)(e)) is in good agreement with the structure observed.
Atomically thin S–Mo–S layers contain mostly covalent bonds. On the other hand, the layers in crystalline MoS2 are held together by much weaker van der Waals forces, figure 3(II), which enable the production of MoS2 materials with different stacking sequences and thus indifferent polytypisms [53]. It is therefore possible to produce high quality nanostructured MoS2 films on wafers at the scale needed for industrial applications, with good control over film thickness and uniformity of structure and composition, and excellent quality of crystal [54]. Figures 3(III) and (IV) shows an example of the experimental furnace-based setup used for synthesis of MoS2 thin films, and correspondence between the surface density of MoO3 vapour and the size and morphology of MoS2 films.
Novel ways to engineer and tailor the electronic properties of these systems in order to obtain direct band gap 3D layered nanoparticles, or Mo doped nanowires can then be proposed from numerical calculations. It has been previously shown that single layered MoS2 nanoparticles do not exhibit significant effects resulting from quantum confinement (even for particles up to ~3.4 nm). The electronic structure in this case is found to be populated dominantly by surface states near the Fermi level. Additionally, a strong dependence of electronic properties on stacking and distance was observed in 3D nanoparticles. Therefore a fusion of these two independent material characteristics was suggested, resulting in MoS2 nanowires with Mo atoms intercalated within MoS2 planes to give rise to metallic-like conducting electronic properties [55].
MoS2 also exhibits very prominent and unique electronic properties, most notably under local deformation as indicated in experiments studying property dependence under the influence of indentation. It was shown that punctual loads only have a negligible effect on quantum transport, thereby substantiating the stable electronic properties of MoS2 under deformation and mechanical loading. This suggests that MoS2 monolayers may serve as viable materials for applications in various versatile flexible or wearable electronic devices. MoS2 also displays numerous highly distinguishable nanostructure morphologies. Some of these nano-organizations include quasi-infinite monolayers, nanonflakes, nanotubes, fullerene-like structures, and other related aggregates and clusters of various sizes and magnitudes in dimension. The array of obtainable structures can be easily obtained through precise tailoring of experimental parameters, with special attention paid to the formation energies of several prototypical species based on MoSs building blocks as shown in figure 3(V). Evidently, MoS2 based fullerenes feature sulfur-terminated corners, which have been widely agreed to serve as sites where catalytic activity is understood to originate from. However, figure 3(V) illustrates the unstable nature of fullerene-like quasi 0D nanostructures, which can be attributed to the extent of edge-induced decomposition effects, as seen in room temperature molecular-dynamics simulations. At the other end of the spectrum, the formation energies of triangular shaped nanoflakes are located almost on top of the reference level. This is expected since they are largely similar in nature to complete MoS2 monolayers which feature edges which are terminated with sulphur. As stated previously, these edges are of interest to many groups working on exploiting unique material phenomena, since they give rise to observed peculiar optical phenomena, as well as account for desirable catalytic activity at these sites [59].
Added focus should be given to quantifying and describing the dependence of optical properties on nanoflake size which can be utilized for applications in biosensing [62] as shown in figure 3(VI). The electronic properties show non-uniformity across the entire nanostructure. As reported, the discrepancies lie in the observation of metallic behaviour at the exterior regions at the flake edges, whereas the interior is observed to possess semiconducting properties. These contrasting location dependent bi-electronic properties indicate a hybrid coexistence of both metallic and semiconducting properties that exist in tandem. Additionally, it is worth noting that the optical absorption spectra in the visible spectrum show distinct red-shifts with the increase of length in the flake edges. This is a clear indication of quantum confinement effects governing the evolution of the materials optical properties at the nanoscale [60].
The results of further investigation on the mechano-structural properties of MoS2 in a graphene-like honeycomb monolayer (g-MoS2) configuration under varied loads using DFT are illustrated in figure 3(VII). It was reported that g-MoS2 has an distinct ability to withstand extensively large strains indicating notable mechanical stability. In these simulations, the ultimate strains for armchair, zigzag and biaxial deformations are given as 0.24, 0.37 and 0.26, respectively. The in-plane stiffness is reported to be as high as 120 N m−1 which is an equivalent of 184 GPa. Given these highly-desirable mechanical properties which include the high tolerable ultimate strains and in-plane stiffness, g-MoS2 has promise as a material for application in devices for clean energy storage of elastic energy. This interest is also driven by the fact that g-MoS2 possesses a theoretical energy storage capacity of up to 1.7 MJ kg−1, which is greater than that of typical Li-ion batteries with the added benefits of the former being more environmentally friendly [61]. Finally, it is also worth mentioning that the hexagonal phase of MoS2 is thermally stable up to temperatures in the excess of 500 °C, presenting a significant advantage for a number of real-life applications [63].
2.2. Physical mechanisms involved in synthesis of nanostructured MoS2
2.2.1. Early stages of MoS2 nucleation.
To study the evolution of MoS2 during the synthesis process, most notably from the nucleation to growth phase in the thermal process, experiments involving the growth of multicrystalline MoS2 monolayers on SiO2 with tailored grain sizes were conducted [64]. It was demonstrated that SiO2 substrates—often utilized as typical substrates in semiconductor technology—could be used for the continuous growth of uniform, homogeneous and high-quality monolayer MoS2. The growth of MoS2 on these substrates bears close resemblance to a typical 2D growth model [65], namely one that follows nucleation, growth, and coalescence in chronological order. With the prolonged and continuous growth processes occurring, the nucleation of the grains cause the formation of larger grains, and a homogeneous film is ultimately formed through the process of coalescence. It is observed that monolayer MoS2 is dominantly observed in most cases, with rare instances of bi- or multilayer MoS2 observed. Finally, the observation of early growth nucleation with it ceasing at later stages as indicated by the uniform size of larger grains can be explained from a thermodynamics perspective.
2.2.2. Role of precursors in MoS2 nucleation.
The influence of the nature of added precursors on the growth of mono- and multilayer MoS2, and their resulting morphologies was investigated [66]. It was reported that the choice of precursors—MoO3 and MoCl5 in this instance—results in dramatic changes in the resulting morphologies of fabricated MoS2. Explicitly, an MoO3 source was reported to give rise to a triangular shaped MoS2 monolayer, whereas MoCl5 precursors resulted in fabrication of uniform MoS2 with an omitted triangular geometry. It is worth noting that in this study, the MoS2 monolayers were grown on 300 nm Si/SiO2 substrates. It is therefore postulated that the reaction pathway involving MoCl5 in excess S may be a single step process. While no clear agreement on the definite reaction pathways have been reached, a possible chemical process is suggested as:
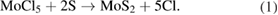
2.3. Chirality control in nanostructured MoS2/which relates to physics of formation
Observation of chirality can be defined as the production of perfectly asymmetric chemical structures that result in non-superimposable enantiomers. Common naturally occurring chiral molecules include DNA molecules, proteins, enzymes, amino acids, and a plethora of drugs including but not limited to ibuprofen and L-3,4-dihydroxyphenylalanine; as well as fragrance and food additives, e.g. aspartame and geraniol. Chirality ultimately plays an important role in chemical activity and recognition by other biomolecules, with significant implications for their use in various fields of chemistry, biology, and medical science [67, 68, 75].
One of the most commonly employed methods in preparation of 2D nanomaterials over large areas with uniform coverage is through sonication-assisted liquid exfoliation [69, 70]. This approach exploits ultrasonic energy to cleave sheets of the material through disruption of the van der Waals forces that hold the sheets together. In this method, choice of solvent plays an important role in the quality of material and its yield. Both the quality and yield can be maximised through selection of a solvent that has a surface tension which closely matches the surface energy of the 2D nanomaterial—with the assumption that entropic contributions from rigid 2D sheet exfoliation is negligible.
Recently, the utilization of specific chiral ligands of cysteine and penicillamine in a liquid-ultrasonic exfoliation process was shown to be effective in the preparation of chiral 2D nanomaterials from MoS2 (figure 4). Specifically, bulk MoS2 was processed with sonication-assisted exfoliation with an inert ambient atmosphere of argon in water in the presence of chiral molecules, which enabled the production of chiral ligand-functionalized MoS2 flakes.
Figure 4. Illustration of chiral 2D MoS2 nanostructures fabricated through sonication-assisted exfoliation in water in presence of chiral ligands such as cysteine and penicillamine. This process resulted in chiral 2D MoS2 nanosheets which exhibit strong circular dichroism signals. Reprinted with permission from [75]. Copyright 2018 American Chemical Society.
Download figure:
Standard image High-resolution imageNot surprisingly, chirality also influences the properties of MoS2 nanotubes, such as electronic property which could be tuned by chiral vectors [71]. Mechanical and electronic properties of monolayer MoS2 are also affected by the chiral vectors [72, 73]. Furthermore, chiral-controlled MoS2 nanostructures have potential applications in biomedical systems as, e.g. chiral sensor arrays [74] or nanosystems for synergistic tumour therapy [18].
- Chirality can be controlled even in the two-dimensional molybdenum sulphate flakes
Indeed, chirality is the property on nanostructures that is exceptionally important for biological and medical applications, since these characteristics directly relate to the biochemical activity of many biomolecules, e.g. enzymes and DNA [19]. Thus, when used in biomedical applications, chirality of nanostructures in some cases may be a critical determinant of their in vitro and in vivo behaviour.
3. Synthesis approaches for nanostructured MoS2
3.1. Chemical synthesis of MoS2
Let us now briefly examine an example of a chemical route that could be easily implemented for the synthesis of MoS2, and then consider several important examples of the technological realization of this approach. Along with the direct deposition of MoS2 building blocks from hot gas environment onto cold wafers, the thermolysis of ammonium thiomolybdates allow for an efficient synthesis of MoS2 nanostructures. One of the examples is the thermal breakdown and treatment of (NH4)2MoS4 under an N2 ambient which converts it to MoS3 at temperatures ranging from 120 °C–360 °C according to the reaction pathways given in equation (1). Subsequently, MoS3 in the environment serves as an intermediate which gets reduced to MoS2 as shown in equation (2). It is worth noting that this process requires annealing at higher temperatures in the excess of 800 °C. A single step mechanism however, is also possible to directly convert (NH4)2MoS4 to MoS2 at a lower temperature of 425 °C under an ambient environment of H2 through the reaction given by equation (3) [76].
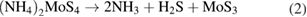
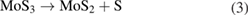
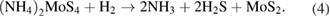
In order to achieve precisely fabricated homogeneous MoS2 films, a reduction of the threshold temperature for thermal decomposition processes has to be obtained. It is worth emphasizing that direct heat treatment of (NH4)2MoS4 films at 1000 °C in inert environments do not produce high quality MoS2. This is attributed to the fact that the reduction of (NH4)2MoS4 to MoS2 involves numerous intermediate chemical pathways, and these intermediates are strongly influenced by the presence of oxygen. Therefore, it is suggested that a H2 ambient environment is of paramount importance in order to mitigate the unwanted oxidation processes occurring intermediately [78].
3.2. Vapour–liquid–solid growth of MoS2 structures
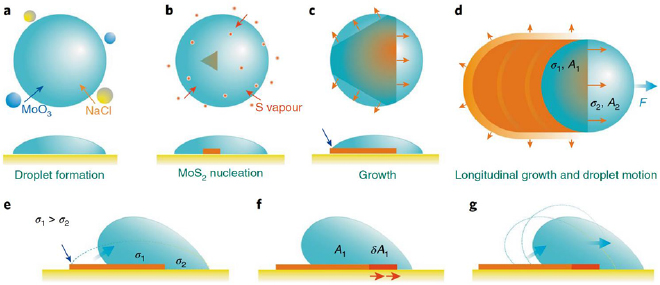
We will discuss briefly this type of growing nanostructured MoS2 using nanoribbons as an example. Li et al developed a mechanism of vapour–liquid–solid growth of monolayer MoS2 nanoribbons [77]. Technology for the growth of monolayers and other related two-dimensional (2D) materials commonly relies on the conversion of precursors in the vapour phase to solid products, a process known as a vapour–solid–solid (VSS) process. Li et al however reports the ability to utilize a vapour–liquid–solid (VLS) process for the growth of monolayered MoS2. The products obtained through this VLS method feature highly crystalline ribbon-shaped structures with widths ranging in the order of nanometres up to micrometres as illustrated in the schematics presented in figure 5. As reported, the VLS growth mode is likely induced by the reaction between MoO3 and NaCl which gives rise to the formation of molten Na–Mo–O intermediates in the form of 'droplets'. These Na–Mo–O intermediates facilitate the growth of MoS2 ribbons in a crawling motion upon saturation with sulfur on a crystalline based substrate. Such a method of growth is reported to yield both straight and kinked ribbons with well-defined local orientation which is indicative of uniform horizontal motion of the droplets in the liquid phase during growth.
Figure 5. Schematic of the mechanism of MoS2 ribbon formation: illustration of VLS growth. (a) Liquid droplet formation due to vapour phase reaction of MoO3 and NaCl; (b) nucleation of MoS2 flake at the droplet–substrate interface; (c) lateral growth of MoS2 flake; (d) lateral horizontal displacement of the droplet and continuous growth of MoS2 ribbon. (e) The droplet motion is induced by the interface energy difference between the droplet-MoS2 ribbon and droplet-substrate interfaces. The droplet exhibits poorer wettability on MoS2 ribbon than on growth substrate. (f) De-wetting of droplet to minimize the total interface free energy in a quasi-equilibrium state. (g) The droplet rolls off the nanoribbon to minimize the interface energy and restore the quasi-equilibrium state. Reprinted from [77] with permission of Springer.
Download figure:
Standard image High-resolution image3.3. Controlled hydrogen-assisted growth of MoS2 structures
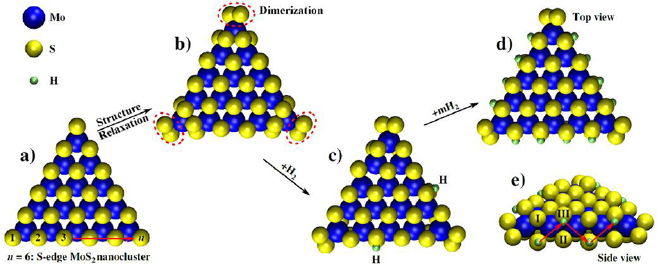
Hydrogenation is one more method for an efficient control of surface stability and for the release of H2S from quantum dots of MoS2 [78], see figure 6. The S atoms at the edges of MoS2 serve as catalytically active sites for nume-rous hydrogen based reactions, such as hydrogen absorption and hydrodesulphurization (HDS) reactions. Hence, a good understanding of the energetic stability of S-edged MoS2 nanoclusters upon hydrogenation is required. Shinde et al employed DFT simulations to explore the electronic properties as well as the energetic stability of 'S-edged' triangular MoS2 quantum dots upon hydrogenation. It was found that the conditions for maximization of the stability of the cluster was a zigzag arrangement for hydrogen absorption at the edges. The theoretical hydrogen coverage required for maximization of the stabilization energy can then be calculated from (n − 2) = 2(n − 1), where n is the given number of S atoms along an edge. Results from the calculations highlight that hydrogenated clusters feature vanishing HOMO–LUMO gaps that are attributed to the presence of unsaturated dangling bonds at the edges. This gives rise to an observable metal-like property. For hydrogenation or HDS of MoS2 nanoclusters, activation of hydrogen molecules in the gas phase is critical. It is thereby found that the energy barrier required for H2S release from MoS2 nanocluster ranges from 0.47 to 0.62 eV.
Figure 6. Optimized hydrogenated triangular MoS2 nanoclusters with S atoms aligning the edges. (a) Depiction of an isolated MoS2 nanocluster, (b) MoS2 nanocluster after undergoing structural relaxation, (c) addition of H2 yielding hydrogenated nanoclusters with NH = 2, and (d) and (e) hydrogenated cluster with NH = 12. Cluster size is indicated by n (given as 6). Reprinted with permission from [78]. Copyright 2018 American Chemical Society.
Download figure:
Standard image High-resolution image4. Technologies for fabrication of nanostructured MoS2
As stated above, techniques used for the synthesis of complex MoS2 nanostructures directly determine chemical, physical, and catalytically properties of these materials [79]. Current experimental approaches could be broadly divided into two major classes, namely bottom-up and top-down techniques. In the top-down method, nanoflakes of monolayered MoS2 may be produced through micromechanical exfoliation of MoS2 crystals. However such a method is notorious for its low throughput as well as the limited control over the dimensions of the exfoliated monolayer flakes [80]. Bottom-up methods, on the other hand, allow for better controllability over the process in MoS2 film production. Therefore, most reports featuring fabrication of MoS2 thin films with pristine quality usually employ chemical vapour deposition (CVD) methods, or feature sulphurization processes of metal or metal oxide films [81]. The following section will outline several important technologies which have proven their efficiency for the synthesis of MoS2 nanostructures.
4.1. Chemical vapour deposition and other heat-enabled methods
4.1.1. Direct sulfurization via CVD.
CVD is one of the fundamental methods for material fabrication and is widely used for the synthesis of various MoS2 nanostructures. However, a diverse set of control parameters intrinsic to CVD technology makes this method sufficiently powerful and flexible to enable fabrication of MoS2 nanostructures with highly controllable structure and properties.
One of the more successful examples is a method of synthesis of large area (2 cm × 2 cm) MoS2 ultrathin nanofilms through direct sulfurization of annealed Mo foils with a view of their application in solar cells. Given its unique band structure, the MoS2 layer primarily serves not just as an effective carrier transport layer, but also as a 'blocking layer' during diffusion processes of photo-generated carriers (holes). Through optimization of the structural properties of the MoS2 nanofilms, a significant increase in photovoltaic response was measured to reach 11.2% in terms of efficiencies for graphene/MoS2/n-Si Shottky junction-based solar cells. A detailed schematic illustration of the device fabrication process is illustrated in figure 7 [82].
Figure 7. (a) Flowchart depicting Mo foil transfer and subsequent MoS2 nanofilm fabrication; (b) schematic illustration of MoS2 nanofilm synthesis; (c) flowchart of the fabrication process of graphene/MoS2/n-Si solar cells. Reprinted with permission from [82], copyright 2018, with permission from Elsevier.
Download figure:
Standard image High-resolution image4.1.2. Atomic layers by CVD.
Lee et al described the synthesis of large-area MoS2 atomic layers with CVD [83]. Such CVD processes can be implemented to directly synthesize MoS2 layers on SiO2/Si substrates with MoO3 and S powder serving as reactants. It is worth mentioning that substrate treatment prior to growth process has a significant impact on the growth of MoS2 and should be addressed appropriately for growth of high quality MoS2. Utilization of graphene-like molecules such as perylene-3,4,9,10-tetracarboxylic acid tetrapotassium salt (PTAS), perylene-3,4,9,10-tetracarboxylic dianhydride and reduced graphene oxide during substrate pre-treatment had been proposed to promote efficient and high quality layer by layer growth of MoS2. In the experiments performed by Lee et al, MoO3 powder was placed in ceramic boats and the SiO2/Si substrate was inverted and mounted on top of the boat.
4.1.3. Morphology control in CVD.
Cao et al studied the evolution of morphology in monolayer MoS2 under different chemical environment in a CVD process [84]. In the series of experiments carried out by the group, monolayer MoS2 nanoflakes were fabricated in a single-step CVD method with solid MoO3 and S as precursors. A dual temperature zoned annealing furnace was used to provide rapid and accurate temperature control of the two precursors independently. An alumina boat containing solid MoO3 was placed in the high-temperature zone, whereas the other boat containing solid S powder was placed upstream in the low-temperature zone. The authors report the transformation of the morphologies of the monolayer MoS2 nanoflakes from a truncated triangular shape, to regular triangular shapes upon increment of the stoichiometric ratio of S:Mo in the process. As a consequence, they are able to precisely tune the optical properties of the MoS2 flakes. These finding point at good potential for engineering the morphology and optoelectronic properties on monolayer MoS2 simply through adjusting chemical environments and reactant quantities during the CVD process.
4.1.4. Role of the seeding promoter in CVD.
Ling et al studied the role of the seeding promoter in MoS2 growth by use of CVD [85]. Using various aromatic ring based molecules to promote seeding, highly crystalline and homogeneous MoS2 monolayers were obtained over large areas through CVD at comparatively low processing temperatures of around 650 °C. Significant dependence of growth quality on seed concentration as well as the effect of different seeding promoters on growth was shown. It was also suggested that it may be possible to derive conditions for optimized concentration of seed molecules to aid and enhance nucleation of MoS2. Additionally, through studies involving the effect of the nature of the seeding molecule in the facilitation of MoS2 growth, it was found that PTAS as seeding promoters will result in the ability to fabricate high quality MoS2 monolayers at low temperatures homogeneously over large areas. Otherwise, MoS2 particles may still be obtained, however the uniformity of the resulting material in the process may be lost using similar low temperature growth conditions without any seeding promoters.
4.1.5. Synchronized carbonization and sulfurization.
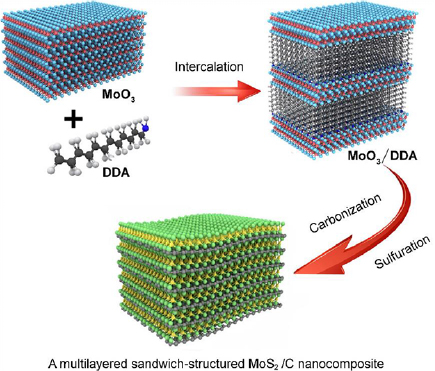
MoS2/C nanocomposites that feature multilayered sandwich-like structures based on MoS2 and C layers were also reported to be successfully synthesized through a synchronized single-step process involving tandem carbonization and sulfurization [86]. Figure 8 illustrates the fabricated MoO3 and MoS2/C nanocomposites, accordingly. In this reported tandem single-step process, an intercalation method was employed to prepare the nanocomposites. A mixture of MoO3, anhydrous ethanol and dodecylamine (DDA) was heated, washed with anhydrous ethanol and then dried after filtration to eventually obtain the MoO3/DDA powdered composites.
Figure 8. Process schematic of the fabrication process of multi-layered sandwich-like MoS2/C nanocomposites. Reprinted with permission from [86]. Copyright 2017, with permission from Elsevier.
Download figure:
Standard image High-resolution image4.1.6. Monolayer MoS2 by CVD.
Özden et al described the CVD growth of monolayer MoS2 with the main interest to present an overview on the role of growth zone configuration and precursor ratio on the resulting process [87]. The authors again note the difficulty of obtaining monolayer 2D material growth over large areas for extended usage, coherent with the challenges faced by other authors. In this study the authors implemented different substrate holders for the growth of monolayers of Mo and S. Not surprisingly, it was reported that the vapour ratio supplied to the growth zones play a critical role in influencing the shape, size, morphology and uniformity of the resulting MoS2 nanostructures. This was confirmed through experimental processing with modulated precursor ratios. Two growth approaches, namely horizontal growth, and face down growth, were investigated under identical controlled growth conditions, whereby S:MoO3 precursor ratio, substrate temperature and pressure conditions were varied in independent series of experiments. Under horizontal growth conditions, it was reported that even though the entire surface was covered with MoS2 monolayer formations, the grown structures were either found to be too small at the central region of the substrate, or they were located in larger densities at the edges of the substrate compared to that in the central region. In contrast, face down growth gave rise to nanoflakes of larger sizes, and films which are homogeneous over larger areas. These results suggest that the local Mo and S vapour concentrations in CVD growth zones have particularly significant influence on morphology as well as size and uniformity of resulting MoS2 nanoflakes and grown films [87].
4.1.7. Thermal vapour sulphurization for synthesis of MoS2 nanostructures.
Thermal vapour sulphurization for the growth of large area, continuous MoS2 atomic layers via vapour-phase mechanism was demonstrated [88]. Firstly the authors utilized a sputtering process in a magnetron sputtering chamber (with Ar/O2 as the working gases in the chamber) for the deposition of thin Mo and MoO3 films on c-plane sapphire and SiO2/Si (0 0 1) substrates. The Ar/O2 ratios were controlled through mass flow controllers. The starting material underwent a thermal vapour sulphurization process in a tube furnace under N2 atmosphere at temperatures under 1000 °C for 20–40 min. A SiO2/bell-shaped MoS2/SiO2 sandwich structure on Si was obtained through Mo based sulphurization of the SiO2/Si (0 0 1) substrates. It is worth noting that Mo sulphurizations that took place on c-plane sapphire substrates under identical conditions have led to the fabrication of the desired MoS2 atomic layers. The coalescence and ability to control the orientation of the MoS2 grains in a singular direction throughout the wafer may then be proposed to promote applications of these MoS2 layers in other practical devices.
4.1.8. Complex MoS2 nanoarchitectures via exfoliation/restacking.
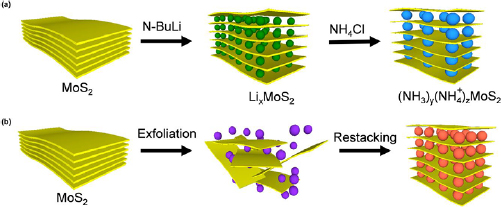
A dual step back-to-back exfoliation/restacking process was conducted to study the possibility to expand the interlayer distance in MoS2 materials to enable their application in energy storage devices as given in figure 9 [89]. In this work, the authors report an electrochemical process that resulted in the intercalation of Li atoms into MoS2. It is interesting to note the ability to tune the interlayer spacing through the modulation of the number of Li ions inserted between the layers. Expansion of the interlayer spacing results in the weakening of the van der Waals forces between the monolayers of MoS2, eventually giving rise to the ability to exfoliate monolayered MoS2 through sonication methods. The sonication then results in MoS2 monolayers which may restack into layered structures owing to the large basal areas. In the event that foreign species are present during the restacking process, these species may then be interwoven and trapped in the gaps of the restacked MoS2 monolayers that form interlayer-expanded (IE) MoS2 as shown in figure 9(b).
Figure 9. Top-down approaches for synthesizing interlayer-expanded MoS2. (a) Schematic illustration of synthesizing IE MoS2 through chemical intercalation of Li+ after the exchange with NH3 and  . (b) Schematic description of the synthesis process of IE MoS2 through back-to-back exfoliation and restacking in presence of foreign species (illustrated in orange) which are different from the species used for exfoliation (illustrated in purple). Reproduced [89]. CC BY 4.0.
. (b) Schematic description of the synthesis process of IE MoS2 through back-to-back exfoliation and restacking in presence of foreign species (illustrated in orange) which are different from the species used for exfoliation (illustrated in purple). Reproduced [89]. CC BY 4.0.
Download figure:
Standard image High-resolution image4.1.9. MoS2 nanostructures by pulsed laser deposition (PLD).
Large-area deposition of MoS2 by pulsed laser deposition was recently reported [90]. In this work, the authors report a large-area scalable process for the deposition of stoichiometric MoS2 with up to ten layers. In this reported work, the authors utilized PLD to deposit MoS2 layers on various substrates, including amorphous, multicrystalline and single crystal substrates. The authors investigated PLD conditions to enable layered deposition of MoS2 including the cooling rate of the processed substrates, the laser fluency, as well as the deposition pressure. It was found that a precise strategic combination of target composition and PLD conditions resulted in high quality deposition of MoS2 films on extra-large substrates of up to 50 mm. A notable advantage of such a processing method is the ability to precisely tune the thicknesses of the deposited MoS2 films uniformly across the entire substrate through variation of the PLD process parameters.
4.1.10. Solvothermal process for growth of MoS2 nanostructures.
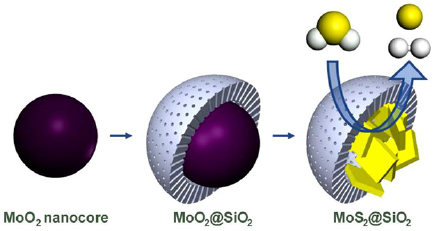
A method for growth of MoS2 nanosheets [91] within a mesoporous silica shell was developed with a view of applying the resultant materials in H2S decomposition [92]. In this work, illustrated in figure 10, a synthesis path for MoS2 nanosheets captured in mesoporous silica was employed. Owing to the fact that MoS2 may be physically confined in the central cavity of the mesoporous silica material, the MoS2 then becomes highly curved with an increased density of chemically active Mo sites. The silica shell promotes the thermal stability of MoS2 and prevents detrimental effects stemming from agglomerate formation. Therefore, the resultant material takes advantage of both catalytic potential of MoS2 active sites at the edges as well as long term stability properties of traditional catalysts. What results is a solvothermal process that effectively fabricates a nanocatalyst comprising of MoS2 in a silica mesoporous encapsulant layer. The encapsulated MoS2 nanosheets are typically observed to be short, with highly curved morphology that results in lower crystallinity and higher strain and defect density [93]. These encapsulated MoS2 sheets feature extraordinarily high catalytic performance suitable for H2S decomposition reactions as compared to MoS2 in the bulk state. Additionally, it was also shown that the encapsulation of the MoS2 nanosheets in silica enhances stability and performance of the nanocatalyst with a reported 54.7% conversion recorded for up to 38 h.
Figure 10. Schematics of synthesis of MoS2 nanosheets captured in mesoporous silica. Hydrogen atoms are given in white and sulfur atoms in yellow. Silica shells promote thermal stability of MoS2. Reprinted with permission from [92]. Copyright 2018 American Chemical Society.
Download figure:
Standard image High-resolution imageA simple, single-step method was used to generate ultra-small MoS2 nanodots. The method relied on solvothermal decomposition of ammonium tetrathiomolybdate. After they have been modified by glutathione (GSH), thus-generated MoS2-GSH nanodots displayed very low level of agglomeration in a broad range of physiological buffers, despite their sub-10 nm hydrodynamic diameter. With limited in vitro toxicity and excellent near-infrared absorbance, such nanodots can be used to generate highly-desirable photothermal ablation of cancer cells, see figure 11 [94].
Figure 11. A single-step solvothermal decomposition of ammonium tetrathiomolybdate was used to produce MoS2 nanodots. (a) Schematics of synthesis and functionalisation MoS2 nanodots. Mo 3d (b) and S 3p (c) XPS spectra of the nanodots. (d) TEM image of MoS2 nanodots prior to their functionalisation. Inset depicts high resolution TEM image of MoS2 nanodots. (e) Dynamic light scattering data used to determine hydrodynamic sizes of MoS2 nanodots as a function of their functionalisation. [94] © Tsinghua University Press and Springer-Verlag Berlin Heidelberg 2016. With permission of Springer.
Download figure:
Standard image High-resolution image4.1.11. Growing MoS2 nanostructures by photolithography.
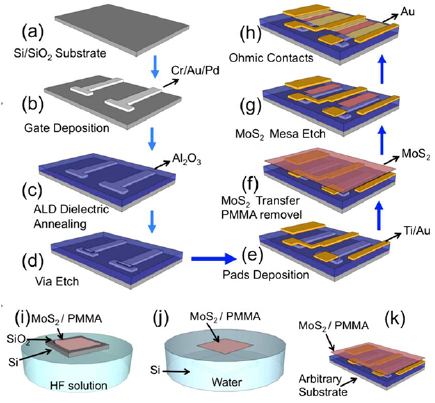
The process of fabrication of field-emitting transistors based on MoS2 application was reviewed by Yu et al [95]. The device fabrication process first begins with a thorough cleaning of the target SiO2/Si substrate using nanostrip and HCl as shown in figure 12. The gate is then formed with a cascading stack of Cr, Au and Pd with dimensions of 5 nm, 30 nm and 30 nm accordingly. Following this, gate patterning is performed through a typical photolithography process. The metal stacks for the gate are strategically chosen to give rise to the best combination of properties which include considerations for adhesion, electrical conductivity as well as work functions. An Al2O3 layer that serves as the gate dielectric is then deposited via atomic layer deposition (ALD). The circuit layout then dictates patterning of VIA holes, and the stack is then etched through a reactive ion etch process. Contact pads from Ti/Au stacks are then deposited before the commencement of the MoS2 transfer process.
Figure 12. Proposed synthesis pathways for the preparation of mesoporous silica encapsulated MoS2. Hydrogen atoms are illustrated in white, and sulphur atoms are illustrated in yellow. Reprinted with permission from [95]. Copyright 2016 American Chemical Society. (a) Si/SiO2 substrate, (b) date deposition, (c) ALD dielectric annealing, (d) via etch, (e) pads deposition, (f) MoS2 transfer PMMA removal, (g) MoS2 mesa etch, (h) ohmic contacts, (i) MoS2/PMMA, (j) MoS2/PMMA, (k) MoS2/PMMA.
Download figure:
Standard image High-resolution imageThe MoS2 samples grown through CVD are then coated with poly(methyl methacrylate) (PMMA) to serve as a supporting layer before they are immersed in dilute HF acid. The SiO2 layer beneath the MoS2 layer is etched away and the MoS2/PMMA stack is transferred to the target substrate. Finally, ohmic contacts are made with the deposition of a 90 nm Au layer. Another example of the lithographic process can be used for producing surface-bonded arrays of MoS2 nanoparticles and ultra-thin structures for specific bio-medical applications, e.g. for biosensors suitable for express-analysis and tests (figure 13). A nanoprinting lithography technology was applied to nanoimprint-assisted shear exfoliation to generate ultrathin monolayer and few-layer MoS2 structures [96].
Figure 13. Generation of patterns of layered nanostructure materials via nanoimprint-assisted shear exfoliation and transfer-printing. Reprinted from [96]. CC BY 4.0.
Download figure:
Standard image High-resolution image4.1.12. MoS2 nanostructures on epitaxial graphene.
Growth of MoS2 on epitaxial graphene (EG) was also reported [97, 98]. Authors report the ability to precisely control the thickness of two dimensionally rotationally commensurate grown MoS2 heterostructures on EG through van der Waals epitaxial growth. In this van der Waals epitaxial growth method, the requirement for a transfer process is omitted, with the synthesis of complex 2D heterostructures. Additionally, it was also reported that the rotational commensurability observed between the EG and MoS2 layers is driven by the energetically favourable alignment of the lattices, thereby resulting in perpetually strain-free MoS2.
4.1.13. Hydrothermal growth of MoS2 nanostructures.
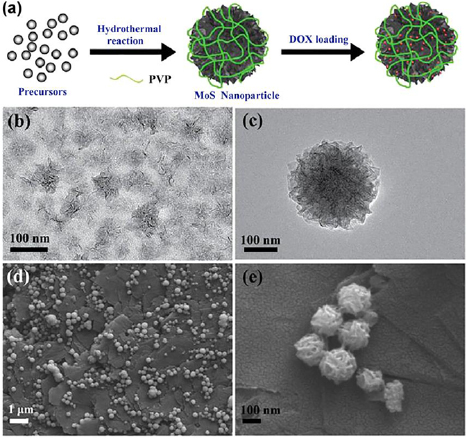
MoS2 nanoparticles with excellent biocompatibility and capacity to carry significant loads for simultaneous chemotherapy drug delivery and tumor photothermal therapy have been produced using a bottom-up hydrothermal method (figure 14). For the single-step hydrothermal decomposition, sodium molybdate and cystamine dihydrochloride were used as precursors, with hydrazine hydrate used as a reductant for MoS2 nanoparticle growth. In the process of hydrothermal synthesis, concomitant grafting of a polyvinyl pyrrolidone (PVP) coating onto MoS2 was achieved due to the chelating-coordinating effect between PVP and Mo. The functionalisation significantly enhanced the colloidal stability of nanoparticles in physiological media. Thus-synthesised MoS2 nanoparticles showed an exceptionally high photothermal-conversion efficiency and excellent ability to withstand photothermal degradation under near infrared irradiation [99].
Figure 14. (a) Generation of MoS2 nanoparticles through hydrothermal synthesis; (b) and (c) TEM and (d) and (e) field emission scanning electron microscope images of MoS2 nanoparticles. Reprinted with permission from [99]. Copyright 2018, with permission from Elsevier.
Download figure:
Standard image High-resolution image4.2. Plasma-based techniques for fabricating MoS2 nanostructures
Plasma is a potent tool for the fabrication of metamaterials, complex nanomaterials and nanoarchitectures [100, 101]. With a powerful ion flux [102, 103] and a broad set of controls, plasma-enabled methods can be used to break or activate bonds [104, 105], remove atoms, intensify surface and bulk diffusion [106], and promote many other processes [107].
4.2.1. Comparative studies on different plasma methods for synthesis of MoS2.
Three distinct plasma processes were studied to gain better understanding of the relative efficiency of plasma assisted methods for the synthesis of MoS2 [108]. Results from this study indicate that processes involving dilute remote H2S plasma (4%) and direct H2S plasma (without dilution) may result in more effective synthesis of MoS2. Findings from this study hint that a critical parameter which affects the synthesis pathways would be the presence of hydrogen radicals produced in the discharge that provide a means to reduce the MoOx film and lower the energy barrier for further reactions. Additionally, plasma enhanced growth from the vapour phase was also demonstrated through the introduction of a volatile vapour phase of MoO3 with the H2S plasma. This resulted in uniform growth of few-layered MoS2 on substrates at low temperatures bordering at 400 °C. The third and final method cited in this study for low temperature MoS2 synthesis was through cyclical sputtering of Mo followed by a H2S plasma immersion process.
4.2.2. Plasma enhanced ALD.
Sharma et al studied the conditions of low-temperature large-area plasma-enhanced ALD of 2D MoS2 with thickness control and tuneable morphology [109]. In this work, control over low temperature synthesis of monolayer-to-multilayer thick MoS2 slabs was demonstrated by the authors through plasma enhanced ALD techniques. It is worth noting that on top of the ability to precisely tailor the morphologies of the deposited layers through varying the process temperatures within a wide operating regime from 150 °C–450 °C, a good control of the layer thickness characteristic of typical ALD processes is maintained. Good control of morphologies and thicknesses is further complemented by highly-desirable wafer scaled uniformity.
4.2.3. Magnetron sputtering of MoS2 monolayers.
Tao et al reported the growth of wafer-scale MoS2 monolayers using magnetron sputtering [110]. A novel process for the synthesis of wafer-scaled atomic MoS2 layers on an array of different substrates through plasma magnetron sputtering was demonstrated by the authors. The MoS2 films were grown at relatively high temperatures in the excess of 700 °C using Mo targets sputtered in a vapourized sulphur ambient containment facility. The vapourization of sulphur was made possible through a heating element encapsulating the sulphur container before it is directed into the chamber. Materials characterization shows that the fabricated MoS2 are highly uniform, crystalline, and homogeneous. Electrical characterization also indicates p-type semiconducting character which is in contrast to the CVD fabricated MoS2 films which are known to be n-type in nature.
4.2.4. Mo sulphurization in fluorocarbon plasmas.
Jeon et al produced MoS2 layers using CF4 plasma [111]. In this work, Mo sulphurization was employed for the synthesis of 2D MoS2 thin films in an inductively coupled plasma (ICP) chemical vapour deposition reactor. The authors explored the potential for controlled etching of six-layer MoS2 through the employment of fluorocarbon based (CF4) plasmas typically used in plasma etch processes. It was eventually demonstrated that CF4 indeed serves as a favourable feedstock for etching of MoS2, with good ability to control layer thickness of MoS2 thin films.
4.2.5. Arc discharge techniques for fabricating MoS2 nanostructures.
There is a wide range of pathways for fabrication of nanostructured molybdenum disulphide nanoparticles and nanocomposites. Among them, those based on high-pressure arc discharges offer several notable advantages. For one, the production yield in arc discharges is relatively high, whereas the level of defects in thus-produced nanoparticles is very small since their synthesis takes place in the very close proximity to a high-pressure arc column, where the temperatures reach in excess of 3000–4000 K [112, 113, 116]. Furthermore, nanomaterials fabricated using this method typically display high flexibility, and as a consequence greater mechanical strength properties compared to similar nanostructures produced via other methods [10, 11, 23]. These and other advantages have led to the extensive use of synthesis approaches based on arcs for growth of single wall carbon nanotubes not only at laboratory but also at industrial scales [10, 11, 24–26].
4.2.6. High pressure arc discharge plasmas for MoS2 nanofabrication.
A typical arc discharge-based setup is shown in figure 15. In this set-up configuration, an anodic arc is generated in helium at approximately half the atmospheric pressure, with the arc current of below 100 A. The substrate is kept approximately 1–2 cm away from the arc source. Setups of this type could be magnetically enhanced, or work without an external magnetic field. Similar technology was used to fabricate hollow MoS2 nanoparticles with ultra-low friction and wear [114]. An atmospheric-pressure plasma torch system was used for treatment of MoS2–N2 composites [117]. Examples of more complex MoS2 nanoparticle-containing composites, such as TiN films with embedded MoS2 fullerenes, have also been successfully produced by employing a combination of cathodic arc reactive evaporation and supersonic cluster beam deposition [115]. The process resulted in films assembled from clusters, where MoS2 nanocages and nanostructures were successfully preserved and incorporated within the TiN matrix. Even at very low concentrations, the entrapped MoS2 nanocages were shown to significantly alter the nanotribological characteristics of the TiN matrix, as shown using by atomic force microscopy.
Figure 15. Examples of arc discharge technologies for the fabrication of MoS2 nanoparticles. (a) Schematic of the arc discharge system. Reproduced from [117]. © IOP Publishing Ltd. CC BY 3.0. (b) Arc discharge processing of MoS2–N2 composites in a plasma torch treatment reactor. Reprinted from [117] with permission of Springer. (c) Modelling of distinct MoS2 nanoparticle shapes from TEM data, with lines and closed circles representing Mo planes and sulphur atoms, respectively. The models were constructed by seamless closing of MoS2 sheets. Reprinted with permission from [118]. Copyright 2003, with permission from Elsevier.
Download figure:
Standard image High-resolution image4.2.7. Arc discharge in liquids to fabricate nanostructured MoS2.
Discharges in liquids could produce high-quality complex nanostructures. An arc discharge generated between a cathode and anode made of graphite and molybdenum carrying MoS2 microparticles immersed in de-ionized water, respectively, it was possible to produce molybdenum disulphide nanoparticles closely resembling the structure of closed caged fullerenes. High resolution microscopy confirmed that thus-produced polyhedral fullerene-like particles had a size of 5–15 nm in diameter and were typically made up of two to three layers of MoS2. These nanoparticles were formed by seamless folding of MoS2 fragments [118].
4.3. Techniques for post processing and functionalization of MoS2 nanostructures
4.3.1. Post-processing after nanofabrication.
After synthesis, MoS2 samples could be post-processed and trimmed in various reactive environments, i.e. in plasma [119]. As an example, flat planar samples can first be deposited on a substrate as a polycrystalline phase with average grain sizes in the order of hundreds of nanometres. The homogeneous as-grown MoS2 films can then be processed with low powered (~25 W) Ar plasma at low temperatures of around 150 °C for 20 min. The films are observed to 'scroll up' from the edges as indicated in the schematic presented in figure 16 once the exposure duration to the Ar plasma was increased to 40 min. The observed heights of these nanoscrolls fabricated through the given plasma process range from a few to tens of nanometres. In this process, Ar plasma is thought to play a role in deconstructing the MoS2 lattice by the removal of sulphur atoms located at the material–air interface when the kinetic energy of the impinging incident Ar ions are greater than the binding energy of Mo–S in the bulk.
Figure 16. Schematic illustration of scrolling up the MoS2 sheets by plasma post-treatment. (a) As-grown MoS2 monolayers. (b) Illustration of the rolling process of MoS2 along the edge plane upon increased exposure to Ar plasma (>40 min). (c) Eventual formation of a MoS2 nanoscroll from this process. [119] John Wiley & Sons. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imageThe dangling bonds created in this process are unable to be saturated due to the inert nature of Ar in the processing environment, which results in the creation of 'out-of-plane' strain. The additional strain induced from this collective process causes the rolling/scrolling of a single layer of MoS2 starting from the edges. While the process of rolling is ongoing, previously formed scrolls are still continuously exposed to Ar plasma. Some of the exposed S atoms may also be removed in this state (indicated by the yellow balls in figure 4) and expedite the scrolling process through the formation of covalent bonds.
4.3.2. Hydrogen plasma for controlling vacancies in of MoS2 nanostructures.
Successful application of plasma-based processes for the fabrication and treatment of MoS2 composites has been demonstrated in a number of studies [120]. One of the better examples illustrating the simplicity and efficiency of plasma-based techniques is in the exploitation of a remote hydrogen process to induce S related vacancies on the basal plane of monolayer crystalline MoS2. This process was reported to generate a high density of S vacancies while maintaining the structural integrity and overall morphologies of the MoS2 monolayers as shown in figure 17(a). The generated defects resulting from the high density of vacancies due to the remote hydrogen plasma process can then be tuned in terms of magnitude accordingly through modulation of process parameters. The ability to tune the density of defects has critical implications on hydrogen evolution reactions as illustrated in the results presented by the authors from spectroscopic and electrical measurements. The H2 plasma processed MoS2 also serves as an excellent testbed for study of the dependence of material properties based on defects in transition metal dichalcogenides that brings information on potential for future disruptive novel applications including optoelectronic and magnetic devices [120, 121].
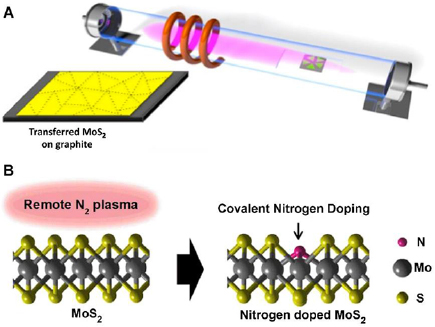
Figure 17. (A) Schematic illustration of the experimental setup for the surface treatment of MoS2 monolayers by inductively coupled H2 plasma. Reprinted with permission from [120]. Copyright 2016, with permission from Elsevier. (B) Schematic of the covalent nitrogen doping in MoS2 upon N2 plasma surface treatment. Reprinted with permission from [122]. Copyright 2016 American Chemical Society.
Download figure:
Standard image High-resolution image4.3.3. Nitrogen doping of MoS2 in plasma.
Successful application of N2 plasma for nitrogen doping of MoS2 nanostructures was recently demonstrated (figure 17(b)). After N2 plasma exposure, the group analysis of the surface chemistry of the resulting MoS2 material indicates that N is prone to typically form a covalent bond with Mo through chalcogen substitution of S. Additionally, it was further pointed out that the N concentration in MoS2 can be tuned precisely through varying duration of exposure to N2 plasma. Results from electrical characterization further indicate that N acts as a p-type substitutional dopant in MoS2 [122].
4.3.4. Remote N2 plasma post-treatment.
Qian et al performed plasma-enhanced dielectric deposition of single-layer MoS2 with low damage using remote N2 plasma treatment [123]. In this work, the authors present a pioneering report on the utilization of remote N2 plasma for promotion of dielectric deposition of single-layer MoS2. It was found that N2 and O2 plasmas tend to cause defects in MoS2 albeit in different ways. As an example, it was shown that single layered MoS2 is notably more stable in N2 plasma as compared to O2 plasma. This is likely due to the fact that O2 plasmas cause defects primarily through oxidation of MoS2 surfaces where defects are already present. These defects usually are present at flake edges which get oxidized rapidly, and it is observed on the macroscopic scale that this has no dependence on the thickness of MoS2. For defect formation in the case of N2 plasmas however, defects are created primarily through strain and distortion effects of the MoS2 layers after adsorption of N on the surface. Since there is known to be a relatively strong MoS2–substrate interaction coupled with the relative chemical inertness of MoS2 in N2 plasma, it is observed that single-layered MoS2 exhibit excellent stability in the presence of N2 plasma. This remains the case even after long term exposure to N2 plasma where only stable point defects are observed.
4.3.5. Effects of plasma on surface properties of ultrathin layered MoS2.
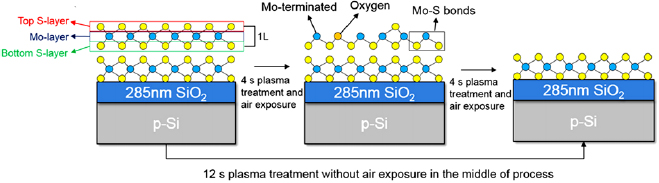
Plasma treatment of surface properties of the ultrathin layered MoS2 is a convenient tool for their functionalization and modification [124]. As reported, O2 plasma treatment is suggested as a method to control the thickness as well as the electronic band structure of MoS2 films. This could be enabled through the adoption of a low intensity ICP source, where the effects of the chemical reaction on the MoS2 surface are relatively mild but still sufficient to induce desired alterations in structural and electronic properties of these materials. In order to do this, the authors first subject virgin MoS2 to O2 plasma for a few seconds. The samples are then exposed to ambient air, and subjected to O2 plasma again, before being exposed to air once more and characterized. The schematic illustration of the process is shown in figure 18. Through this method, exposure to air causes weakening of Mo–S bonds. As a result, subsequent to air exposure, only 4 s of O2 plasma exposure is required for complete removal of 1L of MoS2. Comparatively, a 12 s treatment time is required to completely etch off 1L of MoS2 if exposure to air was not performed intermediately. This study effectively demonstrates the ability to precisely control over layers in the MoS2 material through modulation of duration of exposure to plasma.
Figure 18. Schematic illustration of the atomic states of MoS2 depicting 1L etching based on an intermediate ambient air exposure pathway (requiring a total of 8 s plasma exposure), and a 12 s continuous O2 plasma exposure—both resulting in the same products. Reprinted with permission from [124]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution image4.3.6. Treatment in oxygen and argon plasmas.
Mild oxygen plasma treatment was used to improve the electrical performance of MoS2 [13] to repair the structural defects and improve the electron mobility of monolayer MoS2 by up to an order of magnitude. This is attributed to the easy chemical bonding of oxygen with MoS2 at sulphur vacancy sites. An interesting and very convenient method for tuning the properties MoS2 by Ar plasma was also demonstrated recently [125] using a technology for the layer ordered etching of MoS2 nanosheets. What is most notable of this proposed method is the ability of the complete removal of the top layer of MoS2 with the bottom layer remaining basically unadulterated. This allows one to fabricate 2D heterostructures with periodic single and bilayered MoS2.
The electronic transport properties of FETs based on MoS2 of various layer thicknesses can also be tailored via controlled exposure to oxygen plasma [126]. In this case, high density of defect sites can be induced by O2 plasma, which allows for devices employing up to eight layers of MoS2 to have electronic properties that can be tuned from a semiconductor to a complete insulator. However, it is also worth mentioning that the time required for conversion to a fully insulating material depends on the number of layers inherent in the presented samples.
5. Biomedical applications of MoS2 nanostructures
Nanostructured molybdenum disulphide nanoparticles and composites have found numerous applications in, e.g. catalysis for hydrogen evolution [127, 128], photocatalytic synthesis and degradation [129, 130], advanced bio-sensing platforms [131, 132], wireless environmental monitoring [133] and ultrafast detection [134], optoelectronics [135], solar cells [48], electrode catalysts for dye-sensitized solar cells [136, 137], catalyst for hydrogen-based applications [138], as well as thin flake field effect transistors [139], photo luminescent-based devices [140] and many others. The interested reader could find more information on various applications of the molybdenum disulphide nanostructures in the above cited and other literature. However, in this section we will focus on several examples of the application of molybdenum disulphide nanoparticles and composites in medicine.
5.1. MoS2 nanostructures for photothermal therapy
5.1.1. MoS2 nanoflakes for photothermal oncotherapy.
Cancer therapy is one of the most important applications of MoS2 nanostructures, particularly in photothermal therapy. Recently, one-pot synthesis of MoS2 nanoflakes through a hydrothermal method was reported (see figure 19). Thus-produced MoS2 nanoflakes possessed degradation properties that are critical for many biomedical applications. Poly(acrylic acid) was used as a chemical facilitator that enabled decoration of MoS2 nanoflakes with polyethylene glycol (PEG), as well as playing an important role in controlling degradation of the MoS2 nanoflakes. The PEG-ylated hybrid nanoflakes (MoS2 PPEG) also displayed superior stability when exposed to different media, coupled with exceptional photothermal properties. Interestingly, significantly different degradation rates were observed under different conditions. Rapid degradation of MoS2-PPEG was observed in neutral solutions, whereas the degradation was somewhat slower in under acidic conditions that are typical of tumour microenvironment. Results from this study indicate that the major degradation byproduct of MoS2-PPEG is water soluble Mo-based ions. Favourable in vitro biocompatibility of MoS2-PPEG was demonstrated with cytotoxicity and haemolysis studies. Therefore, with the favourable photothermal performance presented, it can be further suggested that MoS2-PPEG is a potential candidate for in vivo suppression of tumour growth. Finally, the decreased detectable content of MoS2-PPEG in organs (and elemental Mo in urine) of a murine model indicate suitable level of degradability of MoS2-PPEG and subsequent excretion of degradation products, both of which are critical for translation to clinical applications [141].
Figure 19. Schematic illustration of the preparation process of MoS2-PPEG with desired degradation properties required for in vivo photothermal cancer therapy. Reprinted with permission from [141]. Copyright 2017 American Chemical Society.
Download figure:
Standard image High-resolution image5.1.2. Ultra-small MoS2 nanodots for photothermal cancer therapy.
It was also recently demonstrated that ultra-small, activated MoS2 nanodots could also be efficient for the photothermal therapy [94]. A single-step bottom-up approach was adopted by the authors to develop theranostic agents with good clearance based on ultra-small MoS2 nanodots. Specifically, this process involves solvothermal decomposition of ammonium tetrathiomolybdate. The MoS2-GSH nanodots obtained after exposure to GSH exhibited hydrodynamic diameters under 10 nm without aggregation in various buffers. While showing no significant effects in terms of in vitro toxicity, the MoS2-GSH nanodots also exhibit strong near-infrared (NIR) spectral absorbance [142].
This is highly desirable since this characteristic could potentially induce phenomenal photothermal ablation of cancer cells. In a murine model, efficient tumour accumulation of the MoS2-GSH nanodots upon introduction through intravenous (IV) injection was observed through photoacoustic imaging. This phenomena was substantiated by the analysis of the biodistribution of elemental Mo. In comparison to conventional MoS2 with nanoflakes of larger sizes, the MoS2-GSH nanodots exhibited ultra-efficient clearance from the body through urine, where a vast majority of the injected dose was excreted within seven days. Additionally, photothermal ablation of tumours in mice was then studied, with the MoS2-GSH nanodots showing superior therapeutic efficacy compared to other types of nanodots. This landmark study provides further evidence for the potential of MoS2 nanoparticles as efficient agents for direct tumour treatment, with an added benefit of being rapidly degraded and removed from the body which should minimise the potential for long-term toxicity. This makes it a promising candidate for cancer theranostics [94].
5.1.3. Complex MoS2 nanostructures for photothermal therapy.
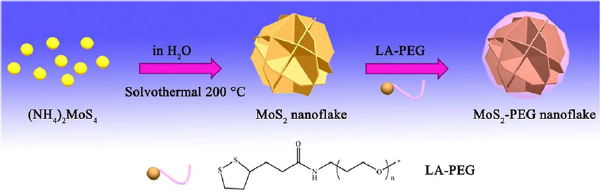
Flower-like MoS2 nanoflakes with high colloidal stability and ultra-low cytotoxicity can be synthesized through a modified hydrothermal method and modification with lipoic acid-terminated polyethylene glycol (LA-PEG) [143]. It is demonstrated that the nanoflakes exhibited commendable abilities to induce high temperatures, with good photothermal stability and conversion efficiencies upon irradiation with an NIR laser (808 nm). The authors also studied the in vitro photothermal effects of MoS2-PEG nanoflakes with varied concentrations under different power densities of the incident 808 nm laser radiation. Results from this study show that low concentration of the MoS2 nanoflakes under a low powered NIR laser irradiation display photothermal efficiency that is sufficient to kill cancer cells. It is expected that in vivo destruction of cancer cells could also be made possible through irradiation induced photothermal effects from the MoS2-PEG nanoflakes (figure 20).
Figure 20. Synthesis pathways for the production of MoS2-PEG. Reprinted with permission from [143]. CC BY 4.0.
Download figure:
Standard image High-resolution image5.1.4. Printed 3D MoS2 nanoarchitectures for photothermal therapy.
The rapid proliferation of 3D printing techniques in biomedical fields have enabled successful fabrication of novel bifunctional MoS2-containing scaffolds (MS-AKT scaffolds) through a hydrothermal complemented process [144]. MoS2 nanosheets were grown on the strut surface of bioceramic scaffolds in a typical hydrothemal process. The presence of nanosheets on the surface of the scaffold enables its application in photothermal therapy, where upon exposure and irradiation from NIR sources, temperatures of the MS-AKT scaffolds can increase rapidly. This effect from temperature increment can be precisely controlled by variation of the MoS2 content, laser power densities, as well as sizes of the MS-AKT scaffolds. This work illustrates how the photothermal temperature may have the potential to inhibit tumour growth, and significantly reduce the viability of osteosarcoma and breast cancer cells in vivo. Additionally, the MS-AKT scaffolds are found to promote in vivo rapid proliferation and osteogenic differentiation of bone mesenchymal stem cells and induce bone regeneration. This multifunctional scaffold that offers strategies in simultaneously treating tumours and facilitating bone growth is therefore has the potential to become a game changer in clinical treatment of tumour-induced bone defects (figure 21).
Figure 21. Schematic representation of the in situ growth and processing of MoS2 nanosheets on 3D printed bioceramic scaffolds (a) and their dual faceted applications in tissue regeneration and tumour therapy (b). MoS2 nanosheets are grown on the surfaces of the bioceramic scaffolds during the hydrothermal processes, forming a core/shell structure with a MoS2 shell. The MoS2 layer then serves to enable the scaffolded collective structure with photothermal therapeutic capabilities. Separately, the release of soluble Ca, Si, Mg and Mo ions may also simultaneously stimulate tissue regeneration. Reprinted with permission from [144]. CC BY 4.0.
Download figure:
Standard image High-resolution image5.2. Other approaches for medical application of MoS2 nanostructures
5.2.1. MoS2 nanoarchitectures for targeted fluorescent imaging in medicine.
Diagnostics is a critical part of a successful oncotherapy. To that effect, functionalization of single-layered MoS2 nanosheets (NS) with fluorescent quantum dots (QD) and arginine–glycine–aspartic (RGD) containing peptides have been shown to result in low dimensional (0D–2D) RGD-QD-MoS2 NS with remarkable fluorescence, photothermal conversion and cancer-targeting properties that can serve a plethora of applications in the biomedical field [145, 146]. The authors show how targeted fluorescent imaging and photothermal therapy of human cervical carcinoma (HeLa) cells may be enabled through the engineering of RGD-QD-MoS2 NS as a theranostic agent. Using a murine model, effective fluorescent imaging and a full eradication of HeLa tumours through photo-thermal irradiation of RGD-QD-MoS2 NS using a low powered NIR laser was shown. RGD-QD-MoS2 NS were found to preferentially accumulate at tumour sites. This is made possible through RGD-integrin targeting and the enhanced penetration and retention (EPR) effect. Coupled with minimal reported toxicity effects to living organisms, RGD-QD-MoS2 NS has exhibit significant potential as a dual-functional theranostic agent for cancer therapy and imaging (figure 22).
Figure 22. (Top) Application of RGD-QD-MoS2 nanosheets in photothermal therapy and cancer imaging. Reproduced from [145] with permission of The Royal Society of Chemistry. (Bottom) Illustration of the high-throughput reaction pathway of MoS2-CS nanosheets. This is proposed to serve as a functional NIR photothermal triggered drug delivery system for cancer therapy. (a) and (b) Single layer MoS2 nanosheet production through Oleum treatment exfoliation processes. (c) Doxorubicin (DOX) loading process, and (d) delivery of drugs through NIR photothermal triggering to tumour sites. Reprinted with permission from [91]. Copyright 2014 American Chemical Society.
Download figure:
Standard image High-resolution image5.2.2. MoS2 for simultaneous diagnostic and therapy.
A nanoplatform consisting of an aptamer (a molecule that selectively binds to another biomolecule) and PEG conjugated with MoS2 NS with Cu1.8S nanoparticles (ATPMC) has also shown good potential for applications in targeted therapy and medical imaging [147]. Examples of the latter include photoluminescence imaging, photoacoustic imaging, as well as in vitro and in vivo tumour cells imaging through photothermal methods. ATPMC also facilitates selective delivery of gene probes to detect intracellular microRNA that is not usually expressed in cancer cells, as well as for delivery of drugs such as doxorubicin (DOX) which is commonly employed for chemotherapy. Additionally, it is worth noting that the synergy between MoS2 and Cu1.8S provides the ATPMC platform with exceptional photothermal conversion efficiencies [148].
5.3. MoS2 nanostructures for drug delivery
5.3.1. Surface-functionalized MoS2 nano-sheets for drug delivery.
Fast targeted delivery of drugs to tumour cells is a critical factor in cancer and other types of therapies. Functionalized MoS2 nanosheets as a multi-functional drug carrier for combination treatment of cancer were recently fabricated and tested [149]. MoS2 was first synthesized through exfoliation from the bulk; functionalization with LA-PEG using thiol reaction was then performed to increase biocompatibility and physiological stability of the material. 2D MoS2-PEG NS exhibit prominent NIR absorbance. The uniquely large surface area-to-mass ratio of MoS2 nanosheets (owing to their atomically-thin structures) enables the high loading efficiency of therapeutic molecules including typical chemotherapy drugs such as DOX, 7-ethyl-10-hydroxycamptothecin (SN38), and a photodynamic agent chlorine e6. In addition to showing minimal toxicity to cells, MoS2-PEG may also be implemented in combined photothermal and chemotherapy after loading with doxorubicin. This was demonstrated both using in vitro cell culture experiments, but also in vivo using a murine tumour model, with high level of anti-cancer activity arising from the synergistic coupling of MoS2-EG and DOX following both intratumour or IV administration [35, 149].
5.3.2. Surface modification of MoS2 NS with copolymers.
Modification of surfaces of MoS2 NS with copolymers through the fusion of mussel inspired chemistry and single-electron transfer living radical polymerization can be performed using 2-methacryloyloxyethyl phosphorylcholine (MPC) and itaconic acid (IA) as monomers. It was shown that the MoS2-PDA-poly(MPC-IA) nanocomposites exhibited promising dispersability and biocompatibility. The drug loading capabilities and controlled drug release characteristics of MoS2-PDA-poly(MPC-IA) towards cis-diamminedichloroplatinum commonly used in chemotherapy was also studied. It was then determined that the drug loading capacity in MoS2-PDA-poly(MPC-IA) nanocomposites reach up to 55.26%. This indicates that the proposed strategy for fabrication of the aforementioned MoS2 based nanocomposites may potentially have significant applications in cancer related therapeutic fields [150].
5.3.3. Photothermally triggered drug release system based on MoS2 nanostructures.
This interesting platform allows efficient control of the drug delivery and release [91]. Functionalized MoS2 NS that have been developed as a chemotherapeutic drug carrier for NIR photothermal-induced drug delivery can also be fabricated through the decoration of MoS2 NS with chitosan. This promotes the integration of both photothermal therapy and chemotherapy into a consolidated all-in-one system for cancer therapy. Controlled release of DOX can be triggered via photothermal effects using irradiation by an 808 nm NIR laser. As compared to standalone chemotherapy or photothermal therapy methods, in vitro and in vivo tumour ablation studies suggest potential synergistic therapeutic effects resulting from the combined treatment.
5.3.4. Anti-angiogenic effect of MoS2 nanoparticles.
During embryonic development, blood vessels are first developed via vasculogenesis, after which point new blood vessels grow from existing vessels to accommodate organism growth and development, as well as wound repair in the process called angiogenesis. This process is tightly controlled through specific signalling pathways, and generally its activity declines with age. However, activation of signalling pathways during tumourigenesis is a critical step in the transformation of benign tumours into malignant counterparts, where the formation of additional blood vessels ensures effective delivery of oxygen and nutrients to the growing tumour and removal of waste products. To initiate and stimulate this process, cancer cells release various growth factors and proteins. Not surprisingly, a number of antiangiogenic drugs have been developed to halt blood supply from the tumours, however when introduced systemically, these drugs are often ineffective due to dilated and often abnormally structured blood vessels within the tumour. Furthermore, the often show detrimental side effects in vivo. Nanomaterial-based therapies and drug delivery platforms offer several advantages, including relatively high capacity for drug loading and controlled release of drugs into tumour microenvironment, higher blood half-life and lower cyto-, organ and systemic toxicity of these structures [151]. For instance, when introduced into a chicken embryo as part of a HeLa cell-extracellular matrix (ECM) gel suspension, MoS2–ZnO nanocomposites have been shown to significantly reduce the expression genes associated with angiogenesis, i.e. VEGF and VEGFR2, preventing vascularization into the tumour intima [152]. Furthermore, exposure to the MoS2–ZnO nanocomposite were also shown to activate caspase-3, a cysteine-aspartic acid protease known for its role in apoptosis.
6. Summary and outlook
Efficient, reliable and affordable anti-cancer therapy approaches are of critical importance to improve the quality of life and extend the lifespan of people globally. Nanoparticle-based techniques including those based on nanostructured MoS2 have the potential to offer a simple yet effective means for killing cancer cells, and could find their applications in various environments, including, e.g. long-term space and interplanetary missions where space radiation presents a considerable risk factor for the development of the disease [153]. A favourable combination of properties of MoS2-based nanostructures make them promising candidates for a wide range of applications spanning catalysis, optoelectronics, and biomedical applications, particularly in detection, visualisation and treatment of tumours. From the examples discussed above, it is evident that synthesis and assembly processes play an important role in defining the structure and properties, and thus the potential applications for MoS2-based nanostructures. This presents opportunities as well as challenges for scale-up. Indeed, micromechanical exfoliation known to provide MoS2-based nanostructures of superior quality typically offers a relatively low yield, which would limit its use in applications that require significant volumes of these nanostructures. On the other hand, processes based on liquid phase exfoliation and solution chemical processes are more affordable and amenable to scale up. CVD and ALD also offer high quality MoS2 nanostructures and thin films, with additional flexibility gained from combining these methods with plasma technologies that can deliver an additional set of process controls for material assembly and functionalisation. The use of plasmas is particularly promising for in situ controlled introduction of defects and dopants for controlling chemical reactivity and optoelectronic properties of MoS2-based nanostructures to enable their application in bio- and chemical sensing, catalysis and optoelectronic devices. Electric fields in plasma environments can also be used to control the nanoassembly of complex multi-component structures, enabling the fabrication of non-toxic, affordable catalytic platforms for chemical processing (owing to highly active edge sites of MoS2), sensing (due to high carrier mobility in MoS2), and visible light photocatalytic degradation of pollutants from aquifers (due to MoS2 ability to produce electron–hole pairs).
However, there are a number of issues that currently face any nanoparticle-based approach used for anti-cancer therapy.
- How to ensure sufficient efficiency of MoS2-based system when applied to tumours of various origins?
Combinational therapy and synergistic approaches could be the most promising way to significantly enhance the selectivity and efficiency of nanoparticle-based therapies.
Careful analysis of the behaviour of active nanoparticles in living tissues evidences a wide range of interrelated processes (see figure 23 that illustrates the diversity of processes at the various stages of therapy), which could participate in the therapy independently but in some cases and under some conditions, they might manifest non-additive, synergistic and combinatorial effects.
Figure 23. Representation of the several events that occur during nanomaterial-mediated photothermal therapy in an animal model, in this example, a murine model. First, nanomaterials are administered to an animal with a tumour and accumulate in the tumour through the so-called EPR effect. Afterward, the tumour zone is irradiated and nanomaterials induce hyperthermia with a high degree of spatial and temporal resolution. Finally, the therapeutic effect is monitored, with one of the possible outcome parameters being animal survival due to tumour elimination. [154] John Wiley & Sons. © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution image6.1. Synergistic therapies
Efficiency of synergistic therapies for treatment of various diseases is well document, e.g. these therapies have been used for the treatment of tuberculosis [155], multidrug-resistant bacterial infections [156, 157], temporomandibular disorder by synergistic laser and ultrasound application [158] and a wide range of diseases that have a microbial origin [159, 160]. Although the nanostructure-based cancer therapy is yet to develop into a mature technology, the synergistic effects of the drug-based cancer treatment using these platforms have already been demonstrated for various treatment technologies and various tumour localizations [161–163]. To achieve the best possible synergistic effects on the nanoparticles-based (including MoS2) methods and finally to translate synergistic MoS2 techniques into clinical applications, it is essential to understand how MoS2 nanoparticles influence and affected by intracellular processes. A new study has presented a systematic experimental effort investigating how MoS2 nanomaterials interact with cells, with a specific focus on pathways for their internalisation, accumulation and removal (i.e. efflux) from the cell, as well as potential strategies to control exocytosis in order to enhance efficacy of MoS2-based cancer therapy. Nanomaterials were determined to be internalized via three possible pathways (figure 24).
- 1.Clathrin → Early endosomes → Lysosomes
- 2.Caveolae → Early endosomes → Lysosomes
- 3.Macropinocytosis → Late endosomes → Lysosomes
Figure 24. Illustration of various therapeutic effects achieved by combining PEGylated MoS2 nanostructures with low doses of a drug with a capacity to inhibit exocytosis pathway-induced loss of nanostructures. (a) Without a drug or (b) with inhibition of Exo1. It is observed that even though similar levels of heat can be generated in both treatment groups, Exo1 inhibition leads to enhanced accumulation of PEGylated MoS2 nanostructures in cancer cells, which, when coupled with generated heat, caused higher degree of damage to organelles and proteins inside the cells. Without the inhibition of Exo1, certain quantities of nanosheets are expelled from the cells into extracellular milieu; the heat generated from these nanostructures is not sufficiently high to induce significant damage to organelles and proteins inside the cells due to the protective action of cell membranes that help maintain cellular homeostasis. Reprinted with permission from [164]. Copyright 2018 American Chemical Society.
Download figure:
Standard image High-resolution imageThe intracellular accumulation of nanoparticles in the lysosome was mediated by autophagy, whereas removal of nanoparticles from the cell was through exocytosis, whereby membrane-bound secretory vesicles containing nanoparticles are transported to the cell membrane, at which point their MoS2 cargo is secreted into the extracellular milieu. The latter process was found to effectively reduce the intracellular concentration of MoS2 nanoparticles, thus reducing the overall efficacy of the thermal treatment once irradiation was applied. Using a low dose of a drug known to interfere with exocytosis, it was possible to prevent efflux of MoS2 NS, resulting in a more efficient killing of cancer cells [164].
In-depth understanding of the entire spectrum of processes would open a door to drastic enhancement in selectivity and efficacy of MoS2-based cancer therapies.
6.2. Combinational therapy
It is also a promising approach which in principle does not rely on synergistic effects, i.e. does not involve a mutual amplification of individual effects but benefits from a simpler additive technology. Being simpler to study and apply, combinational technologies have also demonstrated their efficiency for, e.g. combined photothermal and photodynamic therapy [165, 166] and photo/thermo-based tumor imaging and therapy [167, 168], see figure 25 and detailed comments in the figure caption. Other combinational approaches that utilize various size nanoparticles and different physical and chemical effects [169–172], together with various drug platforms, could also be promising and should be recommended for intensive investigation [173–176].
Figure 25. (Top panel) Combination therapies to selectively target activation and inhibition of pathways associated with the development of vessels that supply blood to tumours, i.e. angiogenic signalling pathways. Reproduced from [151] with permission of The Royal Society of Chemistry. (Bottom panel) MoS2–ZnO nanocomposites can suppress epithelial to mesenchymal transition (EMT), a critical stage in cancer metastasis. The ability of MoS2–ZnO to downregulate EMT was demonstrated by introducing a HeLa cell-ECM gel suspension into fertilized chick embryos, with some gels also containing 236 mg ml−1 MoS2–ZnO, and measuring the expression levels of genes associated with epithelial and mesenchymal states after 5 d of incubation (a). (b) Proposed mechanism of activity of the MoS2–ZnO nanocomposite against different factors of carcinogenesis. Reproduced [152] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageAcknowledgments
This work was supported by the US Department Of Energy, Office of Science, Fusion Energy Sciences program Award #DESC0015767, in part by the OSTIn-SRP/EDB through the National Research Foundation and in part by MoE AcRF (Rp6/16 Xs), Singapore. I L acknowledges support from the School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology. O B acknowledges support from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 766894.