Abstract
Cold atmospheric plasma is thought to be a promising tool for numerous biomedical applications due to its ability to generate a large diversity of reactive species in a controlled way. In some cases, it can also generate pulsed electric fields at the zone of treatment, which can induce processes such as electroporation in cell membranes. However, the interaction of these reactive species and the pulse electric field with cells in a physiological medium is very complex, and we still need a better understanding in order to be useful for future applications. A way to reach this goal is to work with model cell membranes such as liposomes, with the simplest physiological liquid and in a controlled atmosphere in order to limit the number of parallel reactions and processes. In this paper, where this approach has been chosen, 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) small unilamellar vesicles (SUV) have been synthesized in a phosphate buffered aqueous solution, and this solution has been treated by a nanosecond pulsed plasma jet under a pure nitrogen atmosphere. It is only the composition of the plasma gas that has been changed in order to generate different cocktails of reactive species. After the quantification of the main plasma reactive species in the phosphate buffered saline (PBS) solution, structural, surface charge state, and chemical modifications generated on the plasma treated liposomes, due to the interaction with the plasma reactive species, have been carefully characterized. These results allow us to further understand the effect of plasma reactive species on model cell membranes in physiological liquids. The permeation through the liposomal membrane and the reaction of plasma reactive species with molecules encapsulated inside the liposomes have also been evaluated. New processes of degradation are finally presented and discussed, which come from the specific conditions of plasma treatment under the pure nitrogen atmosphere.
Export citation and abstract BibTeX RIS
1. Introduction
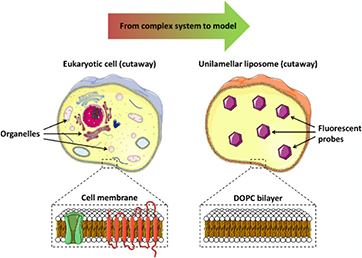
The disinfection of liquids or wet surfaces by the atmospheric plasma (CAP) treatment is seen as a promising field and market [1, 2]. In particular, CAP treatments are envisaged for the disinfection or treatment of biological media due to their biocompatible working conditions, i.e. atmospheric pressure and room temperature, and the use of low quantities of biocompatible gases [3–5]. For therapeutic applications, the behavior of human or plant cells can also be affected or controlled by their interaction with plasma reactive species, which is often naturally present in living organisms. Therefore, numerous teams have studied the interaction of CAP with biological samples and have investigated mechanisms which could explain the antibacterial properties of plasma treatment on solutions or gels containing bacteria or other living cells. The disinfection or therapeutic efficiency of the CAP processes was shown to be due to the generation of several reactive oxygen and nitrogen species (RONS): hydrogen peroxide, peroxynitrite, ozone or hydroxyl and perhydroxyl radicals among others, with the potential contribution of plasma-issued (V)UV photons [6–12] and pulsed electric fields. Recently, hypochlorite ions and chloramine were shown to play a role in the disinfection process at neutral pH when a nanosecond pulsed cold atmospheric microplasma was in contact with a phosphate buffered saline solution under a N2 atmosphere [13]. A way to further our understanding of plasma disinfection processes is to plasma treat suspensions of model cells as it was already made in [14] and as evidenced by the scheme on figure 1. It is well known that unsaturated phospholipids such as DOPC are more present in eukaryotic cell membranes compared to bacterial membranes that are richer in saturated lipids. Unsaturated lipids are also more sensitive to oxidation promoted by UV or RONS species especially compared to saturated phospholipids such as DPPC. However, the presence of a high concentration of cholesterol in eukaryotic cells drastically decreases the damage induced by DOPC oxidation. Even if bacteria have a fewer amount of unsaturated lipids, their oxidation will result into a much higher impact than eukaryote cells due to the lack of cholesterol in the bacterial membrane. Investigations focusing on the interaction between RONS generated by CAP in liquids and DOPC liposomes could also help to better understand the selectivity of CAP treatment during disinfection processes promoted by CAP. A first study using this approach has shown that CAP can induce a disruption of liposomes and the fusion of small liposomes into bigger ones [14]. However the origin of these liposome modifications are still not known and can be due to physical effects (temperature, electric field) or/and chemical effects such an alteration of the phospholipid chemical structure by reactive species generated in solution. In this paper, we have investigated how CAP can chemically and structurally impact the DOPC phospholipids bilayers using small unilamellar vesicles (SUV) loaded with carboxyfluoresce in molecules as a model of a cell. Liposome stability under CAP processes has been investigated using dynamic light scattering and fluorescence spectroscopy. Chemical modification on DOPC induced by CAP has been characterized using atmospheric-pressure matrix assisted laser desorption ionization (APMALDI)-Orbitrap mass spectrometry methods. The impact of CAP processes has been compared with the effect of chemical compounds just added in liposome suspensions in the same conditions used for the treatment of bacteria in our previous work [13] with the main difference being the addition of 5 mM of Mg2+ to stabilize the liposomes [15]. Plasma CAP treatment duration was also increased from 90 to 120 s · ml−1 (i.e. 120 s of plasma treatment per milliliter of treated solution), in order to exacerbate the structural and chemical alteration of the DOPC liposomes.
Figure 1. DOPC unilamellar liposomes are used as the model for eukaryotic cell membrane.
Download figure:
Standard image High-resolution image2. Methods
The following chemicals were purchased and used without further purification: 1,2-dioleoyl-sn-glycero-3-phosphocholine 2.5 mg · ml−1 in chloroform (Avanti Polar Lipids), 5(6)-carboxyfluorescein (Sigma-Aldrich). During the treatment of liposome suspensions, a controlled N2 atmosphere was generated all around the microplasma jet to favor the generation of reactive nitrogen species as shown in figure 1. Liposomes were prepared and treated in a phosphate buffered saline (PBS) solution also containing 5 mM of MgCl2. The interaction between custom-built CAP, with a plasma reactor made at UPPA, in a controlled nitrogen atmosphere or chemicals mimicking reactive species produced by CAP, PBS solutions and liposomes are presented and discussed. He, He + 0.50% N2 and He + 0.50% O2 process gas are used here because these gas mixtures were shown to present very different bacterial inactivation efficiencies [13]. In this paper the effects of hypochlorite (ClO−), whereby hypochlorite will refer to both hypochlorite ion and its acidic counterpart hypochlorous acid, hydrogen peroxide (H2O2), nitrites ( ), peroxynitrites (ONOO−), monochloramine (NH2Cl), ammonium (
), peroxynitrites (ONOO−), monochloramine (NH2Cl), ammonium ( ) and nitryl chloride (NO2Cl) reactive species on the liposome chemical structure were also assessed independently and compared to the CAP treatments in order to better understand the inactivation mechanism discussed in our previous work [13] and to better predict plasma reactive species interaction with eukaryotic cells for future biomedical applications.
) and nitryl chloride (NO2Cl) reactive species on the liposome chemical structure were also assessed independently and compared to the CAP treatments in order to better understand the inactivation mechanism discussed in our previous work [13] and to better predict plasma reactive species interaction with eukaryotic cells for future biomedical applications.
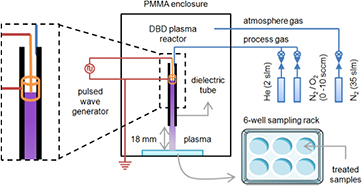
The experimental setup we used for plasma treatments is depicted in figure 2. It has already been described in previous publications [13, 16]. Briefly, the dielectric barrier discharge (DBD) plasma reactor consisted of a tungsten filament powered by a pulsed high voltage (HV) and inserted in an alumina dielectric tube. A metallic grounded ring was enclosed outside the tube. Process gas, fed into the reactor through MKS mass flow controllers, was either He at a 2 standard litres per minute (slm) flow rate, He (2 slm)/N2 (10 standard cubic centime per minute (sccm)) mixture or He (2 slm)/O2 (10 sccm) mixture. Plasma was generated by applying a 5 kV, 15 kHz, 1.5% duty cycle, positive nanosecond pulsed wave potential between the two electrodes. We controlled the atmosphere composition during plasma treatments by providing a constant N2 flow (35 slm) inside a poly(methyl methacrylate) enclosure surrounding the DBD plasma reactor without affecting the plasma treatment zone, thus, creating a low overpressure in the chamber. The DBD reactor sat on a 3-axis robot to ensure reproducible plasma treatments. The liquid samples to be plasma-treated were poured in a 6-well sampling rack (5 ml per well) and placed in the enclosure below the DBD plasma reactor. The vertical distance between the tip of the alumina dielectric tube and the liquid interface was kept constant at 18 mm.
Figure 2. Experimental setup.
Download figure:
Standard image High-resolution imageAll liposome suspensions were prepared from a 1,2- dioleoyl-sn-glycero-3-phosphocholine (DOPC) commercial stock solution in chloroform (2.5 mg · ml−1). 786 µl of this stock was transferred into 4 ml Wheaton sample vial. By tilting and rotating regularly the vial under constant N2 gas flow, the chloroform solvent evaporated to give a white DOPC film on the vial's wall. 2.5 ml of degassed PBS buffer—with or without (5)6-carboxyfluorescein 50 mM—and a stirring magnet was then added. The solution was stirred twice for 30 min at 1000 rpm, with a 60 min break at 4 °C in between. Thereafter, the vial was submitted to a 30 min sonication. The solution is then filtered through a 200 nm polycarbonate filter (one pass) before extrusion through a 100 nm polycarbonate filter (9 passes). The extruded suspension finally undergoes two filtration steps on PD-10 desalting columns filled with Sephadex G25 medium (GE Healthcare) to remove non-encapsulated (5)6-carboxyfluorescein molecules. Before plasma or wet chemistry treatments, the DOPC liposome suspension is diluted 20 times in PBS + Mg2+ 5 mM to reach the desired DOPC concentration of 50 µM.
For chemical treatments, 2 ml of liquid samples in 12-well sampling rack were used. Measurements were carried out 4 h after adding the chemical compounds matching the reactive species generated by CAP in solution, in order to ensure a full reaction between phospholipids and reactive species.
The concentration of the main reactive species in PBS solutions treated by CAP were determined by colorimetric Spectroquant® tests from Merck (1.187 89 test for H2O2, 1.147 76 test for  , 1.147 73 test for
, 1.147 73 test for  1.14752 test for
1.14752 test for  and 1.005 99 test for HOCl and NH2Cl). When necessary, plasma treated PBS solutions were diluted to avoid interference effects with other reactive species. When the dilution approach was insufficient or not possible, chemical compounds have been added before the colorimetric test to eliminate the reactive species generating interferences. Therefore, for these measurements, catalase and amidosulfuric acid (both from Sigma-Aldrich) have been added to eliminate H2O2 and
and 1.005 99 test for HOCl and NH2Cl). When necessary, plasma treated PBS solutions were diluted to avoid interference effects with other reactive species. When the dilution approach was insufficient or not possible, chemical compounds have been added before the colorimetric test to eliminate the reactive species generating interferences. Therefore, for these measurements, catalase and amidosulfuric acid (both from Sigma-Aldrich) have been added to eliminate H2O2 and  , respectively, without inducing any additional interference on the colorimetric tests:
, respectively, without inducing any additional interference on the colorimetric tests:
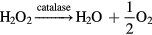
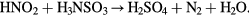
The size distribution and zeta potential of liposomes were measured on a Zetasizer Nano ZS (Malvern Instruments). The samples were poured in DTS1070 disposable cells without further preparation, and the measurements were performed at 25 ± 1 °C. The size distribution of liposomes was obtained by dynamic light scattering measurements. A laser beam was shot into the liposome suspension. The photons encountering a liposome were scattered in all directions. As liposomes moved into the surroundings because of the Brownian motion, the light scattering fluctuated over time. The smaller the liposome the more rapidly the light scattering fluctuated as the liposome was more susceptible to the Brownian motion. The zeta potential of a liposome is the electric potential between the diffuse layer (free ions coming from the surrounding fluid and attracted onto liposome due to its surface charge) and the bulk fluid—here PBS. As zeta potential is related to surface charge, it indicates liposome stability: liposomes with high zeta potential are less prone to aggregation and coalescence phenomena, thanks to a higher electrostatic repulsion between them. The zeta potential of the liposomes is available by electrophoretic light scattering, where the motion of liposomes in a fluid is measured when submitted to a uniform electric field; and this motion called electrophoretic mobility is proportional to the zeta potential.
The fluorescence intensity measurements of 5(6)carboxyfluorescein-loaded samples were performed on an Infinite M1000 Pro UV/vis spectrophotometer (Tecan), with 200 µl of each sample poured in a 96-well sampling rack. In this work, fluorescence intensity of a sample was defined as the area under the curve of fluorescence emission between 500 and 650 nm when this sample is excited at a wavelength of 490 nm, and its value was normalized against a reference untreated sample.
Several of the treated liposome solutions were analyzed with atmospheric pressure matrix-assisted laser desorption/ ionization-Orbitrap mass spectrometry (AP-MALDI-Orbitrap). For analysis, treated samples were diluted 10 times in a methanol/water mixture (50/50, volume/volume). 1 µl droplets of these diluted samples were laid down on a gold-plated steel plate. After evaporation of the solvents, the remaining liposomes and salts from the droplets were rinsed twice with 1 µl of milliQ water to desalt the samples as much as possible. Then, dried and desalted liposome samples were mixed to 1 µl of a matrix, made of 2,5-dihydroxybenzoic acid 15 mg · ml−1 in methanol/trifluoroacetic acid mixture (50/50, volume/volume), to protect the DOPC lipid from excessive photon irradiation, and favour its ionization during AP-MALDI analysis. All measurements were made in triplicates.
3. Results
Under constant N2 atmosphere, we performed a set of 120 s · ml−1 plasma treatments on the PBS + Mg2+ 5 mM solution at neutral pH and room temperature. The colorimetric tests on the plasma treated solutions allowed for the evaluation of their concentration after plasma treatment, as shown on table 1.
Table 1. Evaluation of the quantity of reactive species generated in PBS + Mg2+ 5 mM solutions at neutral pH after 120 s · ml−1 plasma treatments.
| Conc. (µM) | Conc. (µM) | Conc. (µM) | |
|---|---|---|---|
| He + 0.50% O2 plasma | He plasma | He + 0.50% N2 plasma | |
| H2O2 | 150 ± 50 | 550 ± 50 | 350 ± 50 |
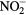 |
500 ± 50 | 2000 ± 100 | 4800 ± 200 |
 |
Not measured, conc. <1000 expected (plasma jet quenching due to ROS) | 1400 ± 100 | 2000 ± 100 |
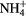 |
<3 (not detected) | <3 (not detected) | 65 ± 3 |
| NH2Cl | <7 (not detected) | 14.6 ± 2.4 | 18.9 ± 1.8 |
| HOCl | 7.2 ± 2.7 | 10.8 ± 0.4 | 18.5 ± 2.5 |
For all the plasma treatments, a low H2O2/NOx ratio was observed due to the pure nitrogen atmosphere used. Due to the plasma jet quenching, which was induced by the highest density of reactive oxygen species generated in the discharge zone, the quantity of the reactive species generated in PBS solution by He + 0.50% O2 plasma was much lower compared with the two other plasma treatments (table 1). Except for H2O2, He plasma treatments generated reactive species in lower amounts compared with He + 0.50% N2 plasma ones. For He + 0.50% N2 plasma treatments, much higher concentrations in NOx and the generation of an additional reactive species (i.e.  ) were detected. HOCl was generated in He + 0.50% N2 plasma, but also with the other gas mixtures, although in lower quantities. NH2Cl was generated in similar quantities for He and He + 0.50% N2 plasmas but was not detected with He + 0.50% O2 plasma.
) were detected. HOCl was generated in He + 0.50% N2 plasma, but also with the other gas mixtures, although in lower quantities. NH2Cl was generated in similar quantities for He and He + 0.50% N2 plasmas but was not detected with He + 0.50% O2 plasma.
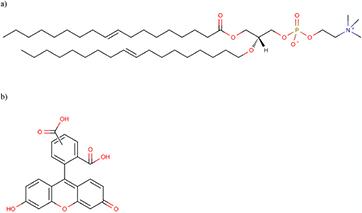
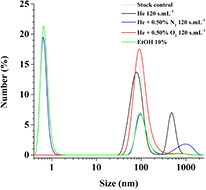
Then, we performed a set of 120 s · ml−1 plasma treatments on 100 nm small unilamellar DOPC vesicles (also called liposomes), containing 50 mM of 5(6)-carboxyfluorescein, suspended in PBS + Mg2+ 5 mM. DOPC and 5(6)-carboxyfluorescein molecules are shown on the figure 3. The dynamic light scattering measurements of these samples allowed for the determination of their size distribution profile, before and after plasma treatment, as shown on figure 4.
Figure 3. Chemical structures of (a) 1,2-dioleoyl-sn-glycero-3-phosphocholine and (b) (5)6-carboxyfluorescein.
Download figure:
Standard image High-resolution imageFigure 4. Size distribution profile of DOPC liposomes, encapsulating (5)6-carboxyfluorescein 50 mM, in PBS + Mg2+ 5 mM after different 120 s · ml−1 plasma treatments or after addition of 10% (v/v) ethanol.
Download figure:
Standard image High-resolution imageOne wide peak centred on 1000 nm, appeared after He + 0.50% N2 120 s · ml−1 plasma treatment (figure 4, blue line), while the initial peak centred on 100 nm decreased. The liposomes coalescence phenomenon observed under He + 0.50% N2 plasma treatment would explain the peak centred on 1000 nm and the decrease of the one centred on 100 nm (liposomes before treatment). The similar profile of the peak centred at 100 nm for the EtOH chemical treatment (10% addition (volume/volume) to the liposome suspension) and the one of He + 0.50% N2 120 s · ml−1 plasma treatment indicates that liposomes leaked a significant part of their 5(6)-carboxyfluorescein molecules. Indeed, the addition of ethanol is known to increase the permeability of liposomes to hydrophilic molecules and in our case it favours the disruption of the SUV and the delivery of the fluorescent molecules [17, 18].
He 120 s · ml−1 plasma treatments (figure 4, black line) result in a gentler decrease of the peak centred on 100 nm indicating that the major part of the SUVs do not break and do not leak. The peak centred on 500 nm is thought to be mainly due to of the agglomeration of several SUVs because no significant leak of the fluorescent molecules is detected (i.e. peak at 100 nm similar to non-treated reference one). 100 nm DOPC liposomes form 500 nm liposomal clusters through weak interactions, without rupture of liposome and leakage of the fluorescent dye. He + 0.50% O2 120 s · ml−1 plasma treatment (figure 4, red line) did not change significantly the size distribution profile of the DOPC liposomes. There was also no structural degradation of liposomes generated with these milder plasma treatments.
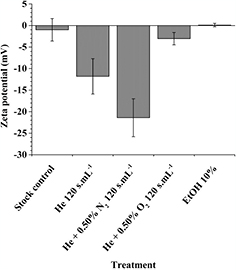
The zeta potential of DOPC liposomes was also determined from their electrophoretic mobility (figure 5). While untreated DOPC liposomes in PBS showed an almost neutral zeta potential (−1.0 ± 2.6 mV), zeta potentials after He 120 s · ml−1 and He + 0.50% N2 120 s · ml−1 plasma treatments both decreased to more negative values (respectively −11.8 ± 4.1 mV and −21.4 ± 4.4 mV). The more negative zeta potential value was obtained after the He + 0.50% N2 120 s · ml−1 plasma treatment indicated that the surface of the treated liposomes is more negatively charged due to a larger number of chemical modifications generated on the phospholipids.
Figure 5. Zeta potential of DOPC liposomes, encapsulating (5)6-carboxyfluorescein 50 mM, in PBS + Mg2+ 5 mM after 120 s · ml−1 plasma treatments and after addition of 10% (v/v) ethanol
Download figure:
Standard image High-resolution imageThe treatment of DOPC liposomes by He + 0.50% O2 120 s · ml−1 plasma and ethanol 10% did not modify significantly the zeta potential (−3.0 ± 1.4 mV). It means that no significant chemical modifications are generated on the phospholipids for these two treatments.
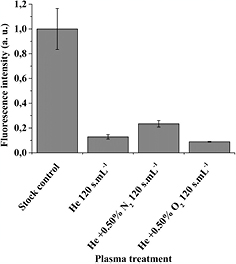
The fluorescence intensity of 5(6)-carboxyfluorescein, encapsulated at a concentration of 50 mM in the DOPC liposomes, was measured after 120 s · ml−1 plasma treatments and normalized against untreated samples. It has to be noted that the fluorescence intensity of non-treated samples (i.e. stock control) is already low due to the self-quenching phenomenon of the fluorescent molecules in liposomes.
All three 120 s · ml−1 plasma treatments unexpectedly led to a significant decrease of the fluorescence of carboxyfluoresce in (figure 6) down to very low values (close to background). The plasma treatments generate a degradation of the fluorescent dye whatever the gas mixture used. This fluorescent probe cannot resist to our plasma conditions even when they are encapsulated in liposomes. Indeed, it has already been shown that HOCl or other reactive species can react with fluorescein or similar dyes and lead to a loss of their fluorescence properties [19–22]. The use of this fluorescent probe is not a good approach in our case to detect the dye release from liposomes due to the novel cocktail of species generated by plasma. However, it shows that reactive species can permeate through the liposomes to react with the molecular probe inside liposomes, which is interesting information for drug delivery or disinfection applications.
Figure 6. Normalized fluorescence intensity of (5)6-carboxyfluorescein 50 mM, encapsulated in DOPC liposomes suspended in PBS + Mg2+ 5 mM after 120 s · ml−1 plasma treatments (n = 3).
Download figure:
Standard image High-resolution imageFigure 7 shows the fluorescence intensity, normalized against untreated samples, of a (5)6-carboxyfluorescein 200 µM solution (not encapsulated in liposomes) in PBS + Mg2+ 5 mM after 0–120 s · ml−1 He, He + 0.50% N2 and He + 0.50% O2 plasma treatments. The fluorescence decrease of non-encapsulated 5(6)-carboxyfluorescein due to the fluorescent molecule degradation is observed for all the plasma treatments even if it is less pronounced for the oxygen-containing one.
Figure 7. Normalized fluorescence intensity of (5)6-carboxyfluorescein 200 µM in PBS + Mg2+ 5 mM after 120 s · ml−1 plasma treatments showing the degradation of dye (i.e. bleaching) by plasma reactive species.
Download figure:
Standard image High-resolution imageIn order to identify which kind of plasma-generated RONS are involved in the degradation of both liposomes and fluorescent dye, we have investigated the degradation of liposomes after 35 min exposure to HOCl (5–500 µM), NH2Cl (5–500 µM), H2O2 (500–5000 µM),  (500 µM),
(500 µM),  (500 µM), NO2Cl (500 µM) and ONOO− (500 µM) as shown on figure 8. These concentration ranges correspond to the quantities generated by the plasma treatments.
(500 µM), NO2Cl (500 µM) and ONOO− (500 µM) as shown on figure 8. These concentration ranges correspond to the quantities generated by the plasma treatments.
Figure 8. Normalized fluorescence intensity of (5)6-carboxyfluorescein 50 mM, encapsulated in DOPC liposomes suspended in PBS + Mg2+ 5 mM after 35 min chemical treatments (n = 2).
Download figure:
Standard image High-resolution imageFrom all those species, only HOCl was able to induce an effect on 5(6)-carboxyfluorescein-loaded DOPC liposomes: the fluorescence intensity decreased to 31 (HOCl 5 µM) and 6% (HOCl 50 and 500 µM) of the original value. Although NH2Cl is potent enough to inactivate Escherichia coli bacteria (5.2log10 reduction after 35 min exposure to 45.2 µM) [13], its degradation power (i.e. fluorescence intensity decrease) on carboxyfluorescein seemed negligible up to 500 µM, which is a much higher amount that the one generated by our plasma treatment (at least 10 times lower). The same observation was made for  ,
,  , NO2Cl and ONOO− up to 500 µM and for H2O2 up to 5000 µM.
, NO2Cl and ONOO− up to 500 µM and for H2O2 up to 5000 µM.
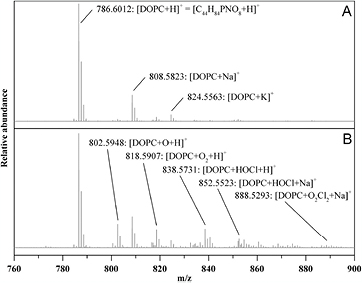
In order to determine more precisely the chemical modifications induced on the phospholipids by the plasma and chemical treatments, we used atmospheric pressure matrix-assisted laser desorption/ionization (AP-MALDI) mass spectrometry to identify the degradation products of DOPC. After a He + 0.50% N2 plasma treatment of 120 s · ml−1 (figure 9), the most potent bactericidal treatment with our set of parameters, the oxidation and the formation of chlorohydrin by electrophilic addition of HOCl on DOPC unsaturated C=C bonds (figure 10) was observed [23, 24]. The direct contribution of nitrating species (e.g. peroxynitrite) is negligible, as no NO nor NO2 functionalization was observed on the phospholipids. This last result shows that HOCl is not only involved in fluorescent dye bleaching but it is also involved in the liposome membrane chemical modification, in addition to other reactive oxygen species.
Figure 9. Positive ion AP-MALDI-Orbitrap mass spectra of DOPC liposomes in PBS + Mg2+ 5 mM before (A) and after He + 0.50% N2 120 s · ml−1 plasma treatment (B).
Download figure:
Standard image High-resolution imageFigure 10. Formation of chlorohydrin by electrophilic addition of HOCl on DOPC unsaturated C=C bonds. R1 and R2 stand for carbon chains of fatty acid.
Download figure:
Standard image High-resolution imageIn order to identify the plasma-generated RONS responsible for the O, OH and Cl functionalizations of DOPC lipid during He + 0.50% N2 plasma treatment, AP-MALDI mass spectra of DOPC liposome suspension in PBS + 5 mM Mg2+ were carried out after 35 min exposure to HOCl (5–500 µM), H2O2 (500–5000 µM),  (500 µM),
(500 µM),  (500 µM),
(500 µM),  (500 µM) and HOCl/H2O2 mixture (500 µM) as previously done with fluorescence measurements. Figure 11 shows the positive ion AP-MALDI mass spectra of DOPC liposome suspension in PBS + 5 mM Mg2+ before and after exposure to HOCl. The degradation products detected match closely those observed after He + 0.50% N2 120 s · ml−1 plasma treatments: DOPC phospholipids mostly underwent one to two electrophilic additions of HOCl molecules, when it was in contact with HOCl solutions.
(500 µM) and HOCl/H2O2 mixture (500 µM) as previously done with fluorescence measurements. Figure 11 shows the positive ion AP-MALDI mass spectra of DOPC liposome suspension in PBS + 5 mM Mg2+ before and after exposure to HOCl. The degradation products detected match closely those observed after He + 0.50% N2 120 s · ml−1 plasma treatments: DOPC phospholipids mostly underwent one to two electrophilic additions of HOCl molecules, when it was in contact with HOCl solutions.
Figure 11. Positive ion AP-MALDI-Orbitrap mass spectra of DOPC liposomes in PBS + Mg2+ 5 mM before (A) and after HOCl wet chemistry treatment (B).
Download figure:
Standard image High-resolution imagePositive ion AP-MALDI-Orbitrap mass spectra of DOPC liposome suspension in PBS + 5 mM Mg2+ after exposure to H2O2 (500–5000 µM),  (500 µM),
(500 µM),  (500 µM),
(500 µM),  (500 µM) and HOCl/H2O2 mixture (500 µM) did not show any degradation of DOPC phospholipids. In the case of exposure to a HOCl/H2O2 mixture (500 µM), oxidation products due to the addition of 3–6 oxygen atoms to DOPC phospholipids were detected. These oxidation products were not found after He + 0.50% N2 120 s · ml−1 plasma treatments. In conclusion, AP-MALDI-Orbitrap measurements show that HOCl-containing solutions give the closest results to the He + 0.50% N2 plasma treated ones in term of chemical modification on the DOPC structure.
(500 µM) and HOCl/H2O2 mixture (500 µM) did not show any degradation of DOPC phospholipids. In the case of exposure to a HOCl/H2O2 mixture (500 µM), oxidation products due to the addition of 3–6 oxygen atoms to DOPC phospholipids were detected. These oxidation products were not found after He + 0.50% N2 120 s · ml−1 plasma treatments. In conclusion, AP-MALDI-Orbitrap measurements show that HOCl-containing solutions give the closest results to the He + 0.50% N2 plasma treated ones in term of chemical modification on the DOPC structure.
4. Discussion
In our conditions where a nanosecond pulsed discharge, a solution buffered to neutral pH and an atmosphere of pure N2 are used, energy/density of electrons and reactive species generated is high, we have observed original cocktails of reactive species formed in solution (table 1). HOCl, NH2Cl and  are generated in higher quantities compared with more conventional discharges, except for He + O2 plasma treatments. In the specific case of He + 0.50% N2 plasma treatments,
are generated in higher quantities compared with more conventional discharges, except for He + O2 plasma treatments. In the specific case of He + 0.50% N2 plasma treatments,  are also generated in significant quantity leading to the formation of NH2Cl through its reaction with HOCl, which is favoured at a neutral pH. Though He plasma treatment generated NH2Cl in quantities similar to He + 0.50% N2, its precursor
are also generated in significant quantity leading to the formation of NH2Cl through its reaction with HOCl, which is favoured at a neutral pH. Though He plasma treatment generated NH2Cl in quantities similar to He + 0.50% N2, its precursor  was not detected. This suggests that, during He plasma treatment,
was not detected. This suggests that, during He plasma treatment,  is generated in relatively low amounts compared to HOCl and reacts quantitatively with HOCl to yield NH2Cl. The potentially remaining
is generated in relatively low amounts compared to HOCl and reacts quantitatively with HOCl to yield NH2Cl. The potentially remaining  would therefore be kept below the 3 µM detection threshold. These novel cocktails of the reactive species generated in pH buffered saline solutions are generating novel degradation processes both on bacteria [13], liposomes and fluorescent probes as discussed below.
would therefore be kept below the 3 µM detection threshold. These novel cocktails of the reactive species generated in pH buffered saline solutions are generating novel degradation processes both on bacteria [13], liposomes and fluorescent probes as discussed below.
4.1. Impact of CAP treatments on the structure and zeta potential of liposomes
The amount of chemical modifications on the phospholipids, which is induced by the reactive species generated by plasma, is proportional to the density of negative charges present at the surface of liposomes. Negative charges are generated on the liposomes mainly due to the nature of chemical groups added on the unsaturations of the phospholipid tail i.e. OH and Cl (figures 9 and 10).  anions generated in large amounts in solution by plasma treatment (table 1) may also be trapped in the liposomal membrane and participate to the decrease of zeta potential. The higher amount of chemical modifications on phospholipids induced by He + 0.50% N2 plasma treatments favour the fusion of liposomes to form larger liposomes, probably due to a higher fluidity and reactivity of the plasma modified liposomal membranes. The lower degradation of liposomes after the He plasma treatments due to the lower amount of reactive species generated in solution, compared with the He + 0.50% N2 plasma (table 1), is leading to the aggregation of the liposomes. It is thought that the fluidity/reactivity of liposomes is not increased enough in this case to lead to a fusion process. These results may be correlated with our previous results on bacterial inactivation by plasma, where He plasma treatments have almost no effect but He + 0.50% N2 ones show a fast and intense bacterial inactivation [13]. In the case of He + 0.50% O2 plasma treatments, no significant modification of the liposomes (i.e. phospholipids) is observed. This results also correlate well with our previous bacterial inactivation results [13]. In summary, critical amounts of reactive species are necessary to lead to the aggregation and then the fusion of liposomes, which are reached only by the He plasma treatment in the first case and by the He + 0.50% N2 in the second case. The generation of high quantities of
anions generated in large amounts in solution by plasma treatment (table 1) may also be trapped in the liposomal membrane and participate to the decrease of zeta potential. The higher amount of chemical modifications on phospholipids induced by He + 0.50% N2 plasma treatments favour the fusion of liposomes to form larger liposomes, probably due to a higher fluidity and reactivity of the plasma modified liposomal membranes. The lower degradation of liposomes after the He plasma treatments due to the lower amount of reactive species generated in solution, compared with the He + 0.50% N2 plasma (table 1), is leading to the aggregation of the liposomes. It is thought that the fluidity/reactivity of liposomes is not increased enough in this case to lead to a fusion process. These results may be correlated with our previous results on bacterial inactivation by plasma, where He plasma treatments have almost no effect but He + 0.50% N2 ones show a fast and intense bacterial inactivation [13]. In the case of He + 0.50% O2 plasma treatments, no significant modification of the liposomes (i.e. phospholipids) is observed. This results also correlate well with our previous bacterial inactivation results [13]. In summary, critical amounts of reactive species are necessary to lead to the aggregation and then the fusion of liposomes, which are reached only by the He plasma treatment in the first case and by the He + 0.50% N2 in the second case. The generation of high quantities of  (>6 mM) and the presence of HOCl (>18 µM),
(>6 mM) and the presence of HOCl (>18 µM),  and NH2Cl species are responsible for the coalescence of liposomes. The generation of intermediate quantities of
and NH2Cl species are responsible for the coalescence of liposomes. The generation of intermediate quantities of  (>3 mM) and the presence of HOCl (10 µM) species are responsible for the aggregation of liposomes. The generation of lower quantities of
(>3 mM) and the presence of HOCl (10 µM) species are responsible for the aggregation of liposomes. The generation of lower quantities of  (<2 mM) and HOCl (7 µM) does not lead to structural modifications of liposomes.
(<2 mM) and HOCl (7 µM) does not lead to structural modifications of liposomes.
4.2. Molecular mechanisms involved in the degradation processes of liposomes and fluorescent molecules
Hypochlorite (like peroxynitrite) is a typical reactive species produced in the organism under pathological conditions or during plasma treatment of water solutions, and having a large range of biological effects such as erythrocyte oxidative damage [25]. HOCl can penetrate into cells and be transformed through a non-enzymatic reaction into other reactive oxygen species (ROS) (e.g. OH and 1O2) [26] to generate local oxidation processes. The degradation effects of HOCl are also explained by the formation of chloramines due to their reactions with the amino group of lipids or proteins [27, 28], and also by the formation of chlorohydrins (figure 10) [23, 24]. Cell exposure to HOCl was shown to lead to structural rearrangement of the membrane, cell swelling and pore formation [27, 28].
When using plasma touching saline water solutions, HOCl can be generated by the reaction between OH radicals generated by plasma reactive species in contact with water and Cl− ions present in our solution at a concentration of 154 mM. In our conditions specifically, it is shown that HOCl seems to play a major role in fluorescent dye, and liposome degradations even at very low concentration of 20 µM. For the liposome degradation, the formation of chlorohydrin by electrophilic addition of HOCl on DOPC unsaturated C=C bonds is one reaction pathway as shown from our high resolution mass spectrometry results (figures 9 and 11) [23, 24]. The generation of ROS in the vicinity of phospholipids from HOCl degradation [26] can also lead to oxidation processes mainly on the C=C unsaturated bond of the phospholipid tail as shown on figure 9 with peaks at 802.6 (O addition), 818.6 (2O additions) and 888.5 (2O + 2Cl2 additions). The formation of chloramine from the reaction of HOCl with amine of the phospholipid head was not observed probably due to the low reactivity of the tertiary amine for DOPC. In regard to the (5)6-carboxyfluorescein, the molecule is degraded whatever the plasma treatment and whatever it isencapsulated or not. HOCl seem to be the main reactive species involved in the degradation of the fluorescent probe (figure 8), which explains why He + 0.50% O2 plasma treatments induces less (5)6-carboxyfluorescein degradation, and the other plasma treatments higher and similar rates of degradation (figure 7) because the HOCl concentrations generated are similar (table 1). The bleaching of fluorescein by oxygen molecules and ROS has already been reported under light irradiation, [29] but the conditions and degradation processes are different to the ones studied in this work. In particular, no significant amount of photons is generated at the vicinity of dye molecules in our case. The (5)6-carboxyfluorescein plasma degradation processes observed here, probably by HOCl species, have been better documented in [30]. The (5)6-carboxyfluorescein degradation by HOCl, can be either due to an electrophilic addition of HOCl on an aromatic C–C bond or an oxidation of C–C aromatic bonds, both processes lead to C–C aromatic bond breaking. Future mass spectrometry measurements will allow us to determine the degradation process in our conditions.
 are known to generate oxidation processes through the formation of reactive oxygen intermediates, which can attack biomolecules present in their vicinity and can lead to lipid peroxidation, protein modification, DNA damage, etc. However, it is shown in our case that nitrite ions are not generating as much damage as expected (e.g. case of He plasma treatments). This last observation can be partly explained by our neutral pH conditions, which inhibit the formation of ONO2H and NO2 intermediate species from
are known to generate oxidation processes through the formation of reactive oxygen intermediates, which can attack biomolecules present in their vicinity and can lead to lipid peroxidation, protein modification, DNA damage, etc. However, it is shown in our case that nitrite ions are not generating as much damage as expected (e.g. case of He plasma treatments). This last observation can be partly explained by our neutral pH conditions, which inhibit the formation of ONO2H and NO2 intermediate species from  species and therefore inhibit the addition of NO2 on the C in α position of the C=C unsaturated bond in the phospholipid tail as explained by Mendiara et al [31]. The lack of molecular fragments with an NO2 addition confirmed this hypothesis (figure 9). However, a part of oxidation processes observed on figure 9 (which is already mentioned as a possible HOCl effect above) can be generated indirectly by nitrite ions, which explains why He + 0.50% O2 plasma treatments are generating higher degradation effects on liposomes. These oxidation processes can also be involved in the (5)6-carboxyfluorescein degradation.
species and therefore inhibit the addition of NO2 on the C in α position of the C=C unsaturated bond in the phospholipid tail as explained by Mendiara et al [31]. The lack of molecular fragments with an NO2 addition confirmed this hypothesis (figure 9). However, a part of oxidation processes observed on figure 9 (which is already mentioned as a possible HOCl effect above) can be generated indirectly by nitrite ions, which explains why He + 0.50% O2 plasma treatments are generating higher degradation effects on liposomes. These oxidation processes can also be involved in the (5)6-carboxyfluorescein degradation.
Finally, effects of NH2Cl and  species, only produced by He + 0.50% N2 plasma treatments, on liposomes and (5)6-carboxyfluorescein degradation are more difficult to discuss because they are masked by the effect of nitrite ions generated in much higher quantities for He + 0.50% N2 plasma treatments. There is also no pertinent work describing their possible role in the degradation of liposomes and dye molecules similar to (5)6-carboxyfluorescein. There will be more experiments carried out to determine their role in the degradation processes.
species, only produced by He + 0.50% N2 plasma treatments, on liposomes and (5)6-carboxyfluorescein degradation are more difficult to discuss because they are masked by the effect of nitrite ions generated in much higher quantities for He + 0.50% N2 plasma treatments. There is also no pertinent work describing their possible role in the degradation of liposomes and dye molecules similar to (5)6-carboxyfluorescein. There will be more experiments carried out to determine their role in the degradation processes.
5. Conclusion
In this work, where novel cocktails of reactive species are generated by CAP in pH buffered aqueous solution containing liposomes loaded with a fluorescent dye, it has been shown that several and novel plasma degradation mechanisms of DOPC small unilamellar liposomes and carboxyfluoresceine dye are generated in suspensions touched by the plasma. HOCl generated in the micromolar range combined with nitrite ions generated in the mM range is mainly responsible for the degradation of the carboxyfluoresceine dye molecules, which make use of this fluorescent probe that is not so pertinent in these conditions. It is probable that no fluorescent dye can resist these plasma treatments, and another method other than fluorescence spectroscopy is needed to follow the permeation of the molecular probe through the liposomal membrane. When a critical quantity of reactive species (i.e. HOCl, NH2Cl,  and other ROS generated locally from HOCl and
and other ROS generated locally from HOCl and  ) generated by the plasma in the suspension is reached, the aggregation of liposomes and the increase of negative charges on liposomes is observed (case of He plasma treatments). When another higher critical quantity of reactive species (i.e. HOCl,
) generated by the plasma in the suspension is reached, the aggregation of liposomes and the increase of negative charges on liposomes is observed (case of He plasma treatments). When another higher critical quantity of reactive species (i.e. HOCl,  ,
,  , NH2Cl and ROS) generated by plasma in the suspension is reached, the fusion of liposomes, an higher increase of negative charges of liposomes and the leakage of fluorescent dye from liposomes is observed (case of He + 0.50% N2 plasma treatments). Reactive species are shown to react with the liposomal membranes through HOCl electrophilic addition and oxidation processes mainly on the unsaturated C=C bond of the phospholipid tail. These chemical modifications make liposomes more permeable leading to the permeation of encapsulated dye through the membrane, and they are also more reactive and more flexible leading to a point where liposome fusion is observed. The reactive species (e.g. HOCl,
, NH2Cl and ROS) generated by plasma in the suspension is reached, the fusion of liposomes, an higher increase of negative charges of liposomes and the leakage of fluorescent dye from liposomes is observed (case of He + 0.50% N2 plasma treatments). Reactive species are shown to react with the liposomal membranes through HOCl electrophilic addition and oxidation processes mainly on the unsaturated C=C bond of the phospholipid tail. These chemical modifications make liposomes more permeable leading to the permeation of encapsulated dye through the membrane, and they are also more reactive and more flexible leading to a point where liposome fusion is observed. The reactive species (e.g. HOCl,  ) generated by plasma are also able to cross the liposomal membrane to react with the fluorescent molecules inside the liposomes without the failure of the liposomes. This work brings novel information about the degradation processes of encapsulated fluorescent molecules and model membranes that can partially explain previous results on bacterial disinfection in similar conditions [13]. These results will be also very useful for future studies dealing with the selectivity of plasma treatments for therapeutic applications and the association of plasma treatments and nanomedicine for novel therapeutic and diagnostic applications.
) generated by plasma are also able to cross the liposomal membrane to react with the fluorescent molecules inside the liposomes without the failure of the liposomes. This work brings novel information about the degradation processes of encapsulated fluorescent molecules and model membranes that can partially explain previous results on bacterial disinfection in similar conditions [13]. These results will be also very useful for future studies dealing with the selectivity of plasma treatments for therapeutic applications and the association of plasma treatments and nanomedicine for novel therapeutic and diagnostic applications.
Acknowledgments
The authors would like to acknowledge, from LIST, M Gerard and O Bouton for technical support, M Hammer from INP Greifswald for liposome preparation and members of COST action TD1208 for supporting discussions. Pictures from figure 1 were modified under Creative Commons Attribution license 2.0 and can be found at http://smart.servier.fr/smart/banque-dimages-powerpoint and http://wikiwand.com/fr/Liposome. This work was partially supported by the Fonds National de la Recherche du Luxembourg and by the European Laboratory LEA-LIPES.