Abstract
Due to its unique physiochemical properties, nano-titanium dioxide (nano-TiO2) is widely used in all aspects of people's daily lives, bringing it into increasing contact with humans. Thus, this material's security issues for humans have become a heavily researched subject. Nano-TiO2 can enter the body through the mouth, skin, respiratory tract or in other ways, after which it enters the blood circulation and is deposited in the liver, changing biochemical indicators and causing liver inflammation. Meanwhile, the light sensitivity of these nanoparticles allows them to become media-generating reactive oxygen species (ROS), causing an imbalance between oxidation and anti-oxidation that leads to oxidative stress and liver damage. Nano-TiO2 can be transported into cells via phagocytosis, where the nanoparticles bind to the mitochondrial membrane, resulting in the disintegration of the membrane and the electron transport chain within the mitochondria. Thus, more ROS are produced. Nano-TiO2 can also enter the nucleus, where it can directly embed into or indirectly affect DNA, thereby causing DNA breakage or affecting gene expression. These effects include increased mRNA and protein expression levels of inflammation-related factors and decreased mRNA and protein expression levels of IκB and IL-2, resulting in inflammation. Long-term inflammation of the liver causes HSC cell activation, and extracellular matrix (ECM) deposition is promoted by multiple signalling pathways, resulting in liver fibrosis. In this paper, the latest progress on murine liver injury induced by environmental TiO2 is systematically described. The toxicity of nano-TiO2 also depends on size, exposure time, surface properties, dosage, administration route, and its surface modification. Therefore, its toxic effects in humans should be studied in greater depth. This paper also provides useful reference information regarding the safe use of nano-TiO2 in the future.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Nanomaterials are defined as substances that measure approximately 1 to 100 nm in at least one dimension of scale. This size is close to the coherence length of the electron, so the properties of nanomaterials undergo great changes. In combination with the specific effects of a large surface area, the small scale of a nanoparticle results in different characteristics, including its melting point and magnetic, optical, thermal, and electrical properties relative to the same substance at a larger scale. As the performance of nanotechnology becomes well understood, the commercial application of nanotechnology products with unique properties is also increasing. The widespread application of nanomaterials has also increased the exposure of nanoparticles and contact between them and humans in the environment. Although in the past far less attention was paid to nanoparticles than to the same compounds at a larger size [1], in recent years scientists and relevant organizations have raised questions regarding the safety of nanomaterials for the environment and human health [2–4].
In nature, titanium (Ti) exists mainly in three crystal phases: anatase, rutile, and brookite. Its most common state is titanium dioxide (TiO2), which is normally a non-flammable, odourless, water-insoluble white powder [5]. Nano-titanium dioxide (nano-TiO2) has a relatively large surface area [6], a strong redox potential [7], photocatalytic properties, and sensitivity to heat and magnetism [8]. These unique properties have resulted in the widespread use of TiO2. In the early 20th century, TiO2 goods had already appeared, and it is widely used as a dye or a sunscreen. TiO2 is an additive in paint, creams, food, toothpaste, and other daily necessities. Today, nanotechnology is developing rapidly because of its advantages. Due to their high stability, resistance to corrosion, and good photocatalytic properties, TiO2 nanoparticles are increasingly replacing their larger counterparts [7–10]. TiO2 is even applied in clinical medicine as a photosensitizer for photodynamic therapy [11, 12], and is used for carrier platforms and targeting strategies in the context of clinical applications [13], for drug delivery [14, 15], and as a photothermal therapy for cancer [16]. Given the widespread use of nano-TiO2, its safety has gained even more attention.
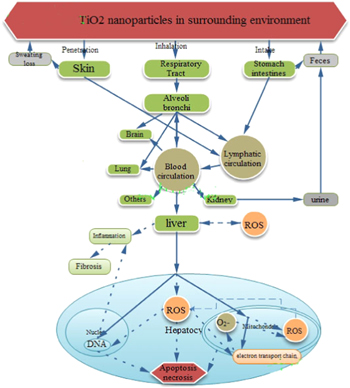
Nano-TiO2 can enter the body in various ways, for example through the respiratory and digestive systems or the skin, and circulate via the blood or lymphatic system, eventually accumulating in various organs [17]. Nano-TiO2 generates toxic effects in multiple organs of the body, causing neurotoxicity, immunotoxicity, reproductive toxicity, and inhalation toxicity, although these nanoparticles can be excreted from the body via sweat or the metabolism (figure 1). The toxicity of nano-TiO2 to the body is related to its particle size. It has been shown that smaller nano-TiO2 particle sizes are more difficult to remove. Early in 1994, Oberdorster et al subjected rats to a 12 week sub-chronic inhalation of nano-TiO2, finding that the half-life of 250 nm TiO2 in the rat lung was 117 days, while the half-life of 20 nm TiO2 was 541 days. These findings indicate that the body has a reduced ability to clear smaller nanoparticles [18]. In the past few years, our laboratory has been engaged in nano-TiO2 toxicological studies. We found that long-term/low-dose and short-term/high-dose exposure to nano-TiO2 damage the heart, liver, spleen, lung, kidney, brain, hippocampal neurons, and testes. Nano-TiO2 can be deposited in these organs, resulting in oxidative damage and inflammatory cell infiltration, atherosclerosis [19], myocardial injury [20], alveoli haemorrhaging [21], angiectasis and hyperaemia of the liver [22], lymphocyte infiltration and fatty degeneration in the spleen [23], and renal tubule apoptosis [24]. This deposition can also reduce sperm count or cause breakages and vacuolation through the blood–testis barrier [25]. Nano-TiO2 can suppress dendritic growth [26], deleteriously affect the ultrastructure of hippocampal neurons cells, and reduce the mitochondrial membrane potential in neuronal cells [27]. Even necrotic cells were observed in various organs of the high-dose group (table 1).
Table 1. Effect of nano-TiO2 on various organs in mice.
| Organs | Pathological changes | Reference |
|---|---|---|
| Atherosclerosis | Intima and arterial media thickening; foamy cell (nucleus shrink, cytoplasm cystose) infiltration, fibrous cap formed at the intima; collagen fibre proliferation and elastic fiber organization | [19] |
| Heart | Sparse cardiac muscle; inflammation on the tunica externa | [20] |
| Lung | Pulmonary emphysema, oedema, congestion, haemorrhage; inflammatory cell infiltration; alveoli haemorrhage | [21] |
| Liver | Angiectasis and hyperaemia, infiltration of inflammatory cells, macrophage aggregation, hepatic tissue crevice, hepatocyte necrosis | [22] |
| Spleen | Lymphocyte proliferation, macrophage infiltration, fatty degeneration, and cell necrosis | [23] |
| Kidney | Infiltration of inflammatory cells, fatty degeneration, and apoptosis; degeneration of superficial adipocytes; apoptosis of renal tubules | [24] |
| Testis | TiO2 nanoparticles able to cross blood–testis barrier and accumulate in the testis, testis and sperm lesions, decreased sperm numbers and sperm motility, imbalance of sex hormones and the alteration of the expression of 142 genes of known function in testis | [25] |
| Hippocampus | Suppressed dendritic growth; Glu release to extracellular region; elevated intracellular calcium; decreased Gln synthetase activity; increased phosphate-activated Gln activity | [26] |
| Hippocampus | Mitochondrial swelling; carina disappearance; nucleus shrinkage; anomalous nuclear membrane; chromatin marginalization and dilation of endoplasmic reticulum | [27] |
In 2008, Fabian et al investigated the deposition and removal cycles of nano-TiO2 in various organs and found that the liver purge cycle is the longest (28 days). The effects of TiO2 in the spleen, lungs, and kidneys are far smaller than in the liver [28]. Thus, many scholars have performed extensive research into the effects of TiO2 particles on the liver. In this paper, we systematically expound upon the toxic effects in the murine liver after exposure to nano-TiO2 (table 2).
Table 2. Effects in mice exposed to nano-TiO2.
| Subjects | Dosage(mg kg−1 BW) | Routes | Time | Result | Reference |
|---|---|---|---|---|---|
| Mice | 5000 | PO | 2 w | Changes of serum biochemistry; hydropic degeneration; hepatocyte necrosis; | [5] |
| Mice | 2.5, 5, 10 | IVGTT | 6 M | liver indices increase; liver dysfunction; inflammation; hepatocyte apoptosis; | [22] |
| congestion; angiectasis and hyperemia, hepatic tissue crevice; | |||||
| mitochondrial swelling, chromatin marginalization, nuclear membrane collapse | |||||
| Mice | 10 | IV | 0.5, 1, 2, 4, 8, 24 h; 1, 3, 7, 15, 30 d | Titanium accumulation in the liver, mainly taken up by macrophages; excretion of TiO2 nanoparticles through urine and faeces | [37] |
| Mice | 5, 10, 50, 100, 150 | IP | 2 w | Lipid peroxidation, oxidative stress; CAT, Apx, SOD, GPXs activated | [45] |
| Rats | 50 | Biochemical parameter changes, pathological lesions; oxidative stress | [46] | ||
| Involvement in mitochondria-mediated cytotoxicity, oxidative DNA damage | |||||
| Mice | 5, 10, 50 | IG | 60 d | Hepatocyte apoptosis, ROS accumulation; changes to stress-related gene expression | [50] |
| Mice | 5, 10, 50, 100, 150 | IP | 2 w | ALT, ALK, Alb; leucine acid peptide, pseudocholinesterase, total protein; inflammation | [52] |
| Mice | 0, 324, 648, 972, | IP | 24, 48 h | Hepatocellular necrosis and apoptosis, hepatic fibrosis | [40] |
| 129, 619, 442, 592 | 7, 14 d | Hydropic degeneration; minor fatty changes; some neutrophilic cells found | |||
| Mice | 150 | PO | 2 w | Serum liver function enzyme activity, liver indices increased | [54] |
| Inflammatory response, causing liver DNA damage; mRNA changes | |||||
| Mice | 100 | IG | 2 w | Alteration to hepatic enzymes, histopathological changes, oxidative stress | [55] |
| DNA damage, tumour suppressor and proapoptotic protein expression in liver cells | |||||
| Mice | 5, 10, 50 | IG | 60 d | Biochemical parameter, histopathological and ultrastructure changes; | [56] |
| hepatocytes apoptosis, increase to mRNA and inflammatory cytokines expression | |||||
| HepG2 | 1 μg ml−1 | Oxidative DNA damage; micronucleus frequency increase; lipid peroxidation; | [60] | ||
| ROS generation; apoptosis | |||||
| Rats | 63, 126, 252 | IP | 24, 48 h | GOT and ALP activity altered; congestion, oxidative stress, apoptosis, necrosis, | [76] |
| hydropic degeneration; cloudy swelling; fatty degeneration; portal and lobular infiltration | |||||
| Mice | 5, 10, 50, 100, 150 | IG | 2 w | Accumulated in liver DNA, liver DNA cleavage | [89] |
| Mice | 2.5, 5, 10 | IG | 90 d | Ti accumulation in the liver; TiO2 nanoparticle aggregation in hepatocyte nuclei; | [90] |
| inflammation, hepatocyte apoptosis, liver dysfunction; gene expression changes | |||||
| HepG2 | DNA strand breaks; oxidized purines; ROS; genotoxic | [96] | |||
| Rats | 5 | IV | 1, 14, 28 d | TiO2 levels are highest in the liver; no detectable inflammatory response or organ toxicity | [138] |
| Mice | 0, 140, 300, 645, or 1387 | IV | 14 d | Death of mice in the highest dose (1387 mg kg−1) group at day 2; at day 7, acute toxicity symptoms in the highest dose group; no significant acute haematological or genetic toxicity except increased WBC in 645 mg kg−1 dose group; lower liver coefficients | [139] |
| Mice | 5000 | PO | 14 d | High coefficients for liver; ALT/AST, LDH and pathology of liver change in female mice only | [140] |
Note: Po: oral administration; IVGTT: intravenously guttae; IV: intravenous; IP: intraperitoneal; IG: intragastrical administration
1. The means by which nano-TiO2 enters the body and is deposited in the liver
1.1. The means of nano-TiO2 entry into the body
1.1.1. Inhalation
Because nano-TiO2 is small, it can easily enter into the body. In the nasal cavity, inhaled nano-TiO2 may be transported via the following routes. First, nano-TiO2 may enter into the respiratory system through the nasopharynx. After being absorbed into the cells, nano-TiO2 may be transferred through the epithelial and endothelial cells to the bloodstream, thereafter circulating to various organs [6, 29]. Second, nano-TiO2 may enter into the nasal cavity along olfactory axons or via the olfactory epithelium into the olfactory bulb, where it is transported to the central nervous system and further interacts with neurons, causing damage [30]. And third, smaller nanoparticles are absorbed by the bronchi and are then deposited in the alveoli. Experiments have shown that when the amount of nanoparticle deposition in the alveoli exceeds the ability of alveolar macrophage phagocytosis to counteract this deposition, the resulting reduction in alveolar macrophage phagocytosis can induce mild but significant pulmonary inflammation, affecting the normal function of the lungs [31]. At the same time, nano-TiO2 also moves through the alveolar gaps into the circulatory system and is subsequently circulated to various organs, including the liver [32].
1.1.2. Skin absorption
As the body's first barrier, skin can stop the invasion of exogenous substances. Intact skin can prevent the penetration of nanoparticles, but at bent and damaged parts of the skin, the possibility that nanoparticles can migrate into the body through the skin is greatly increased. 0.5 and 1.0 micron particles can cross the epidermis to the dermis layer of human skin [33]. Because nanoparticles increase the lipid/water partition coefficients through certain processes, it is almost possible for nanoparticles to penetrate the skin and enter the body via simple diffusion [33]. When TiO2 is present in an oil-in-water emulsion, it can more easily penetrate the skin than when in the presence of an aqueous suspension [34]. Smaller sized nano-TiO2 particles have a greater likelihood of invading the body. For instance, it is entirely possible that a nano-TiO2 particle of 5 nm could cross the skin into the dermis by simple diffusion or penetration. When this particle reaches the lymphatic circulation via skin macrophages and astrocytes, it can then navigate to the regional lymph nodes or be taken up by sensory nerve endings of the dermis, where it is transferred along the nerves [35].
1.1.3. Gastrointestinal absorption
The gastrointestinal tract is another important means by which nano-TiO2 can enter into the body. This can include the intake of foods or drugs containing nanoparticles. Nano-TiO2 particles that enter the body via the gastrointestinal tract are absorbed by the lymphoid tissue of the small and large intestines and subsequently enter the blood circulation, are transferred to the mesentery, and reach the body organs, especially those with abundant reticuloendothelial systems, such as the liver, spleen, and others. Finally, nano-TiO2 is excreted by urine [36] and faeces [37]. Because nanoparticles have high surface activity, they have a good ability to pass through organs. Nano-TiO2 can be transported into various organs in the body through the blood and lymphatic circulatory systems. Moreover, nano-TiO2 can cross the blood–brain barrier, causing cavitation in hippocampal neurons and brain inflammation [5]. It can also pass through the blood–testis barrier, causing testicular damage, inhibition of spermatogenesis, and altered gene expression [25]. Magnetic nanoparticles can enter into the ganglion cell layer of the retina, resulting in changes to the intraocular pressure. These results show that these nanoparticles can also cross the blood–ocular barrier [38].
1.2. Deposition of nano-TiO2 in the liver
The powerful penetrative abilities of nano-TiO2 allow it to enter into various organs of the body. Upon deposition in the internal organs, nano-TiO2 generates toxicity, causing organ damage. ICP-MS (inductively coupled plasma mass spectrometry) analysis showed that upon treatment with increasing doses of nano-TiO2,  accumulation increased in each organ, including the brain, and especially the liver and spleen. Intravenously administered nano-TiO2 primarily accumulates in the livers of rats, with an accumulation of 69% after administration for 5 min and 80% after administration for 15 min [39]. Moreover, a pathological examination by Chen et al revealed that nano-TiO2 is deposited in the liver, where it causes liver cell apoptosis, necrosis, and liver fibrosis, and in the kidney, where it causes glomerular swelling. Nano-TiO2 has also been shown to produce toxicity in the liver and kidneys [40]. Ma et al treated mice with intraperitoneal injections of different doses of nano-TiO2 for 14 days, finding that the liver blood vessels became swollen, causing a series of diseases that included diffuse and focal ischaemic basophilic changes. This research group also observed the submicroscopic swelling of mitochondria and nuclear vacuoles, indicating liver cell apoptosis [41]. When mice were treated with nano-TiO2 (25 and 80 nm) for two weeks, nano-TiO2 accumulation was observed in the liver, kidneys, and spleen, and the liver index significantly increased. 80 nm of TiO2 mostly accumulates in the liver, causing liver inflammation. Finally, a pathological examination also showed liver oedema and liver cell necrosis in the lobules around the central vein, confirming that nano-TiO2 is toxic to the liver [5].
accumulation increased in each organ, including the brain, and especially the liver and spleen. Intravenously administered nano-TiO2 primarily accumulates in the livers of rats, with an accumulation of 69% after administration for 5 min and 80% after administration for 15 min [39]. Moreover, a pathological examination by Chen et al revealed that nano-TiO2 is deposited in the liver, where it causes liver cell apoptosis, necrosis, and liver fibrosis, and in the kidney, where it causes glomerular swelling. Nano-TiO2 has also been shown to produce toxicity in the liver and kidneys [40]. Ma et al treated mice with intraperitoneal injections of different doses of nano-TiO2 for 14 days, finding that the liver blood vessels became swollen, causing a series of diseases that included diffuse and focal ischaemic basophilic changes. This research group also observed the submicroscopic swelling of mitochondria and nuclear vacuoles, indicating liver cell apoptosis [41]. When mice were treated with nano-TiO2 (25 and 80 nm) for two weeks, nano-TiO2 accumulation was observed in the liver, kidneys, and spleen, and the liver index significantly increased. 80 nm of TiO2 mostly accumulates in the liver, causing liver inflammation. Finally, a pathological examination also showed liver oedema and liver cell necrosis in the lobules around the central vein, confirming that nano-TiO2 is toxic to the liver [5].
2. Causing liver oxidative stress and inflammation
2.1. Oxidative stress
Because of its photosensitivity, nano-TiO2 can become a substance that generates reactive oxygen species (ROS) in the body [42]. Under normal conditions, ROS play an important role in anti-bacterial and anti-inflammatory processes and can suppress tumours. However, disease or some exogenous poisons may cause disorders in the body's anti-oxidation system, resulting in radical metabolic imbalances and abnormal increases in ROS. Excessive ROS produce toxicity, resulting in the formation of biofilms and macromolecular substances that induce lipid peroxidation damage. When ROS is harmful, organisms use a variety of enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) to clear ROS.  is converted to H2O2 and O2 by SOD, whereas CAT and GSH-Px can eliminate H2O2 by converting it to H2O and O2 [43, 44]. Accordingly, SOD, CAT, and GSH-Px keep ROS at low levels, effectively protecting cells from the toxic effects. Excessive production of ROS breaks the balance of the liver oxidant/antioxidant system, resulting in lipid peroxidation and liver cell apoptosis. This process may contribute to decreased activity for antioxidant enzymes such as SOD, CAT, and GSH-Px, as well as non-enzyme antioxidants such as ascorbic acid (AsA) and glutathione (GSH) [45].
is converted to H2O2 and O2 by SOD, whereas CAT and GSH-Px can eliminate H2O2 by converting it to H2O and O2 [43, 44]. Accordingly, SOD, CAT, and GSH-Px keep ROS at low levels, effectively protecting cells from the toxic effects. Excessive production of ROS breaks the balance of the liver oxidant/antioxidant system, resulting in lipid peroxidation and liver cell apoptosis. This process may contribute to decreased activity for antioxidant enzymes such as SOD, CAT, and GSH-Px, as well as non-enzyme antioxidants such as ascorbic acid (AsA) and glutathione (GSH) [45].
TEM (transmission electron microscope) images confirmed that nano-TiO2 can enter into the interior of cells through the cell membrane, inducing oxidative stress by interfering with the balance between oxidants and antioxidants or interacting with organelles such as mitochondria, the endoplasmic reticulum and the Golgi. The levels of antioxidant enzymes (GSH-Px activity, CAT, and SOD) are significantly negatively correlated with ROS levels, and significant correlations have been observed between the ROS level and MDA activity. An increase in the MDA content suggests that the interaction of particles and the cell membrane leads to membrane damage, lipid peroxidation, and the generation of ROS, which results in an increase in H2O2 in the intracellular environment and causes DNA damage, protein oxidation, and subsequent liver damage [46].
ROS are produced in the mitochondria, and after nano-TiO2 enters the cell via phagocytosis, it binds to the mitochondria and promotes mitochondrial disintegration. This process results in a large increase to ROS levels, which results in the peroxidation of lipids, proteins, and DNA, as well as apoptosis [47]. In a previous study, Long showed that TiO2 particles can bind to the mitochondrial membrane, causing the disintegration of the mitochondrial membrane electron transport chain and the generation of additional O2 •-. This causes structural damage to the mitochondria in addition to the increased ROS levels produced by nanoparticles. As a result, the permeable pore of the mitochondrial membrane opens and apoptotic or necrotic pathways are activated [48]. Nel et al also proposed a hierarchical model of oxidative stress. With an increase in oxidative stress, nuclear respiratory factor 2 (Nrf-2), mitogen-activated protein kinase (MAPK), and nuclear factor -κB (NF-κB) are activated, while changes in the mitochondrial permeability transition pore result in damage to the endometrium and organelles (figure 2) [49].
Figure 1. Kinetics of poisoning by TiO2 nanoparticles. The solid line represents the means by which nano-TiO2 enters the body; the dashed line represents the nano-TiO2-stimulated biochemical reactions that damage various parts of the body.
Download figure:
Standard image High-resolution imageFigure 2. Process by which TiO2 nanoparticles produce ROS in mitochondria.
Download figure:
Standard image High-resolution imageNano-TiO2 increases the levels of ROS and cytochrome P4501A (Cyp1a1) and decreases the expression levels of oxidative stress-related genes, indicating that it interferes with the regulation of cellular detoxification/metabolism in the liver. Moreover, nano-TiO2 severely inhibits the activity of SOD, CAT, GSH-Px, and ascorbate peroxidase (APx) and drastically reduces the levels of ascorbic acid (AsA), GSH and other antioxidants. Along with the increase in lipid peroxidation induced by nano-TiO2, these data indicate that nano-TiO2 oxidation can cause liver damage [50]. Changes in the levels of SOD, GSH, and MDA indicate the occurrence of oxidative stress and when insulin resistance in the liver is abnormal, further inflammation can occur [51].
2.2. Inflammation
Liu et al tested biochemical indicators in mouse livers, finding that when the levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), leucine peptides (LAP), pseudocholinesterase (PChE), lactate dehydrogenase (LDH), triglycerides, total protein, and albumin all increase, inflammatory cascades are activated and result in liver damage characterised by liver cell apoptosis [52]. The ALT and AST levels were elevated in the nano-TiO2-treated group, which showed loss of functional integrity of the liver cells and membrane leakage [5].
Intraperitoneal injection for 14 days indicated that the titanium content increased in mouse livers, resulting in liver cell damage, mitochondrial swelling, and other pathological changes, while the expression levels of inflammatory factors (NF-κB, MIF, IL-6, IL-1β, CRP, TNF-α, etc) were altered, indicating that nano-TiO2 causes liver inflammation that results in liver injury [41]. After mice were orally treated with nano-TiO2 for 30 days, the liver showed congestion and other diseases, as well as decreased levels of leucocytes and T cells, glutamate aminotransferase, and AST, which are all related to liver function. These results indicate that nano-TiO2 impairs the immunity of the liver and alters liver function [53].
Meanwhile, mice that were orally treated with 5 g kg−1 nano-TiO2 showed an increased ratio of ALT/AST, increased LDH activity and liver weight, and liver cell mortality [5]. In addition, the results of experiments in which different doses of nano-TiO2 in mice were injected intraperitoneally showed significant increases in the levels of ALT and AST, liver cell necrosis and apoptosis, liver inflammation and fibrosis, and other diseases [40]. When mice were treated orally with nano-TiO2 (150 mg kg−1 BW) for two weeks, the nano-TiO2 caused changes to the levels of MDA and GSH in the liver, suggesting the occurrence of oxidative stress and inflammation [54]. Research on the molecular pathogenesis in the liver showed that nano-TiO2 significantly increases the levels of cytokines, mRNA, and proteins related to inflammation, including NF-κB, MIF, TNF-a, IL-6, IL-1ß, CRP, IL-4, and IL-10 [41]. By using an in vitro human immune construct, Schanen et al found that nano-TiO2 increases the expression of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, IFN-γ, and TNF-α) by 5–20-fold. Moreover, the number of DC cells increases and matures, and activated T cells are also produced [42]. A study by Shukla et al showed pathological damage to the liver, including oxidative stress, DNA damage, increased expression of proapoptotic factors (BAX, caspase-3 and -9, etc), and the downregulation of the anti-apoptotic factor Bcl-2, proving that nano-TiO2 induces oxidative damage and apoptosis in the liver [55].
Cui et al also investigated cytokines and biochemical indicators related to liver inflammation and the signalling pathways that are activated by nano-TiO2. Nano-TiO2 can significantly increase the mRNA expression of TLR2 and TLR4 and the levels of several inflammatory cytokines, including IKK1, IKK2, NF-κB, NF-κBP52, NF-κBP65, TNF-a, and NIK. Simultaneously, nano-TiO2 can significantly reduce the mRNA and protein expression of IκB and IL-2. This indicates that the nano-TiO2-activated liver inflammation pathway is TLRs → NIK → IκB kinase → NF-κB → TNF-α → inflammation → apoptosis → liver injury [56].
3. The mode of transport into cells and the effects of nano-TiO2 on cells
3.1. The mode of transport into cells
Nano-TiO2 has a very strong ability to cross the cell membrane into the cytoplasm, where it can damage genetic material due to its high chemical activity. Nano-TiO2 can also enter into cells via endocytosis [57]. A plurality of processes are involved in endocytosis and can be divided into two categories: phagocytosis and pinocytosis. The former, macrophage phagocytosis, is for the intake of large particles (0.2–10 microns) [58]. Monocytes/macrophages and neutrophils have been described as professional phagocytes [59]. Pinocytosis occurs in all types of cells. Of course, the mode of entrance of the nanoparticles into the cells also depends on the presence of an appropriate receptor on the cell membrane as well as the size and surface properties of the nanoparticles. Thus, the mechanisms by which nanoparticles enter into different cell types are not identical. TEM images show that nano-TiO2 particles of a smaller size range can easily be internalised into cells, where they localise in the cytoplasm and the nucleus [60]. Zucker et al used dark field microscopy and flow cytometry, finding that nano-TiO2 often localises to the perinuclear ring, with ER and Golgi juxtaposition [61].
3.2. Effects of nano-TiO2 on cells
3.2.1. Cell membranes
When nano-TiO2 enters the cell through the membrane, its binding and interaction with the membrane results in the generation of ROS. Furthermore, oxidative stress occurs, resulting in a rupture of the membrane lipid layer. In T24 human bladder cell lines treated with nano-TiO2, a significant increase in the  concentration was observed and a large amount of
concentration was observed and a large amount of  leaked into the interior of the cell, showing that damage occurred to the T24 cell membrane [62].
leaked into the interior of the cell, showing that damage occurred to the T24 cell membrane [62].
3.2.2. Causing phagocyte reactions
When nano-TiO2 causes the cell membrane to rupture, the cell contents will flow out, resulting in phagocytosis by fixed or free phagocytes. A previous study showed that nano-TiO2 leads to a reduction in the ability of macrophages to remove foreign matter via phagocytosis. Oberdorster et al studied the clearance mechanisms by which 20 and 250 nm size TiO2 powder can be removed by alveolar macrophages, finding that the scavenging and phagocytic ability of macrophages towards nano-TiO2 particles decreases with smaller nanoparticle sizes [18]. Renwick et al also described the effects of 29 nm nano-TiO2 and 250 nm ultrafine TiO2 particles on macrophage phagocytosis. The results of that study showed that 29 nm TiO2 particles decrease the phagocytic activities of macrophages more significantly than 250 nm TiO2 particles [63].
3.2.3. Mitochondria
The entrance of nano-TiO2 into the cell causes a variety of biochemical reactions, including the expression of inflammatory cytokines and the production of large amounts of ROS. Cell culture experiments showed that in murine glial cells, nano-TiO2 (2.5–120 ppm, a particle size of 25 nm) may exacerbate inflammation and apoptosis, inhibit the cell cycle and energy metabolism, and cause the generation of ROS in the brain [64]. Moreover, nano-TiO2 also has an effect on the mitochondrial membrane. High doses of nano-TiO2 (100–250 mg ml−1) can damage the mitochondrial membrane in rat liver cell lines. Nano-TiO2 also causes liver cell line apoptosis and reduces the stability of the lysosomal membrane, causing the release of cathepsin B and lipid peroxidation [65]. ROS changes the mitochondrial morphology, and mitochondrial dynamics are impaired by the substantial losses resulting from the fusion process [66]. Submicroscopic observations have also shown mitochondrial swelling in mouse livers after 60 days of continuous gavage [50]. Mitochondria are the targeted organelle of intracellular nano-TiO2, which reduces the mitochondrial membrane proteins and leads to apoptosis [47]. The results by Geiser et al and Rothen-Rutishauser et al also proved that nano-TiO2 is membrane-bound, and that there is also a small quantity of nanoparticles in the cytoplasm [67, 68].
3.2.4. Inhibiting the growth of cells
Madrid et al found that nano-TiO2 can inhibit Pseudomonas cell growth after 40 min of UV irradiation, and TEM and XRD (x-ray diffraction) observation indicated that the inhibition rates are from 60% to 72% [69]. Xiong et al added 47 nm nano-TiO2 into the human hepatoma cell line Bel-7402 and found that the number of cells in the G1 phase increased significantly, while the number of cells in the S phase decreased, indicating that nano-TiO2-treated cells can be arrested in the G1 phase of the cell cycle. This results in the inhibition of cell growth [70]. SEM images of nano-TiO2-treated mouse fibroblasts (L929) showed that the cell shrank and became rounded, and only a few cell aggregations and pseudopod protrusions were visible. AO staining (acridine orange staining method) showed significant damage to the DNA, including the scattered chromatin condensation observed in the late stages of apoptosis or necrosis. All these results suggest that nano-TiO2 can inhibit cell adhesion and proliferation [71]. Moreover, nano-TiO2 can change the white and red blood cell counts in young rats after oral administration [72].
3.2.5. Apoptosis
In the spleens of mice treated orally with 50 or 150 mg kg−1 BW nano-TiO2, chromatin condensation, mitochondrial swelling, and the emergence of the nucleolar cap were all observed, proving that nano-TiO2 induces cellular apoptosis [73]. Rahman et al used nano-TiO2 with a diameter of less than 20 nm to treat fibroblasts and, using TEM, observed that the cells underwent morphological changes, nucleolus disappearance, membrane blebbing, and the formation of apoptotic bodies, which also confirmed that nano-TiO2 triggers apoptosis [74]. Shi et al further proved that BEAS-2B cells exposed to nano-TiO2 can undergo mitochondria-dependent apoptosis through an independent caspase 8/t-Bid-pathway [75]. In the livers of mice treated by continuous gavage with nano-TiO2 for 60 days, submicroscopic structures of apoptosis were observed, including chromatin condensation. Moreover, the ROS (H2O2, NO, etc) and MDA levels increased in the cells, while the gene expression levels of SOD, GSH-Px, CAT, GST, HSP-70, and p53 could be associated with changes in oxidative stress. These results suggested that nano-TiO2 can cause oxidative damage and apoptosis in hepatocytes [50], which results in pathological changes such as liver oedema, fatty degeneration, inflammatory cell infiltration, central venous dilation, altered levels of serum aspartate transaminase (GOT) and ALP, and hepatocyte apoptosis [76]. Immunoblot analysis showed increased expression levels of p53, BAX, Cyto-c, Apaf-1, caspase-9, and caspase-3, accompanied by a reduction of the expression of Bcl-2. This explains the nano-TiO2-mediated apoptosis via the caspase-dependent signalling pathway. The pathological results also show that nano-TiO2 induces oxidative damage to the DNA and increases the quantity of apoptotic bodies resulting from lipid peroxidation and oxidative stress. Increased expression levels of p53 are caused by changes in the ratio of Bax/Bcl-2, which leads to the release of apoptotic protease activating factor (Apaf-1). The binding of Apaf-1 to cytochrome C results in the formation of apoptotic bodies. These experimental data also showed that a cascade activation of caspase-9 and caspase-3 causes death in a nano-treated group of HepG2 cells [77]. These results showed that nano-TiO2 can not only induce cell apoptosis through the caspase-dependent signalling pathway described above, but also through the mitochondrial-dependent apoptotic pathway [64].
4. Liver DNA damage and changes in gene expression
4.1. Liver DNA damage
Nano-TiO2 has a direct and an indirect impact on biological DNA. Directly, nano-TiO2 can form covalent linkages with DNA. Indirectly, DNA involves oxidative damage (figure 3).
Figure 3. The impact of nano-TiO2 on DNA: (A) represents the direct effects, while (B) and (C) show the indirect effects.
Download figure:
Standard image High-resolution image4.1.1. Indirect effects
Under UV irradiation, a titanium dioxide electron is excited and electron transition occurs. This results in electron–hole pairs. The electron is captured by the reduction of its hole and then has a hole-trapping oxidation potential. In an aqueous environment, nano-TiO2 will produce hydroxyl radicals that can react with DNA, producing oxide radicals as well as 8-oxo-7, 8-oxodG, and A8OH. As the main product of oxidative damage, 8-OHdG can result in mutations of A:T to G:C or vice versa. This reverse mutation occurs because 8-OHdG can pair adenine bases with cytosine bases [78–80] through purine oxidation, which can also cause mutations and cancer [81]. Thus, UVA radiation can cause DNA breakage [47, 82–86]. Oxidative damage to DNA results in breakage of the DNA helix, thus affecting the structure and expression of DNA. After exposure to ultraviolet light, sunscreen cosmetics containing nano-TiO2 (20 nm–50 nm) will certainly lead to increased DNA helicase activity in human cells as well as the fracture of DNA [87]. This is consistent with the results of Ashikaga et al, which showed that even under otherwise identical experimental conditions, if the light intensity and illumination time are increased, the DNA helicase rate also increases [88]. When nano-TiO2 acted for a period on the labelled 5' end of 32 P DNA, the DNA scission mostly occurred at guanine residues after the light-induced catalytic reaction [47].
4.1.2. Direct effects
In the liver, anatase nano-TiO2 accumulates in the DNA by inserting itself into DNA base pairs or binding to DNA nucleotides by forming bonds with three oxygen or nitrogen atoms and two phosphorous atoms of DNA. These Ti–O/Ti–N and Ti–P bonds have lengths of 1.87 and 2.38 Å, respectively. With increasing doses of nano-TiO2, the liver DNA increases due to the binding of  and the coordination of
and the coordination of  with oxygen/nitrogen and phosphorus in the DNA nucleotide bases. This induces changes in the DNA microenvironment, resulting in the contraction of the DNA molecules and the destruction of their conformation. An electrophoretic analysis showed that DNA breakage occurred in mice treated with high doses of nano-TiO2. Upon entering the body, nano-TiO2 affects the expression of DNA-based genetic information [89]. Meanwhile, long-term exposure to nano-TiO2 can cause changes in the expression levels of genes related to cell proliferation and signal transduction. This suggests that nano-TiO2-mediated liver damage may be associated with the abnormal expression of important genes in the liver [90].
with oxygen/nitrogen and phosphorus in the DNA nucleotide bases. This induces changes in the DNA microenvironment, resulting in the contraction of the DNA molecules and the destruction of their conformation. An electrophoretic analysis showed that DNA breakage occurred in mice treated with high doses of nano-TiO2. Upon entering the body, nano-TiO2 affects the expression of DNA-based genetic information [89]. Meanwhile, long-term exposure to nano-TiO2 can cause changes in the expression levels of genes related to cell proliferation and signal transduction. This suggests that nano-TiO2-mediated liver damage may be associated with the abnormal expression of important genes in the liver [90].
4.2. DNA damage at the genetic level
The types of DNA damage caused by nano-TiO2 are not limited to the DNA level but also extend to the gene level. When peripheral blood lymphocytes were treated with nano-TiO2, p53 protein accumulated and generated ROS, DNA damage, and the formation of micronuclei. This result proved that nano-TiO2-activated ROS can lead to serious damage to proteins, lipids, and DNA, resulting in genotoxic effects [91–94]. Nano-TiO2 causes a mutation in the p53 gene and other genes controlling apoptosis, thereby changing their expression and resulting in genotoxic effects [91, 95]. There are four genes involved in DNA damage, namely p53 and its downstream target genes p21, gadd45a, and mdm2 [96]. p53 is considered to be the main sensor of genotoxic stress and is connected to DNA damage, cell cycle arrest, and apoptosis [97]. Under normal circumstances, the function of p53 is controlled by MDM2, an E3 ubiquitin ligase that can mediate p53 ubiquitination and proteasome-dependent degradation [98]. MDM2 gene expression is a loop tuning process in which p53 positively regulates the expression of MDM2, a negative regulator of p53 [99].
Ziegelbauer et al compared the genotoxic and non-genotoxic carcinogen-induced gene expression profiles in rat livers, showing that genotoxic carcinogens upregulate the expression of MDM2 [100]. When HepG2 cells were exposed to microcystin-LR, the expression of MDM2 also tended to increase. This result indicates that ROS can induce DNA damage [101]. After DNA damage, the growth-inhibiting gene GADD45A, which participates in DNA damage control during the G2-M checkpoint in the cell cycle, plays a significant role in the processes of DNA repair and apoptosis [102]. GADD45A activation is directly regulated by p53 and is related to the oxidative stress-induced pathway [103]. Another target of p53 is p21, a cyclin-dependent kinase inhibitor. After DNA damage, p21 is responsible for cell cycle arrest [104]. In rat livers, genotoxic carcinogens can upregulate p21 [101]. Therefore, cells exposed to nano-TiO2 will produce ROS, which causes DNA oxidative damage and changes in the expression of p53 and its downstream genes, including p21, gadd45a, and mdm2, which are all related to DNA damage. This provides further evidence of the genotoxic effects of nano-TiO2 [96].
4.3. Effects on mRNA
Nano-TiO2 can promote the activity of related enzymes and can affect the mRNA expression of pro-inflammatory cytokines. Just as nano-TiO2 can induce the mRNA expression of nuclear factor erythroid 2-related factor 2, nano-TiO2 can also upregulate NF-κB and Bax while downregulating Bcl-2 [105]. In an analysis by Abdelazim, RT-PCR (real-time PCR) and western blotting showed that nano-TiO2 significantly altered the mRNA and protein expressions of transforming growth factor-β (TGF-β1), Smad-2, and vascular endothelium growth factor (VEGF). A histopathological examination of hepatic tissues reinforced these results [106]. Experiments by Wamer et al showed that RNA and DNA can be isolated from human skin fibroblasts treated with nano-TiO2. Moreover, nano-TiO2 was shown to induce significant levels of photo-oxidation and hydroxylation of guanine bases. These results suggest that injured RNA indirectly influences the expression of genetic information in cells [85].
5. Liver fibrosis
Because inflammation promotes organ parenchymal cell necrosis, the extracellular matrix of a tissue abnormal increases, accumulating excessively during this pathological process. Light damage is called fibrosis, whereas severe damage causes structural damage to the tissue and organ hardening. Fibrosis is a healing response to a variety of wounds induced by chronic stimulation, and it is characterised by the excessive deposition of extracellular matrix (ECM) proteins, including three large families of proteins: glycoproteins, collagen, and proteoglycans [107].
When the liver is damaged, it will proliferate to repair damage sustained previously. This proliferative response is regulated by growth-related genes and is simultaneously mediated by the stimulating and inhibiting signals provided by different growth factors. However, in the long-term presence of liver damage, hepatic stellate cells (HSC) are activated by aSMA, HSC will migrate toward the site of liver injury, and proliferation will occur in the injury site. Meanwhile, extracellular matrix synthesis begins to repair the damage, producing fibrosis [107].
When HSC is stimulated by fibrosis, it is activated from the silent state and the cells begin to produce collagen. Meanwhile, a number of genes associated with the HSC have also been changed. These include many genes involved in intracellular signalling cascades, such as the TGF-β, MAPK, NF-κB, and proliferation signalling pathways, which are all involved in the process of liver fibrosis. These cascades can help maintain the activated phenotype, thereby controlling the value-added state of fibrosis [108]. Nano-TiO2 can effectively induce the expression of TGF-β through IL-1β-dependent mechanisms [109] and can also induce the high expression of inducible transcription factor, Smad proteins, and growth factors [110]. TGF-β is a prominent anti-proliferative and pro-fibrotic cytokine produced mainly by HSC [111]. TGF-β transfers intracellular signals via Smad [112], which is then phosphorylated by TGF-β receptor activation at the SMAD homology 2 domain [113]. This stimulates the synthesis and deposition of ECM components, such as collagen types I, III, and IV, elastin, tenascin, osteonectin, duplexes proteoglycans, biglycan, and decorin [114], promoting fibrosis as follows: chronic inflammatory injury → HSC → TGF-β → SMAD → collagen → fibrosis.
Several experimental animal models of liver disease have also supported the conclusion that HSC is the primary cell responsible for the overproduction of collagen, therefore playing a crucial role in fibrosis [115–117]. Experiments in liver fibrosis models by Milani et al demonstrated that increases in the mRNA levels and location of collagen are associated with the HSC [116, 118, 119]. Moreover, liver fibrosis is associated with the activation of the MAPKs and P70s6k pathways. The extracellular signal-regulated kinases (ERKs) and the stress-activated protein kinase c-Jun terminal kinase (JNK) belong to the MAPKs, which have a pivotal role in transducing signals to the nucleus, thereby resulting in numerous cellular responses such as proliferation, differentiation, and the regulation of specific pathways [120]. Cell proliferation requires an accelerated rate of protein synthesis, which is regulated by several signalling cascades that interact with the translational machinery of the ribosome. The p70S6-kinase (p70S6K) is directly responsible for the phosphorylation of the 40S ribosomal protein S6 and controls the translation of several mRNAs, which encode for proteins necessary for the assembly of the translational machinery [121]. In HSCs, two signalling pathways were observed in an in vitro experiment. The results showed that the activation of these pathways precedes the processes of HSC activation and proliferation, which are associated with tissue remodelling and hepatic fibrosis [122]. Many in vitro experiments have also illustrated that the MAPKs and p70S6K regulate HSC cell activation caused by different stimuli [113, 123–128]. It is more important that the activation of these pathways particularly promotes the development of a myofibroblast-like phenotype [129].
After inhalation of nano-TiO2, this nanoparticle accumulates in the kidneys and causes renal fibrosis via oxidative stress [130]. Fibrosis is intensified by modifications to the ECM, which include the induction of plasminogen activator inhibitor-1 (PAI-1) and the regulation of the tissue inhibitors matrix metalloproteinase 1 (TIMP-1), CTGF, and TGF-β. In the kidneys of mice treated with low doses of nano-TiO2 via repeated tracheal instillation, the ROS/RNS (reactive nitrogen species)-correlated signals nitrotyrosine, iNOS, and HIF-1α, and the renal fibrosis-associated signals HIF-1α, TGF-β, and collagen I were significantly increased [131, 132]. HIF-1α has been found to promote renal fibrosis by inducing inflammation [133–136]. These results show that nano-TiO2 can induce renal fibrosis through oxidative stress according to the following pathway: ROS/RNS → HIF-1a → TGF-β → collagen I → fibrosis.
When the mice were intraperitoneally injected with nano-TiO2, the histology images showed a fibrotic liver tissue phenomenon, which indicated that nano-TiO2 produces liver fibrosis [137]. Moreover, the mechanism described above may also cause liver fibrosis.
6. Perspective
Nano-toxicology has only been studied for several decades. At present, studies on the toxicity of nano-TiO2 are still in their infancy. The toxicity of nano-TiO2 is mainly due to the special physical and chemical properties of nanoscale particles. After the nano-TiO2 enters the body via three main routes, it enters the circulatory system and is deposited in various organs, causing changes in hormone expression and damage to the organs of the body. After nano-TiO2 enters the cell, it damages it and causes it to produce large quantities of free oxidative radicals, thereby breaking the oxidation/de-oxidation balance. Nano-TiO2 can also enter into the nucleus and can directly or indirectly damage DNA, leading to changes in gene expression and even cell apoptosis. If long-term liver damage is present, HSC will be converted into an active state. Accompanied by changes in the activities of several intracellular signalling pathway genes such that the extracellular components become involved in extracellular matrix precipitation, this can ultimately cause fibrosis and, eventually, cirrhosis may develop. However, the detailed mechanism by which nano-TiO2 causes liver fibrosis is still unclear.
To study the molecular mechanisms of liver injury caused by exposure to nano-TiO2, it is necessary to perform experiments involving gene knock-outs and especially biomarker screening, which is crucial for understanding the detailed mechanism of liver injury. Although immune inflammation, apoptosis, and oxidative stress related to liver injury have been investigated, the aspects of energy metabolism, protein metabolism, and lipid metabolism should be studied in detail. Current research into the toxicity of nano-TiO2 has been limited to animal experiments in vivo and in vitro; whether the same results will be observed in humans is still unknown. And the toxicity of nano-TiO2 depends on size, exposure time, surface properties, dosage, administration route, and its surface modification, so differences for these factors may cause different results. For example, there were no remarkable toxic effects in rats for the 5 mg kg−1 TiO2 dose treated by intravenous administration for approximately one month [138]. Xu et al reported acute toxicity for nano-TiO2 in mice using the same administration route [139]. Hematological analysis and micronucleus tests also showed no significant acute hematological or genetic toxicity, except for an increase of the WBC count in mice at 645 mg kg−1 treated for a half-month. A higher (5000 mg kg−1) exposure to nano-TiO2 for 14 days only caused serum biochemical parameter (ALT/AST, LDH) and pathology changes in female mice [140]. So tolerance of nano-TiO2 is also related to species and gender. Therefore, the toxicology of nano-TiO2 must be studied in-depth to improve the quality and safety of this nanoparticle. Research in this area has a long way to go.
Acknowledgments
The authors gratefully acknowledge funding (CARS-22-ZJ0504) from the China Agriculture Research System (CARS) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, People's Republic of China.