Abstract
By investigating the atomic oxygen density in its effluent, two-photon absorption laser-induced fluorescence (TALIF) spectroscopy measurements are for the first time performed in a cold argon/oxygen atmospheric pressure plasma jet. The measurements are carried out in ambient air and quenching by inflowing air species is considered. We propose a novel absorption technique in the VUV spectral range, where emission originating from within the discharge is used as light source to determine the inflow of atmospheric oxygen into the effluent. Furthermore, we propose a modelling solution for the on-axis density of inflowing ambient air based on the stationary convection–diffusion equation.
Export citation and abstract BibTeX RIS
1. Introduction
Due to progress in recent years in the development and research of non-equilibrium atmospheric pressure plasmas with high reactive species densities at low gas temperature, plasma treatment of living tissue has become possible and promises an efficient and improved treatment of infected or chronic wounds [1]. For a thorough analysis of the interaction of cold atmospheric pressure plasmas with biological materials, a detailed quantitative analysis of the output from the discharge's reactive component is required. This work presents atomic oxygen density measurements for the first time in an atmospheric pressure argon plasma jet by two-photon absorption laser-induced fluorescence (TALIF) spectroscopy. At atmospheric pressure collisional quenching of the fluorescence radiation significantly influences the TALIF measurements and renders a quantitative analysis difficult. Previous approaches to operate the plasma in a controlled noble gas atmosphere and thus to reduce the number of relevant quenchers [2, 3] significantly change the chemistry in the effluent. Other approaches include numerical diffusion simulation to solve this problem [4]. In our investigations, an argon plasma jet is operated under realistic conditions in ambient air and quenching by ambient air species (O2 and N2) is considered by experiments and simulations. The densities of the quenching species diffusing into the jet effluent from the surrounding air are determined by novel techniques. Indiffusing molecular oxygen is measured by a new absorption spectroscopic technique, where the vacuum ultraviolet (VUV) radiation emitted from the jet's core plasma region serves as a background light source to determine the O2 density.
The measurements are correlated with a diffusion model, which applies a new analytic solution of the stationary convection–diffusion equation. This analytic solution yields a general expression for the on-axis density of ambient species and in this work applies to molecular oxygen as well as molecular nitrogen.
2. Cold atmospheric pressure argon plasma jet
A concentric atmospheric pressure argon plasma jet—the so-called kINPen—is investigated. In the centre of a quartz capillary (inner radius r0 = 0.8 mm) a pin-type electrode (0.5 mm radius) is mounted (see figure 1). In the continuous working mode, a high-frequency voltage (1.1 MHz, 2–6 kVpp) is coupled to the pin-type electrode [5]. The plasma jet is operated with an argon flux of 1 to 6 standard litres per minute (slm) and in this work with 1% molecular oxygen admixture.
Figure 1. Schematics of the kINPen.
Download figure:
Standard image3. Measurement of atomic oxygen density by TALIF spectroscopy
Atomic oxygen density in the jet's effluent is measured by TALIF spectroscopy, where ground-state oxygen atoms (2p4 3P2) are excited by two UV-photons (225.65 nm) into the 3p 3PJ state and the resulting fluorescence radiation—proportional to the ground-state densities—is detected in the infrared (844.87 nm de-excitation to 3s 3So), as described in the following. The oxygen measurements are calibrated by TALIF measurements on xenon (see section 3.2).
3.1. Experimental setup
Figure 2 illustrates the setup and the principle of the TALIF measurement system. By mounting the jet on a three-axis manipulator, it is possible to move the jet through the detection volume along the axes of a self-defined coordinate system, yielding space-resolved measurements of the oxygen distribution. The detection volume is defined by the overlapping foci of the laser beam (d = 200 µm) and the imaging volume of the fluorescence light (d = 500 µm). The fluorescence is detected by a gated photomultiplier synchronized to the 10 Hz pulsed nanosecond laser beam.
Figure 2. Principle of space resolved TALIF spectroscopy.
Download figure:
Standard imageAs the plasma jet operated in argon produces excited argon species (2P
 4p), radiating at 842.42 nm close to the oxygen fluorescence light, the use of an interference filter (FWHM = 1 nm; T = 50%, λ0 = 844.5 nm) in front of the photomultiplier is necessary in order to reduce stray light and increase the signal-to-noise ratio.
4p), radiating at 842.42 nm close to the oxygen fluorescence light, the use of an interference filter (FWHM = 1 nm; T = 50%, λ0 = 844.5 nm) in front of the photomultiplier is necessary in order to reduce stray light and increase the signal-to-noise ratio.
Figure 3 shows the oxygen resonance and the variation of the laser energy yielding the relative oxygen fluorescence signal normalized over the square of the laser energy. The signal is proportional to the square of the laser energy in the low energy regime. This guarantees that apart from negligible spontaneous emission no competitive processes like photo-dissociation take place which could prohibit a quantitative analysis. The calibration measurements were performed at the defined origin (0,0,0) of the coordinate system close to the nozzle with an argon flow of 5 slm and 50 sccm of O2 admixture defined as standard conditions.
Figure 3. Two-photon absorption resonances of oxygen (a) and xenon (c). Variation of laser energy on oxygen (b) and 1.5 Pa xenon (d).
Download figure:
Standard image3.2. Calibration of the oxygen signal with xenon
For the absolute calibration of the oxygen signal a reference measurement at the same position in a controlled xenon atmosphere is performed [2], which means comparing the atomic oxygen fluorescence signal to the fluorescence signal of a known density of xenon according to

Here SO denotes the normalized oxygen TALIF signal (see figure 3), n are the ground-state densities of the excited species, T and η are optical properties of the experimental setup, a32 the quenching-corrected branching ratios of the observed fluorescence transitions (see section 3.3) and σ are the two-photon excitation cross sections [3]. The lower index denotes the species whereas the upper index refers to the transition. The normalized xenon TALIF signal SXe of the known xenon density nXe is gained from the reference measurements under a variation of the laser energy at different xenon pressures (see figure 3). For high laser energies a non-proportional behaviour due to saturation effects can be observed. From the comparable measurements in xenon using equation (1) the atomic oxygen density under standard conditions and the defined origin is determined to be nO = 2.75 × 1015 cm−3.
3.3. Quenching (fundamentals)
At atmospheric pressure, quenching becomes relevant and needs to be considered. It is implicitly considered in the calibration (see equation (1)) through the effective branching ratios for the oxygen and the xenon excited states:
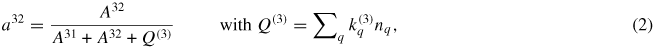
where A denotes the spontaneous emission rate of the excited state and Q(3) the effective quenching rate, depending on quenching partner densities nq and the corresponding quenching coefficients
 taken from [6]. Equation (2) shows that quenching reduces the effective branching ratios of the spontaneous emission of the excited states. In the case of oxygen, neutral ground-state argon atoms and oxygen and nitrogen molecules are considered to be the most efficient quenching partners. In xenon atmospheres only self-quenching by xenon atoms takes place.
taken from [6]. Equation (2) shows that quenching reduces the effective branching ratios of the spontaneous emission of the excited states. In the case of oxygen, neutral ground-state argon atoms and oxygen and nitrogen molecules are considered to be the most efficient quenching partners. In xenon atmospheres only self-quenching by xenon atoms takes place.
4. Inflow of ambient air species
In order to study quenching by inflowing atmospheric species, the jet's effluent is investigated with a novel VUV absorption spectroscopic technique, where the VUV radiation generated by the core plasma is used as background source for absorption spectroscopy. Using this technique, the axial optical depth profile of molecular oxygen is determined. Furthermore, a model is developed that describes the oxygen concentration along the axial position of the effluent and which correlates well with the measured axial optical depth profile.
4.1. Experimental setup and VUV radiance measurements
The spectrum of the jet between 115 and 140 nm is measured with a 0.5 m VUV scanning monochromator (Acton Research Corp., VM 505) with a grating of 1200 g mm−1 blazed at 150 nm. A photomultiplier (PM) tube (Thorn/EMI, 9635 QB) with a sodium salicylate entrance window is used to detect the VUV radiation. The optical emission is measured end on by placing the jet nozzle in the axis of the optical system, consisting of a measuring aperture, imaging mirror and entrance slit, which are located in a vacuum chamber with a pressure in the range of 2 × 10−6 mbar. The plasma jet is positioned in ambient air at different distances axially to the MgF2 window, which seals the vacuum chamber. A detailed description of the VUV setup is given in [7].
When the jet is operated in ambient air, ambient air species diffuse into the jet's effluent from the ambient atmosphere leading to photo-absorption of the VUV radiation generated inside the core plasma. This results in intensity dips in the measured
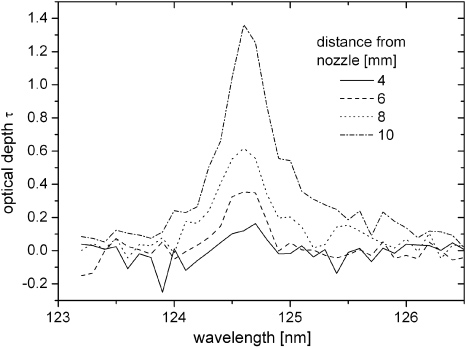
 -excimer second continuum in the region of 120–135 nm (figure 4). By increasing the distance between the jet nozzle and the MgF2 window the intensity of these dips decreases, which means that more VUV photons are absorbed. On the one hand, this is due to the longer absorption path length. On the other hand, the oxygen concentration inside the effluent increases in the axial direction, which also leads to a higher absorption rate. The effect of an increasing nozzle distance is displayed in figure 4. While at 4 mm distance hardly any intensity dips were detected, at 10 mm distance significant absorption dips are noticeable. From the dip intensity I located at 124.6 nm the optical depth τ = −ln(I/I0) is calculated for varying nozzle distances. In order to determine the background intensity I0 the Ar excimer continuum was fitted by a polynomial as exemplarily displayed in figure 4.
-excimer second continuum in the region of 120–135 nm (figure 4). By increasing the distance between the jet nozzle and the MgF2 window the intensity of these dips decreases, which means that more VUV photons are absorbed. On the one hand, this is due to the longer absorption path length. On the other hand, the oxygen concentration inside the effluent increases in the axial direction, which also leads to a higher absorption rate. The effect of an increasing nozzle distance is displayed in figure 4. While at 4 mm distance hardly any intensity dips were detected, at 10 mm distance significant absorption dips are noticeable. From the dip intensity I located at 124.6 nm the optical depth τ = −ln(I/I0) is calculated for varying nozzle distances. In order to determine the background intensity I0 the Ar excimer continuum was fitted by a polynomial as exemplarily displayed in figure 4.
Figure 4. Typic VUV-spectrum of the jet plasma produced by the kINPen for distances of 4 and 10 mm between nozzle and MgF2 window. Additionally the fitted background of the spectrum at 4 mm distance is shown.
Download figure:
Standard imageIn figure 5 the calculated optical depths in the wavelength range 123–126.5 nm are shown for different nozzle window distances. It can be observed that the optical depth steeply increases for longer distances. This disproportionately high slope of the optical depth reveals that the oxygen density must increase in the axial direction.
Figure 5. Optical depth spectra for different distances from the nozzle.
Download figure:
Standard imageA verification that the absorption around 124.6 nm is due to molecular oxygen is achieved by the following experiment. An absorption cell with two magnesium fluoride windows at each end is mounted between the plasma jet and the entrance of the VUV emission measuring system (figure 6).
Figure 6. Schematic presentation of the experimental system with absorption cell. The jet operates with an argon gas flow rate of 5 slm, expanding in open air. The total length of the unperturbed effluent reaches 6 mm.
Download figure:
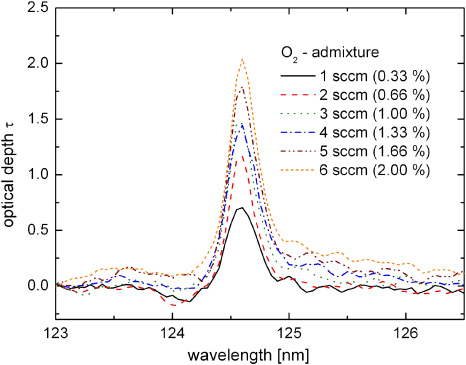
Standard imageThe gap spacing L between the two MgF2 windows is 5 mm. Either pure argon (0.3 slm) or argon mixed with a minority flow of oxygen (1 sccm to 6 sccm) is fed into this region. The gas flux and the geometry of the cell assembly prevent air leaking into the absorption path, and the plasma jet positioned on one side of the cell acts as a background radiation source. The distance between the jet nozzle and the MgF2 window is 4 mm and hence the visible effluent touches the cell window. The transmitted VUV radiation of the jet, especially that around the argon excimer second continuum, is detected spectrally resolved after passing the absorption cell. The calculated optical depths, for different flows of oxygen mixed to the constant argon flow rate of 0.3 slm, are presented in figure 7.
Figure 7. Optical depth spectra for different O2 admixtures in the absorption cell. The argon flow rate is 300 sccm.
Download figure:
Standard imageA comparison of the optical depth spectra, measured along the downstream axis and in the absorption cell shows almost identical values and shape. Hence, it is most likely that molecular oxygen diffuses into the effluent from the ambient air.
From the maxima of the profiles in figure 7 obtained in the absorption cell the photo-absorption cross section σ for the given experimental setup is calculated using the relation σ = 1/L · dτ/dn, where L and n are the absorption length (5 mm) and the density of admixed molecular oxygen, respectively. The cross section calculated from this measurement is 9.3 × 10−18 cm2 with an uncertainty of about 10%. The cross section value of oxygen at 124.6 nm found in the literature is 10.2 × 10−18 cm2 [8].
4.2. Modelling ambient air species diffusion into the effluent
Diffusion of molecular oxygen into the effluent of the jet with a velocity field u is described by the stationary convection–diffusion equation (u · ∇)n = DΔn, where D is the diffusion coefficient for diffusion of air into argon. Assuming a velocity field u = u(r, z)ez directed in the axial direction with a flux of 5 slm through a tube of radius r0 and neglecting transport by diffusion over convectional transport in the axial direction, we obtain the expression

Here Δr denotes the radial component of the Laplace operator in cylindrical coordinates. The axial dependence of the factor u/D is approximated by the function u/D = u0/D0 (1−sz) with slope s, while the radial velocity profile and the initial values u(z = 0) and D(z = 0) determine the factor u0/D0. Setting the boundary condition n(z = 0) = n0θ(r − r0), where θ(r) denotes the Heaviside step function, equation (3) yields the on-axis solution
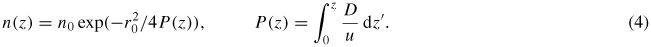
To obtain an integrable expression, P−1(z) is expanded to first order in z, yielding

where
 . Integration from z = 0 to the nozzle distance zn and multiplication with σ provides a theoretical expression for the optical depth
. Integration from z = 0 to the nozzle distance zn and multiplication with σ provides a theoretical expression for the optical depth
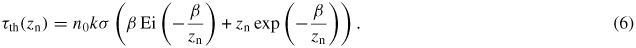
Here Ei(x) is the exponential integral function, and
 is a numerical constant which can be used as a fitting parameter.
is a numerical constant which can be used as a fitting parameter.
A numerical solution for the density of ambient species was obtained solving the isothermal Navier–Stokes equations with the standard k–ε turbulence model [9] using COMSOL 4.2. The diffusion of ambient species is described by the equation for the conservation of mass
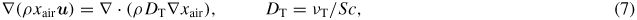
where ρ is the mass density, xair is the mole fraction of air and νT is the turbulent viscosity. The turbulent Schmidt number is estimated by Sc = 0.7 [10]. Equation (7) was decoupled from the Navier–Stokes equations assuming identical molar masses of argon and ambient air. In figure 8 the on-axis density obtained from a simulation of the free jet—as is the case for the TALIF measurements—is compared with simulations with a wall placed in front of the jet at various distances from the nozzle—as is the case for the UV-absorption measurements. It is observed that the wall does not change the on-axis density of ambient species and the theoretical formula (5) is applicable for distances up to 11 mm. With the presented solution of the convection–diffusion equation, the on-axis density of indiffusing ambient species into both laminar and turbulent flows from a cylindrical tube can be estimated.
Figure 8. On-axis density of ambient air obtained by simulations with wall, without wall and by the theoretical estimation according to formula (5).
Download figure:
Standard image4.3. Ambient air species in the jet effluent: correlation of model and measurement
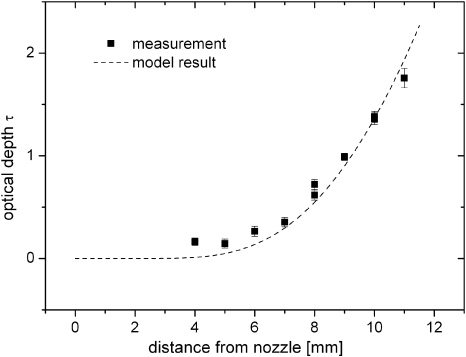
The fit of τth to the measured optical depth according to (6) is displayed in figure 9. The factor n0kσ is calculated assuming k = 1.
Figure 9. Axial optical depth measurements on jet without oxygen admixture (rectangles) and model result (dashed line).
Download figure:
Standard imageThe on-axis density profile of oxygen corresponding to the fit is displayed in figure 10. Within the first 3 mm from the nozzle hardly any molecular oxygen is found in the effluent. For distances greater than 4 mm a steep oxygen density increase is obtained. Its concentration lies in the same order of magnitude as is intentionally admixed to the argon feed gas. For greater distances the density function saturates to the ambient air oxygen concentration level.
Figure 10. Oxygen and nitrogen concentration in the effluent, obtained by differentiation of the fit to the optical depth (figure 9) and division by σ.
Download figure:
Standard imageCompared with the numerical simulation, the measured density is lower by a factor of 0.5 in the range 6–12 mm. This is in good agreement, as the simulations are of a more qualitative nature, neglecting gas heating and electrohydrodynamic forces. Furthermore it is noted that the value of the Schmidt number can be tuned to fit the experimental data, but should be determined by independent measurements.
5. Atomic oxygen density and influence of ambient species on the measurement
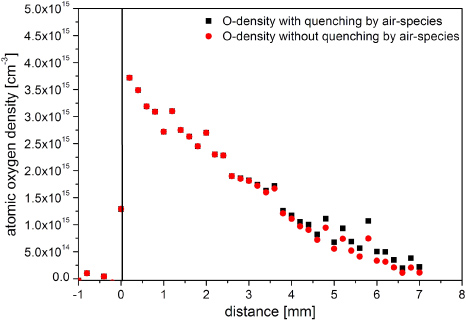
In figure 11, absolutely calibrated TALIF measurements of atomic oxygen in the effluent of the kINPen, operated with 5 slm argon and 1% oxygen admixture, are shown. The atomic oxygen density is in the order of 1015 cm−3. It decreases with the distance to the nozzle by up to an order of magnitude. The atomic oxygen density is higher than for comparable conditions in atmospheric pressure plasma jets operated in helium [11]. This may be due to the differing electric field geometry in the kINPen resulting in a higher charged species density in the jet effluent.
Figure 11. Atomic oxygen density in the effluent of the kINPen operated with 5 slm argon and 1% O2 admixture. Data with and without quenching by ambient indiffusing O2 and N2 are presented.
Download figure:
Standard imageBlack rectangles represent the measured atomic oxygen density with the indiffusing air species oxygen and nitrogen with the above-determined densities taken into account. The effect of ambient air species quenching on the measured atomic oxygen density can be clearly seen. Figure 10 shows that the nitrogen density is higher than the molecular oxygen density. Since the quenching coefficient is less than half of the molecular oxygen quenching coefficient, the influence of nitrogen on the TALIF measurements is about the same as that of molecular oxygen. The ambient species density in the percentage range leads to a quenching effect on the oxygen density only by a factor of below 1.8.
6. Summary and conclusion
A cold atmospheric pressure argon plasma jet (kINPen) was investigated for the atomic oxygen and ambient air species density in its effluent. TALIF spectroscopy on atomic oxygen was for the first time performed on an argon plasma jet. Furthermore, the plasma jet was operated in ambient air and thus under realistic conditions, such as envisaged in medical treatment applications. A novel VUV absorption spectroscopy method was presented, in which the core plasma of the jet was used as a background light source and is applied to determine the density of molecular oxygen diffusing from the ambient air into the effluent, in order to account for collisional quenching. Furthermore, a model was developed which describes the ambient air species diffusion into the jet's effluent. The measured axial optical depth and the result from the model are in excellent agreement. Knowledge of ambient species densities in plasma jet effluents is vital for effluent chemistry and will help to achieve a designed plasma jet surface interaction.
In conclusion, it can be stated from our investigations that although there is detectable collisional quenching by ambient air species in the plasma jet's effluent, it will prove irrelevant for most applications.
Acknowledgments
The authors gratefully acknowledge the spectroscopic measurements by Peter Holtz. The TALIF measurements at the RUB were supported by the DFG (FOR 1123). This work is funded by the German Ministry of Education and Research (BMBF, grant number 03Z2DN12).