Abstract
As the first part of this series of papers, a new calculation method for composition and thermodynamic properties of 2-temperature plasma considering condensed species under local chemical equilibrium (LCE) and local phase equilibrium assumption is presented. The 2-T mass action law and chemical potential are used to determine the composition of multiphase system. The thermo-physical properties of CO2–CH4 mixture, which may be a possible substitution for SF6, are calculated by this method as an example. The influence of condensed graphite, non-LTE effect, mixture ratio and pressure on the thermo-physical properties has been discussed. The results will serve as reliable reference data for computational simulation of CO2–CH4 plasmas.
Export citation and abstract BibTeX RIS
1. Introduction
The deviation from local thermal equilibrium (LTE) is a common phenomenon in the switching arc extinguishing, especially during the 'current-zero zone' and dielectric recovery period. In non-LTE plasma, the inefficiency of electron–heavy-species collisions and the large temperature gradient limit the redistribution of energy from the electrons and cause the electron temperature differing from that of the heavy species. In order to simulate non-LTE plasma, the 2-T thermo-physical properties, namely composition, thermodynamic properties and transport coefficients, are necessary. A large number of papers deal with 2-T thermodynamic properties and transport coefficients for homonuclear gases (Ar, O2, N2, H2, He, Cu) and their mixtures [1–5]. Some research also focused on the properties of 2-T SF6 and SF6-based plasmas [6–10].
Besides the non-LTE effect, the multiphase effect can also significantly influence the plasma properties. For LTE plasma system, the Gibbs free energy minimization method is common used in multiphase plasma calculation [11, 12]. The multiphase effect can also be significant in non-LTE plasma system, due to low heavy species temperature Th. Recently André et al [13] presented a paper focusing on composition of multiphase non-LTE air-aluminum plasma in which showed the influence of non-LTE effect, mixture proportion and pressure on phase transition process. However, the 2-T Gibbs free energy minimization method is still debatable [1, 14–16] while the widely used classic 2-T mass action law cannot take condensed phase into account [17]. Besides, to our knowledge, the influence of condensed species in non-LTE plasma system is neglected in existing 2-T thermo-physical property calculation. In summary, a new calculation method which can take both the non-LTE effect and condensed species into account is needed for plasma thermo-physical properties. In this series of papers, a new calculation method for 2-temperature plasma thermo-physical properties considering condensed phases is presented. The first paper (this paper) focuses on the composition calculation and thermodynamic properties. With these data, the transport coefficients of multiphase non-LTE system are calculable, which are presented in the second paper.
The section 2 of this paper presents a new calculation method for composition and thermodynamic properties of 2-temperature plasma considering condensed species under local chemical equilibrium (LCE) and local phase equilibrium assumption. According to the second law of thermodynamics, when a multi-component system reaches phase equilibrium, the chemical potential of condensed particle equals to that of corresponding gaseous particle. Using this theorem with consideration of 2-T mass action law, Dalton's law, the species conservation law and the quasi-neutrality condition, the composition of multiphase plasma system can be obtained. The widely used 2-T mass action law proposed by van de Sanden [17] have been applied in this work. The thermodynamic properties can be evaluated through a classical statistical mechanics approach once composition is obtained.
Sulfur hexafluoride (SF6) is widely adopted in electric power industry, especially in high-voltage circuit breakers and gas-insulated switchgear, due to its superior arc-quenching ability and dielectric strength. However the high global warming potential (GWP) of SF6, which is 23 900 times that of CO2, limits its wide application. Much research has been conducted on CO2 and CO2 based mixture as a substitute gas for SF6 [18–22]. In section 3, the composition and thermodynamic properties of CO2–CH4 mixture, which may be a possible substitution for SF6 due to its environmental friendliness and good electrical properties [23], are calculated by this new method as an example. The results are presented for different temperatures (300–30 000 K), pressures (0.1–10 atm), non-equilibrium degrees (1–5), and CH4 molar proportions (0–100%). The results of higher non-equilibrium degree systems are not presented in this paper considering the little influence of condensed phase in this system, which is explained in detail in section 3. Results for LTE composition and thermodynamic transport properties are in good agreement with previously published data. The influence of condensed graphite, non-LTE effect, mixture ratio and pressure on the thermo-physical properties has been discussed. The results will serve as reliable reference data for computational simulation of the behavior of CO2–CH4 plasmas. Some of the calculation results are tabulated in appendix.
2. Calculation method
2.1. Composition for non-LTE multiphase plasma system
The determination of the plasma composition is a prerequisite for the calculation of the thermodynamic and the transport properties. In a non-LTE plasma system, due to the inefficiency of electron–heavy-species collisions, the electron temperature differs from that of heavy species. A parameter of thermal non-equilibrium can be defined as θ = Te/Th, where Te and Th are respectively the electron and heavy-species temperatures.
The classic composition calculations for 2-T gaseous system were based on the laws of mass action (Saha law and Guldberg–Waage law), the conservation of the chemical elements, Dalton's law and electrical quasi-neutrality [24, 25]. For the mass action law, the derivation proposed by van de Sanden [17] is widely used. In this method, the dissociation reaction ab ↔ a + b and ionization reaction ar+ ↔ a(r+1)+ + e− are described as follow:
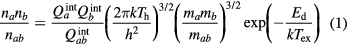
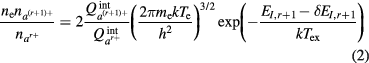
where  , ni and mi are the internal partition function, species number density and mass of the ith species, respectively; Ed and EI,r+1 are the dissociation energy of molecule ab and ionization energy of species ar+, respectively; δEI,r+1 is the lowering of the ionization energy due to the Coulomb interactions, and k and h are the Boltzmann and Planck constants, respectively, and Tex is the excitation temperature. In this paper, for monatomic species ionization, which is governed by electron, we let Tex = Te. On the other hand, for the molecular ionization, which occurs in the temperature range where there is basically no electrons in the system, we let Tex = Th. This definition of Tex consists with previous research work [26, 27].
, ni and mi are the internal partition function, species number density and mass of the ith species, respectively; Ed and EI,r+1 are the dissociation energy of molecule ab and ionization energy of species ar+, respectively; δEI,r+1 is the lowering of the ionization energy due to the Coulomb interactions, and k and h are the Boltzmann and Planck constants, respectively, and Tex is the excitation temperature. In this paper, for monatomic species ionization, which is governed by electron, we let Tex = Te. On the other hand, for the molecular ionization, which occurs in the temperature range where there is basically no electrons in the system, we let Tex = Th. This definition of Tex consists with previous research work [26, 27].
However, the phase transition process cannot be considered by this method and therefore this method could be inaccurate for plasma system where the condensing effect is not negligible [13]. According to the second law of thermodynamics, when a multi-component system reaches phase equilibrium, the chemical potential of condensed particle equals to that of corresponding gaseous particle, which is given by [28]

where μi-gas and μi-cond are chemical potential of gaseous particle and corresponding condensed particle respectively. By using this equation, the composition of condensed species is calculable.
Calculating chemical potential of condensed particle and its corresponding gaseous particle is the crux. The chemical potential of gaseous particle is calculated by [11, 29]
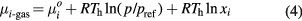
where xi is molar fraction of ith particle in the gaseous phase. pref is reference pressure which is 1 atm in current calculation.  is the chemical potential of the ith particle in the standard state, which is given by
is the chemical potential of the ith particle in the standard state, which is given by
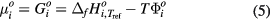
where  is molar weighted Gibbs energy, which can be calculated by internal partition function
is molar weighted Gibbs energy, which can be calculated by internal partition function  and translational partition function
and translational partition function  [13, 29]. For monatomic species, the internal partition function is only governed by electron temperature [13] and therefore the
[13, 29]. For monatomic species, the internal partition function is only governed by electron temperature [13] and therefore the  is given by
is given by
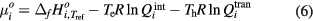
The calculation method of internal partition function of gaseous species is presented in the next part.
The chemical potential of condensed species cannot be established by calculation with sufficient accuracy and therefore the data are derived from measurement data. In the present calculation, we use the fitting data of molar tempered Gibbs energy presented by Coufal [29] on the assumption that the chemical potential of condensed particle (like graphite in this paper) is governed by Th.
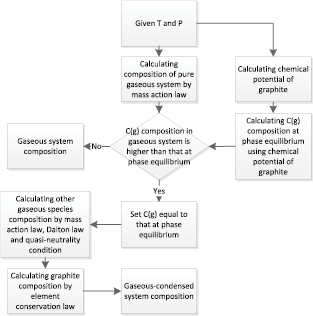
Figure 1 shows the calculation flow of composition of multiphase 2-T plasma system containing graphite. By knowing the chemical potential of graphite at given Th, the chemical potential of gaseous C is obtained by equation (3) according to the second law of thermodynamics. With the knowledge of  of gaseous C, which is derived from formation enthalpy and partition function, the molar fraction of gaseous species at phase equilibrium can be obtained by equation (4). If the molar fraction of gaseous C in pure gaseous system is higher than that at phase equilibrium, it means the phase transition process is not completed at given condition. By using the molar fraction of gaseous C at phase equilibrium, mass action law, the conservation of the chemical elements, Dalton's law and electrical quasi-neutrality with consideration of the Debye–Huckel corrections, the molar fraction of other species in the multiphase system is obtained. Otherwise, if the molar fraction of gaseous C in pure gaseous system is lower than that at phase equilibrium, which means the graphite sublimation is completed in this given condition, the composition result of pure gaseous system is adopted.
of gaseous C, which is derived from formation enthalpy and partition function, the molar fraction of gaseous species at phase equilibrium can be obtained by equation (4). If the molar fraction of gaseous C in pure gaseous system is higher than that at phase equilibrium, it means the phase transition process is not completed at given condition. By using the molar fraction of gaseous C at phase equilibrium, mass action law, the conservation of the chemical elements, Dalton's law and electrical quasi-neutrality with consideration of the Debye–Huckel corrections, the molar fraction of other species in the multiphase system is obtained. Otherwise, if the molar fraction of gaseous C in pure gaseous system is lower than that at phase equilibrium, which means the graphite sublimation is completed in this given condition, the composition result of pure gaseous system is adopted.
Figure 1. Calculation flow chart of composition of multiphase 2-T plasma system containing graphite.
Download figure:
Standard image High-resolution image2.2. Partition function of gaseous species
The calculation of the partition function is a prerequisite for calculating the plasma composition and thermodynamic properties. Following the recommendation of Gleizes and some other previous work [4, 6, 25, 27, 30–32], we assumed that translational, rotational and vibrational motions of heavy species were governed by Th while electronic excitation (of both heavy species and electrons) and translational motion of electrons were governed by Te. The calculation method of partition functions are summarized as follows.
For monatomic species, the internal partition function is given by
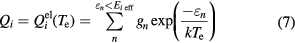
where  (Te) is the electronic internal partition function of species i, gn and εn are the degeneracy and energy of the nth electronic levels, and Ei eff is the effective ionization limit considering the Debey correction at which the sum over electronic levels is cut off. The data of energy levels and degeneracy are mainly taken from NIST database [33]. The data of high energy levels with high principal quantum number, which are not given by Moore or NIST database, was estimated using the hydrogenic approximation based on the last principal quantum number [34].
(Te) is the electronic internal partition function of species i, gn and εn are the degeneracy and energy of the nth electronic levels, and Ei eff is the effective ionization limit considering the Debey correction at which the sum over electronic levels is cut off. The data of energy levels and degeneracy are mainly taken from NIST database [33]. The data of high energy levels with high principal quantum number, which are not given by Moore or NIST database, was estimated using the hydrogenic approximation based on the last principal quantum number [34].
For diatomic and polyatomic species, the internal partition function is the product of electronic, vibration and rotation partition functions. The expression is given by
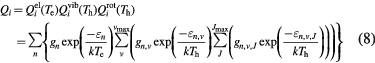
where  (Th) and
(Th) and  (Th) are respectively the vibrational and rotational partition functions of species i. The subscript n, v indicates the vth vibration energy level of the nth electron energy level. And similarly, the subscript n, v, J indicates the Jth rotation energy level which belongs to vth vibration energy level of the nth electron energy level. The Morse potential minimization method has been used to limit the calculation on the vibrational and rotational levels [35]. All the data needed have been taken from the most recent data of NIST [33] and the JANAF thermochemical tables [36].
(Th) are respectively the vibrational and rotational partition functions of species i. The subscript n, v indicates the vth vibration energy level of the nth electron energy level. And similarly, the subscript n, v, J indicates the Jth rotation energy level which belongs to vth vibration energy level of the nth electron energy level. The Morse potential minimization method has been used to limit the calculation on the vibrational and rotational levels [35]. All the data needed have been taken from the most recent data of NIST [33] and the JANAF thermochemical tables [36].
For polyatomic species, due to the lack of accurate spectroscopic data, the internal partition functions were calculated by a simplified method, in which the discrete summations over rotational quantum numbers are replaced by an integral form [34]
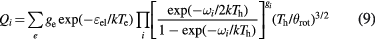
where  is the rotational constant. For the polyatomic species, the rotational energy can be expressed as a function of the three principal moments of inertia IA, IB and IC:
is the rotational constant. For the polyatomic species, the rotational energy can be expressed as a function of the three principal moments of inertia IA, IB and IC:
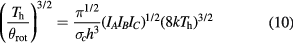
2.3. Thermodynamic properties for non-LTE multiphase plasma system
After obtaining the composition, the thermodynamic properties can be evaluated through a classical statistical mechanics approach. The method of thermodynamic property calculation presented by Coufal [29] is applied in this paper.
The mass density is given by

where ci and mi are the amount and mass of species i in the system, respectively. Vg is the volume of the gaseous phase. The volume of condensed phase is neglected.
The specific enthalpy of system is summation of specific heat of every single species. For gaseous species, the translational contributions, and reactive and internal contributions due to electronic excitation are taken into account. For monatomic species, the specific enthalpy is given by
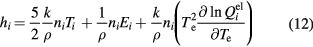
where ni is the number density of species i and Ei is the formation energy of species i. For molecular species, the specific enthalpy is given by
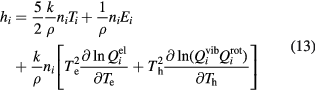
The specific heat for condensed species can be calculated through standard thermodynamic function as proposed by Coufal [29].
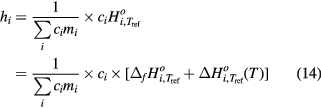
where  is the summation of standard molar enthalpy change Δ
is the summation of standard molar enthalpy change Δ (T) and standard molar formation enthalpy ▵f
(T) and standard molar formation enthalpy ▵f . All the data needed have been taken from NIST Atomic Spectra Database [33] and the JANAF thermochemical tables [36]. According to the JANAF table, the graphite, O2 and H2 are chosen as the reference state particles.
. All the data needed have been taken from NIST Atomic Spectra Database [33] and the JANAF thermochemical tables [36]. According to the JANAF table, the graphite, O2 and H2 are chosen as the reference state particles.
Taking total pressure p, electron temperature Te, and non-equilibrium parameter θ = Te/Th as independent variables, differentiation of the total enthalpy gives [31, 37]
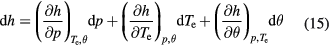
The second term of this equation can be interpreted as the total specific heat at constant pressure and constant non-equilibrium parameter.
3. Sample calculation
3.1. Description of calculation model
CO2–CH4 mixture is now being considered as a possible substitution for SF6 due to its environmental friendliness and good electrical properties. In this part, the composition and thermodynamic properties of CO2–CH4 mixture are calculated using the method presented above. Totally 48 species have been considered in the present calculation as given in table 1. The species C(c) is the condensed carbon, namely graphite in this calculation. The chemical potential of graphite is derived from standard molar tempered Gibbs energy using the measurement results which is fitted by Coufal [29]
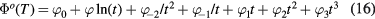
where t = T/(10 000 K). The coefficients  ,
,  ,
,  ,
,  ,
,  ,
,  and
and  of graphite are given in table 2.
of graphite are given in table 2.
Table 1. Species in the calculation of CO2–CH4 plasma composition.
| Elements | Species |
|---|---|
| C | C3, C2, C, C+, C2+, C3+, C(c) |
| H | H2, H, 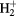 , , 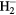 , H−, H+ , H−, H+ |
| O | O3, O2, O, 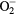 , , 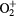 , O2−, O−, O+, O2+, O3+ , O2−, O−, O+, O2+, O3+ |
| CH | C2H6, C2H4, C2H2, C2H, CH4, CH3, CH2, CH |
| CO | CO2, C2O, CO, CO+ |
| HO | H2O2, H2O, HO2, OH, OH+, OH− |
| Others | e−, COOH, H2CO, HCOOH, CH3OH, HCO, C2H4O, C2H5OH, HCO+ |
Table 2. Coefficients of graphite in equation (16).
| Th (K) | 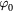 |
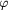 |
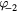 |
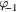 |
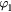 |
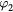 |
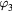 |
|---|---|---|---|---|---|---|---|
| 298–1600 | −4.1845 | 0.697 29 | −0.000 8680 | 0.128 911 | 195.3163 | −369.2265 | 359.1611 |
| 1600–5000 | 61.9368 | 28.351 58 | −0.021 45 | 1.854 69 | −16.2256 | 17.3267 | −0.6276 |
| 5000–6000 | 78.8933 | 27 | 0 | −9.064 66 | 0 | 0 | 0 |
3.2. Influence of condensed phase under LTE assumption
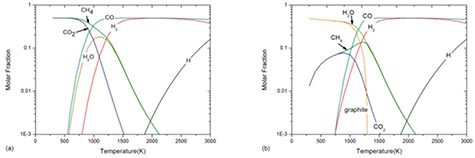
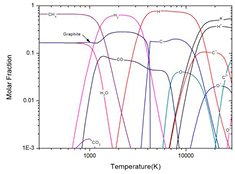
As presented by many previous published papers [12, 19, 21], the presence of condensed species can significantly influence the plasma composition and further influence the plasma properties under LTE. Figure 2 shows the molar fraction of 50%CO2–50%CH4 mixture at atmospheric pressure under LTE assumption for temperature lower than 3000 K with and without the consideration of condensed graphite. For a pure gaseous system, as shown in figure 2(a), the CH4 and CO2 dominate the mixture before CO2 dissociation which occurs at about 800 K. However, once condensed carbon, namely graphite, is taken into account, the composition at low temperature is different. As shown in figure 2(b), due to the presence of graphite, the reaction CO2 + CH4 ↔ 2H2O + 2C(c) makes H2O and graphite being the dominant species before the graphite sublimation at around 1300 K. This discrepancy in composition, which can make difference in thermo-physical properties, shows the importance of considering condensed species in plasma simulation.
Figure 2. Molar fraction of 50%CO2–50%CH4 mixture at atmospheric pressure under LTE assumption for temperature lower than 3000 K (a) without graphite and (b) with graphite.
Download figure:
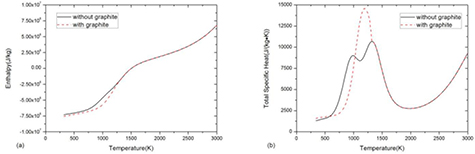
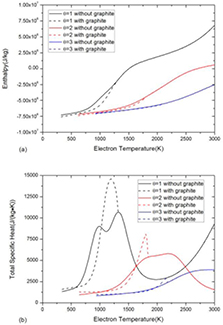
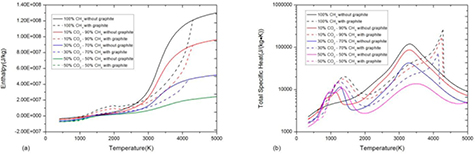
Standard image High-resolution imageFigure 3 shows the comparison of specific enthalpy and total specific heat between the system with and without considering graphite. For mass density, the presence of graphite makes no difference because the reaction CO2 + CH4 ↔ 2H2O + 2C(c) introduced by graphite does not change the system volume or total mass.
Figure 3. Comparison of (a) specific enthalpy and (b) total specific heat of 50%CO2–50%CH4 mixture at atmospheric pressure under LTE assumption between the system with and without considering graphite.
Download figure:
Standard image High-resolution imageThe presence of graphite decreases the specific enthalpy as shown in figure 3(a), which is also due to the difference of composition and formation enthalpy. In the system with graphite, the composition consists of 50%H2O–50%C(c) before graphite sublimation at 1300 K due to the reaction CO2 + CH4 ↔ 2H2O + 2C(c). The lower formation enthalpy of 50%H2O–50%C(c) mixture comparing to 50%CO2–50%CH4 mixture leads to lower value of specific enthalpy.
The presence of graphite changes the peaks of specific heat which are closely associated with chemical reactions, as shown in figure 3(b). In a pure gaseous system without graphite, there are two peaks in specific heat corresponding to CO2 dissociation at 800 K and CH4 and H2O dissociation at around 1300 K. In a multiphase system considering graphite, the sharp peak at 1200 K is due to the graphite sublimation. The inflection at 1300 K is corresponding to the fully sublimation point where phase transition completes. For temperature higher than that point, the mixture is no longer a multiphase system but a gaseous system.
3.3. Influence of non-equilibrium parameter
Figure 4 shows the temperature dependence of 50%CO2–50%CH4 mixture composition with different non-equilibrium parameter. In order to make the figure more readable, only the species whose molar fraction is higher than 10−3 are presented. For LTE system, as shown in figure 4(a), the reaction CO2 + CH4 ↔ 2H2O + 2C(c) makes H2O and graphite being the dominant species at low temperature (lower than 1000 K). After the sublimation of graphite at around 1200 K, the reaction CO2 + CH4 ↔ 2CO + 2H2 leads the domination of CO and H2. At 3200 K and 6800 K, H2 and CO dissociate respectively. The first order ionization of C, H and O occur at 12 500 K, 14 700 K and 15 700 K respectively.
Figure 4. Electron temperature dependence of 50%CO2–50%CH4 mixture composition at atmospheric pressure with (a) θ = 1, (b) θ = 3 and (c) θ = 5.
Download figure:
Standard image High-resolution imageIn a two-temperature CO2–CH4 mixture, the non-LTE effect may significantly affect both the dissociation and ionization process and further affect the thermo-physical properties. In a non-LTE system, the excitation temperature of dissociation is Th while the excitation temperature of monatomic particle ionization is Te as given in equations (1) and (2). Therefore from the perspective of Te, the dissociation reactions are shifted to higher electron temperature in non-LTE system [9, 10], namely 'delay of dissociation reaction'. When θ = 3 (figure 4(b)), the reaction of CO2 + CH4 ↔ 2CO + 2H2 occurs at electron temperature range from 2500 K to 3000 K with H2O as intermediate product. H2 dissociates into H atoms at Te = 10 000 K. Due to the delay of CO dissociation which occurs at Te = 17 000 K, H is almost the only monatomic neutral species in the Te range from 6000 K to 15 000 K and therefore the system ionization is dominated by ionization of H atoms at temperature below 14 000 K. This phenomenon is different from the LTE system in which the system ionization is dominated by C+ in low temperature range. At Te = 17 000 K, the dissociation of CO and ionization of C and O atoms occur at same time due to the high electron temperature, which causes the low molar fraction of C and O atoms in this system.
As the non-equilibrium parameter increases further (see the θ = 5 in figure 4(c)), the producing of CO and H2 are shifted to higher electron temperature (around Te = 4000 K to 5000 K). The dissociation of H2 and ionization of H atom occur at same time due to the delay of dissociation reactions (at around Te = 15 000 K). The H+ dominates the system ionization process until Te = 20 000 K at which CO dissociates and C and O atoms ionize.
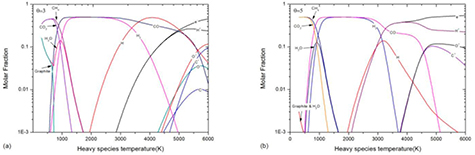
On the other hand, from the perspective of Th, the influence of non-LTE effect on dissociation reactions can be different or even opposite, as shown in figure 5. In non-LTE system, the high electron temperature promotes the ionization of dissociation product and therefore promotes the dissociation process. For instance, the reaction CO ↔ C + O occurs at 6800 K at LTE system, while in non-LTE system this reaction is shifted to Th = 5700 K when θ = 3 and Th = 4600 K when θ = 5. This is because the ionization of C and O consumes the dissociation product and further promotes the dissociation process. Similar phenomenon can be found in H2 dissociation which occurs at T = 3300 K when LTE and at Th = 3100 K when θ = 5. However, when θ = 3 the Th of H2 dissociation is basically same as that when LTE because the H ionization does not happen at this temperature.
Figure 5. Heavy species temperature dependence of 2-T 50%CO2–50%CH4 mixture composition at atmospheric pressure for Th < 6000 K with (a) θ = 3 and (b) θ = 5.
Download figure:
Standard image High-resolution imageBesides the chemical reactions, non-LTE effect can also affect the phase transition process, as shown in figure 6. At same Te, the Th leads to higher chemical potential of graphite μi-cond and further increases the gaseous carbon molar fraction and lowers the graphite molar fraction. At the meantime, similar to the dissociation reaction, the non-LTE effect shifted the sublimation process to higher Te, as shown in the figure 6(a). On the other aspect, at same Th, the high electron temperature increases the internal partition function and therefore decreases the  in equation (4), which further increases the gaseous carbon molar fraction and lowers the graphite molar fraction. As a consequence, in non-LTE system, the molar fraction of graphite is lower than that in LTE system and the sublimation process occurs in lower heavy species temperature, as shown in figure 6(b). This trend is consistent with results of condensed metal species presented by André et al [13] who found the heavy species temperature of sublimation decreases as θ increases.
in equation (4), which further increases the gaseous carbon molar fraction and lowers the graphite molar fraction. As a consequence, in non-LTE system, the molar fraction of graphite is lower than that in LTE system and the sublimation process occurs in lower heavy species temperature, as shown in figure 6(b). This trend is consistent with results of condensed metal species presented by André et al [13] who found the heavy species temperature of sublimation decreases as θ increases.
Figure 6. Molar fraction of graphite in 50%CO2–50%CH4 mixture at atmospheric pressure with different non-equilibrium parameter versus (a) electron temperature and (b) heavy species temperature.
Download figure:
Standard image High-resolution imageFigure 7 shows the thermodynamic properties of 50%CO2–50%CH4 mixture at atmospheric pressure with different non-equilibrium parameter. As θ increases, the sharp decrease of the mass density, which is due to the successive dissociations of polyatomic species, is shifted to higher electron temperature, because of the delay of dissociation reactions.
Figure 7. Thermodynamic properties of 50%CO2–50%CH4 mixture against electron temperature at atmospheric pressure with different non-equilibrium parameter (a) mass density, (b) enthalpy and (c) total specific heat.
Download figure:
Standard image High-resolution imageThe non-LTE effect also affects the enthalpy and specific heat, as shown in figures 7(b) and (c). The enthalpy increases strongly in the temperature ranges in which chemical reactions occur. This is associated with the peaks in the specific heat. Under LTE, the first peak at 1200 K is due to the reaction phase transition and CO2 + CH4 ↔ 2CO + 2H2. The other peaks at 3300 K, 6800 K, 15 000 K and 30 000 K are due to H2 dissociation, CO dissociation, first and second order ionization respectively. In a two-temperature plasma system, the delay of dissociation reaction can change the presence temperature of monatomic species and therefore change the ionization order of system [9]. As a consequence, two-temperature CO2–CH4 plasmas can show different properties from plasmas in LTE. For θ = 3, as shown in blue dot curve in figure 7(c), the first peak due to CO2 + CH4 ↔ 2CO + 2H2 reaction is shifted to Te = 3000 K. The gentle peak at around Te = 12 000 K is due to the combination of H2 dissociation at 10 000 K and H ionization at Te = 14 000 K. Besides, the CO dissociation reactions are shifted to higher electron temperature, higher than that required for C and O ionization reactions. Once CO dissociation takes place, the ionization of C and O occur at the same time, which leads to sharp peaks in specific heat at Te = 17 000 K. As the degree of non-equilibrium increases further (see the θ = 5 curve), the electron temperature at which H2 dissociates is higher than that of H ionization. It leads to a sharp peak at Te = 16 000 K rather than a gentle peak shown in θ = 3 curve.
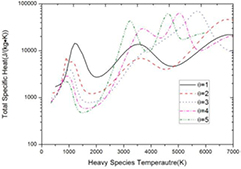
On the other hand, from the perspective of Th, the influence of non-LTE effect on thermodynamic properties can be different, as shown in figure 8. In non-LTE system, the high electron temperature promotes the ionization of dissociation product and therefore promotes the dissociation process (see figure 5). Therefore, the peaks caused by dissociation reactions can be shifted to lower Th in non-LTE system. For the peaks caused by CO dissociation, when θ ⩽ 2, the CO dissociation occurs at Th = 6800 K while the Te is not high enough to trigger the C and O ionization. However, when θ ⩾ 3, the extremely high Te promotes the ionization of dissociation product and shifts the peaks caused by CO dissociation to lower Th. For H2 dissociation, this phenomenon occurs only when θ ⩾ 5, due to its relatively low dissociation temperature.
Figure 8. Total specific heat of 50%CO2–50%CH4 mixture against heavy species temperature at atmospheric pressure with different non-equilibrium parameter.
Download figure:
Standard image High-resolution imageAs shown in figure 6, the molar fraction of graphite decreases as non-equilibrium parameter increases. Therefore, the influence of condensed phase species, which is closely related to the condensed species composition, is negligible in high non-LTE system, see figure 9. For the non-LTE system whose θ is higher than 3, the molar fraction of graphite is too small to make difference in thermodynamic properties.
Figure 9. Influence of considering condensed phase in 2-T 50%CO2–50%CH4 mixture at atmospheric pressure on (a) specific enthalpy and (b) total specific heat.
Download figure:
Standard image High-resolution image3.4. Influence of mixture ratio in LTE and 2-T system
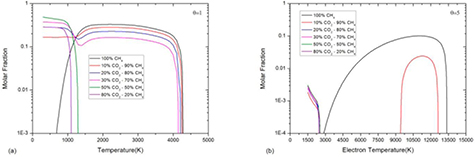
The mixture ratio can significantly affect chemical reaction and composition, especially the condensed species. Figure 10 shows the composition of 10%CO2–90%CH4 mixture at atmospheric pressure under LTE. Similar to 50%CO2–50%CH4 mixture, at low temperature (lower than 1000 K), the reaction CO2 + CH4 ↔ 2H2O + 2C(c) produces graphite and H2O. However, due to the low ratio of CO2, CH4 is the dominant species in this temperature range. As temperature increases, the CH4 dissociates into graphite and H2 and therefore there is an increase of graphite molar fraction at 1300 K. At around 4100 K, the graphite fully sublimates and phase transition process completes.
Figure 10. Temperature dependence of 10%CO2–90%CH4 mixture composition at atmospheric pressure under LTE assumption.
Download figure:
Standard image High-resolution imageAs shown in figure 11, the composition of graphite is drastically affected by mixture ratio in CO2–CH4 plasma. For pure CH4, which is relatively stable for temperature lower than 1000 K, there is barely any graphite in low temperature. As temperature increases, the molar fraction of graphite increases sharply and remains relatively high due to CH4 dissociation. At around 4100 K, the graphite fully sublimates and phase transition process completes.
Figure 11. Molar fraction of graphite in CO2–CH4 mixture of different ratio at atmospheric pressure with (a) θ = 1 and (b) θ = 5.
Download figure:
Standard image High-resolution imageFor CO2–CH4 mixture, there are two gentle peaks in graphite molar fraction. The first peak at temperature lower than 1000 K is caused by reaction CO2 + CH4 ↔ 2H2O + 2 C(c). The largest value of this peak can be found in 50%CO2–50%CH4 mixture (see green curve in figure 11(a)) in which the ratio between H element and O element is 2 : 1. The second peak in temperature range from 1400 K to 4100 K, which is due to the CH4 dissociation, can only be found in the mixture whose CH4 proportion higher than 50%. For the mixture whose CH4 proportion lower than 50%, there are enough O elements for C to produce CO which is more stable than graphite in this temperature range.
In non-LTE system, which is able to contain more gaseous species than LTE system at same electron temperature and pressure, the molar fraction of graphite is significantly decreases (see figure 11(b)). The first peak of graphite molar fraction is lower than 3‰. The second peak of graphite is still high in mixture with high CH4 proportion. The peak values are 0.1 in pure CH4 and 0.02 in 10%CO2–90%CH4 with θ = 5 respectively.
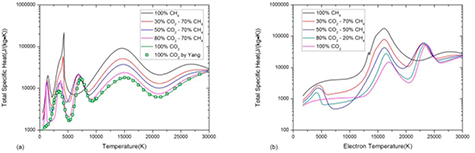
Figure 12 shows the influence of mixture ratio on specific enthalpy in LTE and non-LTE system. Generally the specific enthalpy increases as CH4 proportion due to its low molar mass. The inflection at 4200 K in mixture with CH4 proportion higher than 50% is due to the graphite sublimation. Our enthalpy results for pure CO2 under LTE show good agreement with that of Yang et al [21]. In a non-LTE system, there is no visible inflection in specific enthalpy even in mixture with high CH4 proportion because the multiphase effect is weak in non-LTE system as discussed above.
Figure 12. Specific enthalpy of CO2–CH4 mixture with different ratio at atmospheric pressure with (a) θ = 1 at low temperature, (b) θ = 1 at high temperature and (c) θ = 5. The results of Yang et al [21] are shown with symbols for comparison.
Download figure:
Standard image High-resolution imageFigure 13 shows the influence of mixture ratio on specific enthalpy in LTE and non-LTE system. Due to the complicate reaction process in the CO2–CH4 mixture at low temperature, the first two peaks under LTE at around 1000 K and 4000 K are the consequence of combination of several reactions (see figure 13(a)). The first peak at around 1000 K can only be found in pure CH4 and CO2–CH4 mixture. For the pure CH4 system, this peak is the cause of CH4 dissociation. For CO2–CH4 mixture, this peak is due to the reaction CO2 + CH4 ↔ 2CO + 2H2. The peaks at around 4000 K in different mixtures are due to the different chemical process. For the pure CO2, this peak is caused by CO2 dissociation. For CO2–CH4 mixture whose CO2 proportion is higher than 50%, this peak is due to the combination of CO2 and H2 dissociation. For 50% CO2–50%CH4 mixture, in which nearly all the CO2 is consumed by reaction CO2 + CH4 ↔ 2CO + 2H2 and there is no CO2 at 4000 K, this peak is the consequence of H2 dissociation only. For the pure CH4 and CO2–CH4 mixture whose CH4 proportion is higher than 50%, the dramatic sharp peak at 4000 K corresponding to the sharp increase in specific enthalpy is due to the H2 dissociation and graphite sublimation. The third peak which cannot be seen in pure CH4 is due to the CO dissociation. The highest peak value can be found in 50%CO2–50%CH4 mixture in which the molar fraction of CO is highest because of the reaction CO2 + CH4 ↔ 2CO + 2H2. The rest two peaks at around 14 000 K and 30 000 K are caused by first and second order ionization.
Figure 13. Specific heat of graphite in CO2–CH4 mixture with different ratio at atmospheric pressure with (a) θ = 1 and (b) θ = 5. The results of Yang et al [21] are shown with symbols for comparison.
Download figure:
Standard image High-resolution imageIn non-LTE system, as shown in figure 13(b), the influence of condensed can only be found in pure CH4 at Te = 13 000 K. The three peaks at 4000 K, 16 000 K and 23 000 K are caused by CO2 + CH4 ↔ 2CO + 2H2, H2 ↔ 2 H+ + 2e− and CO ↔ C+ + O+ + 2e−, respectively.
The multiphase effect on thermodynamic properties is significant especially in CO2–CH4 mixture with high CH4 proportion due to the large production of graphite during CH4 dissociation process, as shown in figure 14. When CH4 proportion is lower than 50%, the influence of condensed phase is weak and can be neglected in thermodynamic property calculation, especially in non-LTE system.
Figure 14. Influence of considering condensed phase in CO2–CH4 mixture with different ratio at atmospheric pressure under LTE on (a) specific enthalpy and (b) total specific heat.
Download figure:
Standard image High-resolution image3.5. Influence of pressure in LTE and 2-T system
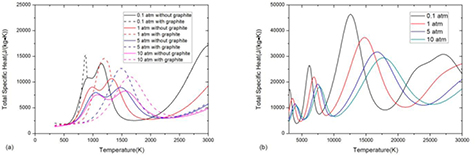
As shown in figure 15, the influence of pressure on phase transition shows in two ways. Firstly, according to Le Chatelier's law, the increase of the pressure opposes changes to the original state of equilibrium, so that reactions, including phase transition, are shifted to higher electron temperature as the pressure increases. Secondly, the increase of pressure enlarges the  in equation (4). Since the pressure does not influence chemical potential of condensed phase which is equal to chemical potential of corresponding gaseous species μi-gas, the increase of
in equation (4). Since the pressure does not influence chemical potential of condensed phase which is equal to chemical potential of corresponding gaseous species μi-gas, the increase of  leads to decrease of gaseous carbon and therefore increase the molar fraction of graphite according to equations (3) and (4). This trend is consistent with results of condensed metal species presented by André et al [13].
leads to decrease of gaseous carbon and therefore increase the molar fraction of graphite according to equations (3) and (4). This trend is consistent with results of condensed metal species presented by André et al [13].
Figure 15. Molar fraction of graphite in 50%CO2–50%CH4 mixture at different pressure with (a) θ = 1 and (b) θ = 5.
Download figure:
Standard image High-resolution imageFigure 16. Total specific heat of 50%CO2–50%CH4 mixture at different pressure under LTE at temperature (a) lower than 3000 K and (b) higher than 3000 K. The influence of graphite is also shown.
Download figure:
Standard image High-resolution imageAccording to Le Chatelier's law, the reactions, including dissociation, ionization and phase transition, are shifted to higher electron temperature as the pressure increases. Therefore, the peaks in specific heat which are closely related to reactions are shifted to high temperature in high pressure system, as shown in figure 16. The multiphase effect on thermodynamic properties is strong in high pressure system, consistent with that on composition.
4. Conclusion
In this paper, a new method of considering condensed phase in LTE and non-LTE plasma in composition and thermodynamic property calculation has been presented. This method is based on LCE and local phase equilibrium assumption and derived from second law of thermodynamics and mass action law, the conservation of the chemical elements, Dalton's law and electrical quasi-neutrality. The composition and thermodynamic properties of CO2–CH4, which may be a possible substitution for SF6, are calculated by this method as an example. The results are presented for electron temperatures from 300 to 30 000 K, pressures from 0.1 to 10 atm and non-equilibrium parameter from 1 to 5. The results can be used to research the influence of condensed species on LTE and non-LTE plasma and can be served as reliable reference data for computational simulation of the behavior of CO2–CH4 plasmas. Some of the calculation results are tabulated in appendix tables A1–A4.
The properties of CO2–CH4 mixture are significant affected by condensed species, especially in low non-equilibrium parameter, high CH4 proportion and high pressure system. In LTE system, the high heavy species temperature leads to low chemical potential of condensed species and therefore causes low saturated vapor pressure of gaseous species which increases the molar fraction of graphite. In mixture with high CH4 proportion, the reaction CH4 ↔ 2 H2 + C(c) produces graphite and further increases the influence of condensed phase. When system pressure is high, the delay of phase transition and increase of  in gaseous species chemical potential strengthen the influence of condensed phase.
in gaseous species chemical potential strengthen the influence of condensed phase.
Besides the condensed species, the influence of non-LTE effect, mixture ratio and pressure on composition and thermodynamic properties of CO2–CH4 mixture is also significant. From the perspective of Te, the delay of dissociation reactions in non-LTE system changes dissociation and ionization process both. On the other side, from the perspective of Th, the influence of non-LTE effect on dissociation reactions can be different or even opposite. In non-LTE system, the high electron temperature promotes the ionization of dissociation product and therefore promotes the dissociation process. Therefore the enthalpy and specific heat show different properties in LTE and non-LTE system. The different mixture ratio introduces different reactions in system and therefore changes the thermodynamic properties. Generally the specific enthalpy increases as CH4 proportion due to its low molar mass. As the increase of pressure, the reactions are shifted to higher temperature according to Le Chatelier's law.
Acknowledgment
This work is supported by the National Key Basic Research Program of China (973 Program, No. 2015CB251002), the National Natural Science Foundation of China (No. 51521065, 51577145, 51377128).
Appendix.: Tabular thermodynamic and transport properties
Table A1. Thermodynamic properties of CO2–CH4 mixture at atmospheric pressure under LTE.
| Te (K) | 20%CO2–80%CH4 | 50%CO2–50%CH4 | 80%CO2–20%CH4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | |
| 300 | 8.79 × 10−1 | −5.80 × 106 | 1.52 × 103 | 1.22 × 100 | −7.60 × 106 | 1.50 × 103 | 1.56 × 100 | −8.36 × 106 | 1.02 × 103 |
| 400 | 6.59 × 10−1 | −5.59 × 106 | 1.78 × 103 | 9.15 × 10−1 | −7.43 × 106 | 1.60 × 103 | 1.17 × 100 | −8.24 × 106 | 1.12 × 103 |
| 600 | 4.39 × 10−1 | −5.16 × 106 | 2.30 × 103 | 6.10 × 10−1 | −7.07 × 106 | 1.81 × 103 | 7.80 × 10−1 | −7.98 × 106 | 1.33 × 103 |
| 800 | 3.28 × 10−1 | −4.60 × 106 | 3.05 × 103 | 4.55 × 10−1 | −6.65 × 106 | 2.27 × 103 | 5.81 × 10−1 | −7.65 × 106 | 1.81 × 103 |
| 1000 | 2.49 × 10−1 | −3.58 × 106 | 6.12 × 103 | 3.37 × 10−1 | −5.71 × 106 | 6.13 × 103 | 4.10 × 10−1 | −6.65 × 106 | 6.92 × 103 |
| 1200 | 1.73 × 10−1 | −1.21 × 106 | 1.39 × 104 | 2.16 × 10−1 | −3.20 × 106 | 1.43 × 104 | 2.79 × 10−1 | −5.03 × 106 | 3.26 × 103 |
| 1400 | 1.17 × 10−1 | 2.00 × 106 | 1.59 × 104 | 1.49 × 10−1 | −7.80 × 105 | 1.05 × 104 | 2.39 × 10−1 | −4.71 × 106 | 1.53 × 103 |
| 1600 | 8.93 × 10−2 | 4.43 × 106 | 1.06 × 104 | 1.18 × 10−1 | 6.38 × 105 | 5.79 × 103 | 2.09 × 10−1 | −4.40 × 106 | 1.56 × 103 |
| 1800 | 7.53 × 10−2 | 5.87 × 106 | 6.34 × 103 | 1.03 × 10−1 | 1.37 × 106 | 3.22 × 103 | 1.86 × 10−1 | −4.08 × 106 | 1.59 × 103 |
| 2000 | 6.65 × 10−2 | 6.90 × 106 | 4.97 × 103 | 9.17 × 10−2 | 1.92 × 106 | 2.73 × 103 | 1.67 × 10−1 | −3.76 × 106 | 1.63 × 103 |
| 2200 | 5.99 × 10−2 | 6.79 × 106 | 4.30 × 103 | 8.29 × 10−2 | 2.49 × 106 | 2.91 × 103 | 1.52 × 10−1 | −3.42 × 106 | 1.69 × 103 |
| 2400 | 5.43 × 10−2 | 7.80 × 106 | 5.36 × 103 | 7.55 × 10−2 | 3.16 × 106 | 3.54 × 103 | 1.39 × 10−1 | −3.07 × 106 | 1.79 × 103 |
| 2600 | 4.92 × 10−2 | 9.16 × 106 | 7.40 × 103 | 6.87 × 10−2 | 4.04 × 106 | 4.69 × 103 | 1.28 × 10−1 | −2.68 × 106 | 2.03 × 103 |
| 2800 | 4.43 × 10−2 | 1.11 × 107 | 1.06 × 104 | 6.23 × 10−2 | 5.23 × 106 | 6.45 × 103 | 1.18 × 10−1 | −2.18 × 106 | 2.74 × 103 |
| 3000 | 3.94 × 10−2 | 1.39 × 107 | 1.52 × 104 | 5.60 × 10−2 | 6.87 × 106 | 8.78 × 103 | 1.07 × 10−1 | −1.36 × 106 | 4.66 × 103 |
| 3500 | 2.77 × 10−2 | 2.56 × 107 | 2.87 × 104 | 4.16 × 10−2 | 1.29 × 107 | 1.37 × 104 | 7.60 × 10−2 | 3.49 × 106 | 1.33 × 104 |
| 4000 | 1.92 × 10−2 | 4.49 × 107 | 5.16 × 104 | 3.24 × 10−2 | 1.88 × 107 | 9.51 × 103 | 5.25 × 10−2 | 1.04 × 107 | 1.07 × 104 |
| 4500 | 1.43 × 10−2 | 6.76 × 107 | 9.68 × 103 | 2.75 × 10−2 | 2.21 × 107 | 5.15 × 103 | 4.39 × 10−2 | 1.30 × 107 | 3.19 × 103 |
| 5000 | 1.26 × 10−2 | 7.11 × 107 | 5.99 × 103 | 2.44 × 10−2 | 2.45 × 107 | 4.93 × 103 | 3.91 × 10−2 | 1.42 × 107 | 2.11 × 103 |
| 5500 | 1.14 × 10−2 | 7.39 × 107 | 5.68 × 103 | 2.17 × 10−2 | 2.76 × 107 | 7.54 × 103 | 3.54 × 10−2 | 1.53 × 107 | 2.45 × 103 |
| 6000 | 1.04 × 10−2 | 7.75 × 107 | 8.84 × 103 | 1.92 × 10−2 | 3.29 × 107 | 1.29 × 104 | 3.20 × 10−2 | 1.72 × 107 | 5.26 × 103 |
| 6500 | 9.41 × 10−3 | 8.40 × 107 | 1.59 × 104 | 1.67 × 10−2 | 4.14 × 107 | 1.96 × 104 | 2.80 × 10−2 | 2.18 × 107 | 1.25 × 104 |
| 7000 | 8.48 × 10−3 | 9.30 × 107 | 1.81 × 104 | 1.45 × 10−2 | 5.22 × 107 | 2.18 × 104 | 2.37 × 10−2 | 3.02 × 107 | 1.88 × 104 |
| 8000 | 7.16 × 10−3 | 1.06 × 108 | 9.72 × 103 | 1.16 × 10−2 | 6.77 × 107 | 9.95 × 103 | 1.78 × 10−2 | 4.57 × 107 | 1.04 × 104 |
| 9000 | 6.25 × 10−3 | 1.16 × 108 | 1.02 × 104 | 9.99 × 10−3 | 7.53 × 107 | 7.14 × 103 | 1.51 × 10−2 | 5.25 × 107 | 5.53 × 103 |
| 10 000 | 5.47 × 10−3 | 1.28 × 108 | 1.53 × 104 | 8.73 × 10−3 | 8.37 × 107 | 9.82 × 103 | 1.31 × 10−2 | 5.85 × 107 | 6.75 × 103 |
| 11 000 | 4.75 × 10−3 | 1.48 × 108 | 2.36 × 104 | 7.58 × 10−3 | 9.60 × 107 | 1.47 × 104 | 1.14 × 10−2 | 6.68 × 107 | 9.75 × 103 |
| 12 000 | 4.07 × 10−3 | 1.78 × 108 | 3.50 × 104 | 6.49 × 10−3 | 1.14 × 108 | 2.14 × 104 | 9.75 × 10−3 | 7.87 × 107 | 1.37 × 104 |
| 13 000 | 3.42 × 10−3 | 2.20 × 108 | 4.82 × 104 | 5.48 × 10−3 | 1.40 × 108 | 2.90 × 104 | 8.27 × 10−3 | 9.48 × 107 | 1.82 × 104 |
| 14 000 | 2.86 × 10−3 | 2.74 × 108 | 5.89 × 104 | 4.59 × 10−3 | 1.73 × 108 | 3.54 × 104 | 6.97 × 10−3 | 1.15 × 108 | 2.22 × 104 |
| 15 000 | 2.40 × 10−3 | 3.35 × 108 | 6.06 × 104 | 3.87 × 10−3 | 2.10 × 108 | 3.72 × 104 | 5.90 × 10−3 | 1.39 × 108 | 2.40 × 104 |
| 16 000 | 2.07 × 10−3 | 3.92 × 108 | 5.17 × 104 | 3.33 × 10−3 | 2.45 × 108 | 3.28 × 104 | 5.08 × 10−3 | 1.62 × 108 | 2.22 × 104 |
| 17 000 | 1.84 × 10−3 | 4.36 × 108 | 3.85 × 104 | 2.95 × 10−3 | 2.74 × 108 | 2.53 × 104 | 4.49 × 10−3 | 1.82 × 108 | 1.78 × 104 |
| 18 000 | 1.67 × 10−3 | 4.68 × 108 | 2.74 × 104 | 2.68 × 10−3 | 2.95 × 108 | 1.83 × 104 | 4.07 × 10−3 | 1.97 × 108 | 1.32 × 104 |
| 19 000 | 1.55 × 10−3 | 4.91 × 108 | 2.03 × 104 | 2.49 × 10−3 | 3.10 × 108 | 1.37 × 104 | 3.76 × 10−3 | 2.09 × 108 | 9.92 × 103 |
| 20 000 | 1.46 × 10−3 | 5.09 × 108 | 1.65 × 104 | 2.33 × 10−3 | 3.23 × 108 | 1.11 × 104 | 3.52 × 10−3 | 2.17 × 108 | 8.07 × 103 |
| 22 000 | 1.31 × 10−3 | 5.40 × 108 | 1.57 × 104 | 2.08 × 10−3 | 3.43 × 108 | 1.07 × 104 | 3.14 × 10−3 | 2.33 × 108 | 7.83 × 103 |
| 24 000 | 1.18 × 10−3 | 5.76 × 108 | 2.11 × 104 | 1.88 × 10−3 | 3.69 × 108 | 1.49 × 104 | 2.82 × 10−3 | 2.52 × 108 | 1.14 × 104 |
| 26 000 | 1.06 × 10−3 | 6.26 × 108 | 2.82 × 104 | 1.68 × 10−3 | 4.05 × 108 | 2.10 × 104 | 2.51 × 10−3 | 2.80 × 108 | 1.70 × 104 |
| 28 000 | 9.59 × 10−4 | 6.86 × 108 | 3.01 × 104 | 1.51 × 10−3 | 4.52 × 108 | 2.50 × 104 | 2.22 × 10−3 | 3.19 × 108 | 2.19 × 104 |
| 30 000 | 8.73 × 10−4 | 7.45 × 108 | 2.84 × 104 | 1.35 × 10−3 | 5.04 × 108 | 2.71 × 104 | 1.96 × 10−3 | 3.68 × 108 | 2.61 × 104 |
Table A2. Thermodynamic properties of CO2–CH4 mixture at atmospheric pressure with θ = 5.
| Te (K) | 20%CO2–80%CH4 | 50%CO2–50%CH4 | 80%CO2–20%CH4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | |
| 1500 | 8.79 × 10−1 | −4.89 × 106 | 8.98 × 102 | 1.22 × 100 | −6.76 × 106 | 7.08 × 102 | 1.56 × 100 | −7.81 × 106 | 6.00 × 102 |
| 1600 | 8.24 × 10−1 | −4.79 × 106 | 9.40 × 102 | 1.14 × 100 | −6.68 × 106 | 7.37 × 102 | 1.46 × 100 | −7.75 × 106 | 6.22 × 102 |
| 1800 | 7.32 × 10−1 | −4.59 × 106 | 1.02 × 103 | 1.02 × 100 | −6.53 × 106 | 7.95 × 102 | 1.30 × 100 | −7.62 × 106 | 6.67 × 102 |
| 2000 | 6.59 × 10−1 | −4.37 × 106 | 1.11 × 103 | 9.15 × 10−1 | −6.36 × 106 | 8.56 × 102 | 1.17 × 100 | −7.48 × 106 | 7.10 × 102 |
| 2200 | 5.99 × 10−1 | −4.13 × 106 | 1.21 × 103 | 8.31 × 10−1 | −6.18 × 106 | 9.18 × 102 | 1.06 × 100 | −7.33 × 106 | 7.53 × 102 |
| 2400 | 5.49 × 10−1 | −3.88 × 106 | 1.32 × 103 | 7.62 × 10−1 | −5.98 × 106 | 9.88 × 102 | 9.75 × 10−1 | −7.17 × 106 | 8.00 × 102 |
| 2600 | 5.06 × 10−1 | −3.59 × 106 | 1.44 × 103 | 7.03 × 10−1 | −5.77 × 106 | 1.07 × 103 | 8.99 × 10−1 | −7.00 × 106 | 8.50 × 102 |
| 2800 | 4.70 × 10−1 | −3.29 × 106 | 1.57 × 103 | 6.51 × 10−1 | −5.55 × 106 | 1.15 × 103 | 8.33 × 10−1 | −6.82 × 106 | 9.12 × 102 |
| 3000 | 4.37 × 10−1 | −2.95 × 106 | 1.72 × 103 | 6.05 × 10−1 | −5.30 × 106 | 1.26 × 103 | 7.74 × 10−1 | −6.63 × 106 | 9.90 × 102 |
| 3200 | 4.07 × 10−1 | −2.58 × 106 | 1.89 × 103 | 5.62 × 10−1 | −5.03 × 106 | 1.40 × 103 | 7.19 × 10−1 | −6.42 × 106 | 1.09 × 103 |
| 3400 | 3.79 × 10−1 | −2.17 × 106 | 2.09 × 103 | 5.22 × 10−1 | −4.72 × 106 | 1.56 × 103 | 6.66 × 10−1 | −6.18 × 106 | 1.21 × 103 |
| 3600 | 3.52 × 10−1 | −1.72 × 106 | 2.31 × 103 | 4.81 × 10−1 | −4.38 × 106 | 1.74 × 103 | 6.15 × 10−1 | −5.92 × 106 | 1.34 × 103 |
| 3800 | 3.26 × 10−1 | −1.23 × 106 | 2.52 × 103 | 4.41 × 10−1 | −4.01 × 106 | 1.92 × 103 | 5.63 × 10−1 | −5.63 × 106 | 1.46 × 103 |
| 4000 | 3.00 × 10−1 | −7.00 × 105 | 2.70 × 103 | 4.01 × 10−1 | −3.60 × 106 | 2.08 × 103 | 5.11 × 10−1 | −5.33 × 106 | 1.55 × 103 |
| 4500 | 2.41 × 10−1 | 7.34 × 105 | 2.94 × 103 | 3.08 × 10−1 | −2.51 × 106 | 2.21 × 103 | 3.99 × 10−1 | −4.57 × 106 | 1.38 × 103 |
| 5000 | 1.96 × 10−1 | 2.13 × 106 | 2.57 × 103 | 2.36 × 10−1 | −1.46 × 106 | 1.99 × 103 | 3.36 × 10−1 | −4.08 × 106 | 7.53 × 102 |
| 5500 | 1.72 × 10−1 | 3.21 × 106 | 1.98 × 103 | 1.88 × 10−1 | −6.20 × 105 | 1.42 × 103 | 3.04 × 10−1 | −3.75 × 106 | 6.47 × 102 |
| 6000 | 1.57 × 10−1 | 4.19 × 106 | 1.97 × 103 | 1.61 × 10−1 | −9.72 × 104 | 8.35 × 102 | 2.79 × 10−1 | −3.42 × 106 | 6.59 × 102 |
| 6500 | 1.45 × 10−1 | 5.19 × 106 | 2.05 × 103 | 1.44 × 10−1 | 2.30 × 105 | 5.70 × 102 | 2.57 × 10−1 | −3.09 × 106 | 6.75 × 102 |
| 7000 | 1.34 × 10−1 | 6.24 × 106 | 2.13 × 103 | 1.32 × 10−1 | 4.87 × 105 | 4.90 × 102 | 2.39 × 10−1 | −2.74 × 106 | 6.92 × 102 |
| 8000 | 1.18 × 10−1 | 8.45 × 106 | 2.27 × 103 | 1.15 × 10−1 | 9.67 × 105 | 4.88 × 102 | 2.09 × 10−1 | −2.03 × 106 | 7.31 × 102 |
| 9000 | 1.04 × 10−1 | 1.08 × 107 | 2.45 × 103 | 1.02 × 10−1 | 1.49 × 106 | 5.54 × 102 | 1.86 × 10−1 | −1.27 × 106 | 7.82 × 102 |
| 10 000 | 9.32 × 10−2 | 1.34 × 107 | 2.74 × 103 | 9.12 × 10−2 | 2.12 × 106 | 7.13 × 102 | 1.67 × 10−1 | −4.52 × 105 | 8.61 × 102 |
| 11 000 | 8.28 × 10−2 | 1.64 × 107 | 3.28 × 103 | 8.20 × 10−2 | 3.01 × 106 | 1.08 × 103 | 1.51 × 10−1 | 4.79 × 105 | 1.00 × 103 |
| 12 000 | 7.14 × 10−2 | 2.02 × 107 | 4.40 × 103 | 7.29 × 10−2 | 4.50 × 106 | 1.94 × 103 | 1.36 × 10−1 | 1.61 × 106 | 1.26 × 103 |
| 13 000 | 5.57 × 10−2 | 2.63 × 107 | 8.33 × 103 | 6.21 × 10−2 | 7.39 × 106 | 3.91 × 103 | 1.21 × 10−1 | 3.14 × 106 | 1.82 × 103 |
| 14 000 | 3.15 × 10−2 | 4.29 × 107 | 2.77 × 104 | 4.81 × 10−2 | 1.36 × 107 | 8.78 × 103 | 1.00 × 10−1 | 5.63 × 106 | 3.28 × 103 |
| 15 000 | 1.34 × 10−2 | 9.26 × 107 | 6.58 × 104 | 3.12 × 10−2 | 2.85 × 107 | 2.18 × 104 | 7.11 × 10−2 | 1.10 × 107 | 8.02 × 103 |
| 16 000 | 6.84 × 10−3 | 1.79 × 108 | 1.02 × 105 | 1.70 × 10−2 | 6.24 × 107 | 4.24 × 104 | 3.82 × 10−2 | 2.62 × 107 | 2.29 × 104 |
| 17 000 | 4.43 × 10−3 | 2.69 × 108 | 6.86 × 104 | 1.09 × 10−2 | 1.00 × 108 | 2.93 × 104 | 2.09 × 10−2 | 5.19 × 107 | 2.15 × 104 |
| 18 000 | 3.63 × 10−3 | 3.15 × 108 | 3.27 × 104 | 8.94 × 10−3 | 1.20 × 108 | 1.43 × 104 | 1.64 × 10−2 | 6.60 × 107 | 9.86 × 103 |
| 19 000 | 3.24 × 10−3 | 3.39 × 108 | 1.94 × 104 | 7.95 × 10−3 | 1.31 × 108 | 9.69 × 103 | 1.44 × 10−2 | 7.34 × 107 | 5.94 × 103 |
| 20 000 | 2.98 × 10−3 | 3.55 × 108 | 1.38 × 104 | 7.21 × 10−3 | 1.41 × 108 | 1.03 × 104 | 1.32 × 10−2 | 7.84 × 107 | 4.42 × 103 |
| 22 000 | 2.55 × 10−3 | 3.89 × 108 | 2.91 × 104 | 5.39 × 10−3 | 1.83 × 108 | 3.83 × 104 | 1.06 × 10−2 | 9.38 × 107 | 1.84 × 104 |
| 24 000 | 2.02 × 10−3 | 4.72 × 108 | 3.18 × 104 | 3.35 × 10−3 | 2.89 × 108 × 106 | 4.12 × 104 | 5.38 × 10−3 | 1.84 × 108 | 4.64 × 104 |
| 26 000 | 1.77 × 10−3 | 5.18 × 108 | 2.21 × 104 | 2.80 × 10−3 | 3.34 × 108 | 1.74 × 104 | 4.18 × 10−3 | 2.31 × 108 | 1.50 × 104 |
| 28 000 | 1.57 × 10−3 | 5.67 × 108 | 2.60 × 104 | 2.46 × 10−3 | 3.73 × 108 | 2.12 × 104 | 3.60 × 10−3 | 2.64 × 108 | 1.85 × 104 |
| 30 000 | 1.40 × 10−3 | 6.20 × 108 | 2.57 × 104 | 2.16 × 10−3 | 4.19 × 108 | 2.40 × 104 | 3.10 × 10−3 | 3.05 × 108 | 2.30 × 104 |
Table A3. Thermodynamic properties of 50%CO2–50%CH4 mixture under LTE at different pressure.
| Te (K) | 0.1 atm | 5 atm | 10 atm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | |
| 300 | 1.22 × 10−1 | −7.48 × 106 | 1.21 × 103 | 6.10 × 100 | −7.62 × 106 | 1.37 × 103 | 1.22 × 101 | −7.62 × 106 | 1.35 × 103 |
| 400 | 9.15 × 10−2 | −7.30 × 106 | 1.48 × 103 | 4.57 × 100 | −7.45 × 106 | 1.49 × 103 | 9.15 × 100 | −7.45 × 106 | 1.47 × 103 |
| 600 | 6.09 × 10−2 | −6.93 × 106 | 1.85 × 103 | 3.05 × 100 | −7.12 × 106 | 1.73 × 103 | 6.10 × 100 | −7.12 × 106 | 1.71 × 103 |
| 800 | 4.32 × 10−2 | −6.16 × 106 | 5.42 × 103 | 2.28 × 100 | −6.73 × 106 | 1.98 × 103 | 4.57 × 100 | −6.74 × 106 | 1.95 × 103 |
| 1000 | 2.68 × 10−2 | −3.77 × 106 | 1.09 × 104 | 1.80 × 100 | −6.23 × 106 | 2.78 × 103 | 3.62 × 100 | −6.29 × 106 | 2.43 × 103 |
| 1200 | 1.74 × 10−2 | −1.21 × 106 | 1.35 × 104 | 1.38 × 100 | −5.28 × 106 | 5.64 × 103 | 2.90 × 100 | −5.60 × 106 | 3.90 × 103 |
| 1400 | 1.33 × 10−2 | 3.55 × 105 | 5.67 × 103 | 9.77 × 10−1 | −3.38 × 106 | 1.08 × 104 | 2.21 × 100 | −4.37 × 106 | 7.08 × 103 |
| 1600 | 1.15 × 10−2 | 9.83 × 105 | 2.76 × 103 | 6.84 × 10−1 | −9.12 × 105 | 1.21 × 104 | 1.60 × 100 | −2.40 × 106 | 1.06 × 104 |
| 1800 | 1.02 × 10−2 | 1.49 × 106 | 2.51 × 103 | 5.40 × 10−1 | 7.80 × 105 | 7.12 × 103 | 1.19 × 100 | −2.93 × 105 | 1.01 × 104 |
| 2000 | 9.11 × 10−3 | 2.04 × 106 | 2.88 × 103 | 4.67 × 10−1 | 1.69 × 106 | 3.99 × 103 | 9.77 × 10−1 | 1.18 × 106 | 6.44 × 103 |
| 2200 | 8.21 × 10−3 | 2.75 × 106 | 3.89 × 103 | 4.19 × 10−1 | 2.33 × 106 | 3.17 × 103 | 8.54 × 10−1 | 2.03 × 106 | 4.44 × 103 |
| 2400 | 7.38 × 10−3 | 3.82 × 106 | 5.90 × 103 | 3.81 × 10−1 | 2.95 × 106 | 3.12 × 103 | 7.70 × 10−1 | 2.77 × 106 | 3.54 × 103 |
| 2600 | 6.55 × 10−3 | 5.48 × 106 | 9.22 × 103 | 3.49 × 10−1 | 3.63 × 106 | 3.53 × 103 | 7.03 × 10−1 | 3.46 × 106 | 3.49 × 103 |
| 2800 | 5.71 × 10−3 | 7.96 × 106 | 1.35 × 104 | 3.20 × 10−1 | 4.45 × 106 | 4.32 × 103 | 6.46 × 10−1 | 4.22 × 106 | 3.92 × 103 |
| 3000 | 4.92 × 10−3 | 1.12 × 107 | 1.68 × 104 | 2.93 × 10−1 | 5.48 × 106 | 5.48 × 103 | 5.94 × 10−1 | 5.12 × 106 | 4.69 × 103 |
| 3500 | 3.64 × 10−3 | 1.82 × 107 | 9.99 × 103 | 2.31 × 10−1 | 9.39 × 106 | 9.49 × 103 | 4.77 × 10−1 | 8.34 × 106 | 7.78 × 103 |
| 4000 | 3.07 × 10−3 | 2.11 × 107 | 4.21 × 103 | 1.79 × 10−1 | 1.50 × 107 | 1.15 × 104 | 3.75 × 10−1 | 1.32 × 107 | 1.06 × 104 |
| 4500 | 2.70 × 10−3 | 2.31 × 107 | 4.38 × 103 | 1.45 × 10−1 | 1.99 × 107 | 8.49 × 103 | 3.01 × 10−1 | 1.83 × 107 | 9.63 × 103 |
| 5000 | 2.39 × 10−3 | 2.62 × 107 | 7.98 × 103 | 1.25 × 10−1 | 2.32 × 107 | 5.69 × 103 | 2.55 × 10−1 | 2.23 × 107 | 6.78 × 103 |
| 5500 | 2.08 × 10−3 | 3.25 × 107 | 1.61 × 104 | 1.11 × 10−1 | 2.60 × 107 | 5.64 × 103 | 2.25 × 10−1 | 2.52 × 107 | 5.69 × 103 |
| 6000 | 1.76 × 10−3 | 4.35 × 107 | 2.55 × 104 | 9.95 × 10−2 | 2.94 × 107 | 7.88 × 103 | 2.01 × 10−1 | 2.83 × 107 | 6.80 × 103 |
| 6500 | 1.50 × 10−3 | 5.62 × 107 | 2.28 × 104 | 8.87 × 10−2 | 3.45 × 107 | 1.20 × 104 | 1.81 × 10−1 | 3.25 × 107 | 9.75 × 103 |
| 7000 | 1.33 × 10−3 | 6.43 × 107 | 1.23 × 104 | 7.83 × 10−2 | 4.20 × 107 | 1.69 × 104 | 1.61 × 10−1 | 3.86 × 107 | 1.39 × 104 |
| 8000 | 1.12 × 10−3 | 7.27 × 107 | 7.90 × 103 | 6.09 × 10−2 | 6.03 × 107 | 1.65 × 104 | 1.26 × 10−1 | 5.57 × 107 | 1.76 × 104 |
| 9000 | 9.57 × 10−4 | 8.29 × 107 | 1.28 × 104 | 5.11 × 10−2 | 7.16 × 107 | 8.08 × 103 | 1.04 × 10−1 | 6.93 × 107 | 9.99 × 103 |
| 10 000 | 8.03 × 10−4 | 1.00 × 108 | 2.14 × 104 | 4.49 × 10−2 | 7.86 × 107 | 6.84 × 103 | 9.07 × 10−2 | 7.69 × 107 | 6.64 × 103 |
| 11 000 | 6.57 × 10−4 | 1.28 × 108 | 3.28 × 104 | 3.98 × 10−2 | 8.63 × 107 | 8.76 × 103 | 8.07 × 10−2 | 8.38 × 107 | 7.38 × 103 |
| 12 000 | 5.29 × 10−4 | 1.67 × 108 | 4.38 × 104 | 3.52 × 10−2 | 9.68 × 107 | 1.21 × 104 | 7.19 × 10−2 | 9.23 × 107 | 9.72 × 103 |
| 13 000 | 4.29 × 10−4 | 2.13 × 108 | 4.51 × 104 | 3.08 × 10−2 | 1.11 × 108 | 1.66 × 104 | 6.39 × 10−2 | 1.04 × 108 | 1.31 × 104 |
| 14 000 | 3.62 × 10−4 | 2.52 × 108 | 3.42 × 104 | 2.68 × 10−2 | 1.31 × 108 | 2.19 × 104 | 5.63 × 10−2 | 1.19 × 108 | 1.72 × 104 |
| 15 000 | 3.20 × 10−4 | 2.79 × 108 | 2.15 × 104 | 2.31 × 10−2 | 1.56 × 108 | 2.71 × 104 | 4.93 × 10−2 | 1.39 × 108 | 2.17 × 104 |
| 16 000 | 2.93 × 10−4 | 2.96 × 108 | 1.37 × 104 | 1.99 × 10−2 | 1.85 × 108 | 3.08 × 104 | 4.30 × 10−2 | 1.63 × 108 | 2.60 × 104 |
| 17 000 | 2.72 × 10−4 | 3.07 × 108 | 1.01 × 104 | 1.73 × 10−2 | 2.16 × 108 | 3.16 × 104 | 3.75 × 10−2 | 1.91 × 108 | 2.88 × 104 |
| 18 000 | 2.55 × 10−4 | 3.17 × 108 | 9.17 × 103 | 1.52 × 10−2 | 2.47 × 108 | 2.91 × 104 | 3.29 × 10−2 | 2.20 × 108 | 2.93 × 104 |
| 19 000 | 2.40 × 10−4 | 3.26 × 108 | 1.02 × 104 | 1.36 × 10−2 | 2.74 × 108 | 2.45 × 104 | 2.92 × 10−2 | 2.48 × 108 | 2.74 × 104 |
| 20 000 | 2.27 × 10−4 | 3.38 × 108 | 1.31 × 104 | 1.24 × 10−2 | 2.95 × 108 | 1.97 × 104 | 2.63 × 10−2 | 2.74 × 108 | 2.38 × 104 |
| 22 000 | 2.00 × 10−4 | 3.74 × 108 | 2.23 × 104 | 1.08 × 10−2 | 3.27 × 108 | 1.30 × 104 | 2.23 × 10−2 | 3.13 × 108 | 1.63 × 104 |
| 24 000 | 1.76 × 10−4 | 4.24 × 108 | 2.69 × 104 | 9.68 × 10−3 | 3.50 × 108 | 1.10 × 104 | 1.98 × 10−2 | 3.41 × 108 | 1.21 × 104 |
| 26 000 | 1.55 × 10−4 | 4.81 × 108 | 2.97 × 104 | 8.78 × 10−3 | 3.73 × 108 | 1.27 × 104 | 1.79 × 10−2 | 3.64 × 108 | 1.15 × 104 |
| 28 000 | 1.38 × 10−4 | 5.41 × 108 | 2.98 × 104 | 7.98 × 10−3 | 4.03 × 108 | 1.68 × 104 | 1.63 × 10−2 | 3.89 × 108 | 1.36 × 104 |
| 30 000 | 1.25 × 10−4 | 5.94 × 108 | 2.32 × 104 | 7.23 × 10−3 | 4.41 × 108 | 2.10 × 104 | 1.48 × 10−2 | 4.20 × 108 | 1.74 × 104 |
Table A4. Thermodynamic properties of 50%CO2–50%CH4 mixture at different pressure with θ = 5.
| Te (K) | 0.1 atm | 5 atm | 10 atm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | ρ (kg m−3) | h (J kg−1) | Cp (J kg−1 K−1) | |
| 1500 | 1.22 × 10−1 | −6.76 × 106 | 7.05 × 102 | 6.10 × 100 | −6.76 × 106 | 7.15 × 102 | 1.22 × 101 | −6.77 × 106 | 7.23 × 102 |
| 1600 | 1.14 × 10−1 | −6.68 × 106 | 7.35 × 102 | 5.72 × 100 | −6.68 × 106 | 7.43 × 102 | 1.14 × 101 | −6.69 × 106 | 7.50 × 102 |
| 1800 | 1.02 × 10−1 | −6.52 × 106 | 7.94 × 102 | 5.08 × 100 | −6.53 × 106 | 7.99 × 102 | 1.02 × 101 | −6.53 × 106 | 8.03 × 102 |
| 2000 | 9.15 × 10−2 | −6.36 × 106 | 8.56 × 102 | 4.57 × 100 | −6.36 × 106 | 8.57 × 102 | 9.15 × 100 | −6.36 × 106 | 8.60 × 102 |
| 2200 | 8.31 × 10−2 | −6.17 × 106 | 9.24 × 102 | 4.16 × 100 | −6.18 × 106 | 9.19 × 102 | 8.31 × 100 | −6.18 × 106 | 9.20 × 102 |
| 2400 | 7.61 × 10−2 | −5.98 × 106 | 9.99 × 102 | 3.81 × 100 | −5.99 × 106 | 9.81 × 102 | 7.62 × 100 | −5.99 × 106 | 9.81 × 102 |
| 2600 | 7.02 × 10−2 | −5.77 × 106 | 1.09 × 103 | 3.52 × 100 | −5.78 × 106 | 1.05 × 103 | 7.03 × 100 | −5.78 × 106 | 1.04 × 103 |
| 2800 | 6.48 × 10−2 | −5.53 × 106 | 1.22 × 103 | 3.26 × 100 | −5.56 × 106 | 1.13 × 103 | 6.53 × 100 | −5.56 × 106 | 1.12 × 103 |
| 3000 | 5.99 × 10−2 | −5.26 × 106 | 1.40 × 103 | 3.04 × 100 | −5.31 × 106 | 1.23 × 103 | 6.08 × 100 | −5.33 × 106 | 1.21 × 103 |
| 3200 | 5.51 × 10−2 | −4.94 × 106 | 1.65 × 103 | 2.83 × 100 | −5.06 × 106 | 1.32 × 103 | 5.67 × 100 | −5.06 × 106 | 1.33 × 103 |
| 3400 | 5.02 × 10−2 | −4.56 × 106 | 1.96 × 103 | 2.64 × 100 | −4.78 × 106 | 1.43 × 103 | 5.31 × 100 | −4.79 × 106 | 1.40 × 103 |
| 3600 | 4.50 × 10−2 | −4.12 × 106 | 2.31 × 103 | 2.46 × 100 | −4.47 × 106 | 1.56 × 103 | 4.96 × 100 | −4.50 × 106 | 1.50 × 103 |
| 3800 | 3.98 × 10−2 | −3.61 × 106 | 2.64 × 103 | 2.29 × 100 | −4.14 × 106 | 1.68 × 103 | 4.62 × 100 | −4.18 × 106 | 1.61 × 103 |
| 4000 | 3.48 × 10−2 | −3.0 × 1064 × 106 | 2.88 × 103 | 2.12 × 100 | −3.79 × 106 | 1.79 × 103 | 4.30 × 100 | −3.84 × 106 | 1.71 × 103 |
| 4500 | 2.48 × 10−2 | −1.62 × 106 | 2.64 × 103 | 1.70 × 100 | −2.84 × 106 | 1.92 × 103 | 3.52 × 100 | −2.94 × 106 | 1.84 × 103 |
| 5000 | 1.95 × 10−2 | −6.74 × 105 | 1.36 × 103 | 1.35 × 100 | −1.91 × 106 | 1.80 × 103 | 2.84 × 100 | −2.04 × 106 | 1.73 × 103 |
| 5500 | 1.69 × 10−2 | −2.36 × 105 | 6.64 × 102 | 1.09 × 100 | −1.07 × 106 | 1.59 × 103 | 2.31 × 100 | −1.23 × 106 | 1.55 × 103 |
| 6000 | 1.53 × 10−2 | 3.34 × 104 | 4.89 × 102 | 8.96 × 10−1 | −3.78 × 105 | 1.24 × 103 | 1.91 × 100 | −5.21 × 105 | 1.31 × 103 |
| 6500 | 1.41 × 10−2 | 2.66 × 105 | 4.56 × 102 | 7.67 × 10−1 | 1.20 × 105 | 8.46 × 102 | 1.61 × 100 | 3.96 × 104 | 9.87 × 102 |
| 7000 | 1.31 × 10−2 | 4.93 × 105 | 4.57 × 102 | 6.83 × 10−1 | 4.64 × 105 | 6.07 × 102 | 1.41 × 100 | 4.43 × 105 | 7.04 × 102 |
| 8000 | 1.14 × 10−2 | 9.69 × 105 | 4.99 × 102 | 5.79 × 10−1 | 9.83 × 105 | 4.93 × 102 | 1.17 × 100 | 1.00 × 106 | 5.03 × 102 |
| 9000 | 1.01 × 10−2 | 1.53 × 106 | 6.47 × 102 | 5.10 × 10−1 | 1.49 × 106 | 5.34 × 102 | 1.03 × 100 | 1.51 × 106 | 5.26 × 102 |
| 10 000 | 8.99 × 10−3 | 2.41 × 106 | 1.15 × 103 | 4.58 × 10−1 | 2.08 × 106 | 6.38 × 102 | 9.18 × 10−1 | 2.08 × 106 | 6.20 × 102 |
| 11 000 | 7.75 × 10−3 | 4.31 × 106 | 2.78 × 103 | 4.14 × 10−1 | 2.81 × 106 | 8.27 × 102 | 8.31 × 10−1 | 2.78 × 106 | 7.78 × 102 |
| 12 000 | 6.00 × 10−3 | 9.47 × 106 | 8.06 × 103 | 3.76 × 10−1 | 3.81 × 106 | 1.18 × 103 | 7.56 × 10−1 | 3.70 × 106 | 1.04 × 103 |
| 13 000 | 3.54 × 10−3 | 2.64 × 107 | 2.82 × 104 | 3.38 × 10−1 | 5.31 × 106 | 1.82 × 103 | 6.87 × 10−1 | 4.96 × 106 | 1.49 × 103 |
| 14 000 | 1.58 × 10−3 | 7.57 × 107 | 5.83 × 104 | 2.97 × 10−1 | 7.74 × 106 | 3.04 × 103 | 6.17 × 10−1 | 6.83 × 106 | 2.25 × 103 |
| 15 000 | 1.07 × 10−3 | 1.12 × 108 | 2.04 × 104 | 2.48 × 10−1 | 1.20 × 107 | 5.44 × 103 | 5.39 × 10−1 | 9.76 × 106 | 3.60 × 103 |
| 16 000 | 9.29 × 10−4 | 1.25 × 108 | 9.01 × 103 | 1.90 × 10−1 | 1.99 × 107 | 1.05 × 104 | 4.47 × 10−1 | 1.46 × 107 | 6.12 × 103 × 102 |
| 17 000 | 8.44 × 10−4 | 1.33 × 108 | 7.35 × 103 | 1.28 × 10−1 | 3.57 × 107 | 2.11 × 104 | 3.41 × 10−1 | 2.32 × 107 | 1.11 × 104 |
| 18 000 | 7.64 × 10−4 | 1.42 × 108 | 1.33 × 104 | 7.87 × 10−2 | 6.41 × 107 | 3.32 × 104 | 2.35 × 10−1 | 3.90 × 107 | 2.03 × 104 |
| 19 000 | 6.41 × 10−4 | 1.67 × 108 | 3.89 × 104 | 5.39 × 10−2 | 9.56 × 107 | 2.74 × 104 | 1.52 × 10−1 | 6.50 × 107 | 2.97 × 104 |
| 20 000 | 4.67 × 10−4 | 2.30 × 108 | 7.80 × 104 | 4.35 × 10−2 | 1.17 × 108 | 1.65 × 104 | 1.07 × 10−1 | 9.38 × 107 | 2.59 × 104 |
| 22 000 | 3.33 × 10−4 | 3.14 × 108 | 1.97 × 104 | 3.43 × 10−2 | 1.41 × 108 | 1.09 × 104 | 7.55 × 10−2 | 1.29 × 108 | 1.23 × 104 |
| 24 000 | 2.85 × 10−4 | 3.57 × 108 | 2.37 × 104 | 2.69 × 10−2 | 1.73 × 108 | 2.50 × 104 | 6.09 × 10−2 | 1.53 × 108 | 1.39 × 104 |
| 26 000 | 2.46 × 10−4 | 4.07 × 108 | 2.64 × 104 | 1.77 × 10−2 | 2.57 × 108 | 5.11 × 104 | 4.53 × 10−2 | 1.99 × 108 | 3.52 × 104 |
| 28 000 | 2.14 × 10−4 | 4.63 × 108 | 2.83 × 104 | 1.36 × 10−2 | 3.25 × 108 | 1.98 × 104 | 3.06 × 10−2 | 2.87 × 108 | 4.02 × 104 |
| 30 000 | 1.89 × 10−4 | 5.15 × 108 | 2.34 × 104 | 1.20 × 10−2 | 3.59 × 108 | 1.70 × 104 | 2.53 × 10−2 | 3.37 × 108 | 1.71 × 104 |