Abstract
We have directly measured the thickness distribution of the tunnel barriers in state-of-the-art Al/AlOx/Al tunnel junctions. From the distribution we can conclude that less than 10% of the junction area dominates the electron tunnelling. The barriers have been studied by transmission electron microscopy, specifically using atomic resolution annular dark field (ADF) scanning transmission electron microscopy (STEM) imaging. The direct observation of the local barrier thickness shows a Gaussian distribution of the barrier thickness variation along the junction, from ~1 to ~2 nm. We have investigated how the thickness distribution varies with oxygen pressure ( ) and oxidation time (
) and oxidation time ( ) and we find, in agreement with resistance measurements, that an increased
) and we find, in agreement with resistance measurements, that an increased  has a larger impact on barrier thickness and its uniformity compared to an increased
has a larger impact on barrier thickness and its uniformity compared to an increased  .
.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Aluminium oxide (AlOx) layers of nanometre thickness are widely used as tunnel barriers in tunnel junctions that have a wide range of applications in devices such as radiation detectors, single-electron transistors, and superconducting qubits [1–6]. Magnetic tunnel junctions also often include ultrathin AlOx layers as the tunnel barrier between two magnetic layers [7]. The quality and structural characteristics of these oxide barriers may directly determine the tunnelling characteristics of the charge carriers across the barrier and consequently the performance of the junctions. The most relevant physical parameter for characterizing the structure of the oxide barrier is the barrier thickness, since the tunnelling current decreases exponentially with increasing barrier thickness. The thinnest region in the barrier will give rise to the highest tunnel current and become the preferential tunnelling channel for the charge carriers. Consequently, the even small variations of the barrier thickness will result in inhomogeneity of the tunnel current across the barrier, and the conductance per unit area becomes much smaller than if the tunnel barrier had been equally thick everywhere. In several experiments there is indirect evidence that only a small fraction of the junction area is active [8–10]. A tunnel barrier with varying thickness in a superconducting tunnel junction changes the ratio between multiparticle currents, based on Andreev processes, and the single-particle current. This leads to excess current in the subgap region where single-particle tunnelling is suppressed. Excess subgap current has been found in various superconducting tunnel junctions and may become a limitation for the future applications of electron pumps [11, 12]. The origin of excess subgap current has been suggested to be the variation of the local junction transparency resulting from a distribution of barrier thickness, or the existence of 'pinholes' in the junctions [13–16]. However, more recent studies based on low-temperature measurements of current–voltage characteristics of superconducting tunnel junctions have ruled out 'pinholes' as an explanation for the excess current, but confirmed the role of the uneven distribution of the barrier thickness [10].
If the local transparency fluctuates in time, this may also lead to resistance noise in the tunnel junction and to critical-current noise in Josephson junctions. The latter may limit the coherence times in superconducting qubits [17, 18].
Transport measurements and theoretical modelling have provided strong indications of the influence of barrier thickness on the performance of the tunnel junctions, and it is therefore of great importance to directly measure the thickness distribution of the oxide barriers in the junctions in order to evaluate the effects of detailed, local structure on the transport properties of the junctions.
Here we report on a cross-section transmission electron microscopy (TEM) study on the microstructure of the AlOx barriers in Al/AlOx/Al Josephson junctions with the main focus on the direct measurement of the barrier thickness distribution. While techniques such as conductive atomic force microscopy (CAFM), scanning tunnelling microscopy (STM), and x-ray photoelectron spectroscopy (XPS) are usually used to measure the barrier thickness, the information they provide is usually indirect and/or has relatively low spatial resolution [19–22]. CAFM and STM are used to measure the tunnel current locally (~nm2 scale) and then derive the tunnel barrier parameters (barrier thickness, barrier height, etc) by fitting the current–voltage curve with some physical model. For XPS measurements, the results are usually averaged over a sample surface area with the size in ~µm2 scale. In the present study, with atomic resolution scanning TEM (STEM) imaging, we directly show a local thickness variation of the barrier thickness along the junction and also that partial oxygen pressure ( ) and oxidation time (
) and oxidation time ( ) during fabrication affect the barrier thickness distribution. The discussion also extends to the tunnel current distribution as a result of the variation in the barrier thickness. The results provide important information for the understanding of the transport properties of superconducting devices such as excess subgap conductance in tunnel junctions and decoherence in qubits. Previously we have reported a study on the detailed atomic structure at the interface between the Al/AlOx/Al Josephson junctions and the substrate by TEM [23]. That study and the results presented here could both demonstrate the importance of understanding microscopic structure of the junction in order to explain some of the critical phenomena observed in Josephson junction based superconducting devices. The work presented here could also shed light on the pathways whereby further improved tunnel barriers can be produced with respect to the homogeneity in barrier thickness.
) during fabrication affect the barrier thickness distribution. The discussion also extends to the tunnel current distribution as a result of the variation in the barrier thickness. The results provide important information for the understanding of the transport properties of superconducting devices such as excess subgap conductance in tunnel junctions and decoherence in qubits. Previously we have reported a study on the detailed atomic structure at the interface between the Al/AlOx/Al Josephson junctions and the substrate by TEM [23]. That study and the results presented here could both demonstrate the importance of understanding microscopic structure of the junction in order to explain some of the critical phenomena observed in Josephson junction based superconducting devices. The work presented here could also shed light on the pathways whereby further improved tunnel barriers can be produced with respect to the homogeneity in barrier thickness.
2. Experimental details
The samples used in this study were grown on Si/SiO2 substrates in high vacuum by thermal evaporation. The base pressure of the evaporation system is less than 5 × 10−7 mbar. The SiO2 layer is 400 nm thick. A bottom Al film with nominal thickness of 15 nm was deposited with a deposition rate ranging from 9 to 12 Å s−1. Ultra high purity source pellets of Al-6N (purity = 99.9999%) were placed in tungsten boat for Al evaporation. The source–substrate distance is about 20 cm. The sample stage was not cooled or heated up intentionally during the evaporation and oxidation of the Al film. The Al film was thereafter exposed to high-purity (99.99%) O2 with fixed pressure and time. Subsequently the top Al layer with a nominal thickness of 60 nm was deposited with the same deposition rate as that for the bottom Al film. The tunnelling characteristics of the junctions used in this study are representative of large numbers of the junctions analysed in another study [10]. Three sets of unpatterned samples (large area trilayer junctions) were used in this study. The area size of the trilayer junctions is ~7 mm × 7 mm. They were made with the same film deposition parameters but with different barrier oxidation parameters, namely, with  /
/ being 0.1 mbar/3 min (sample 1), 0.1 mbar/30 min (sample 2), and 1 mbar/3 min (sample 3), as shown in table 1.
being 0.1 mbar/3 min (sample 1), 0.1 mbar/30 min (sample 2), and 1 mbar/3 min (sample 3), as shown in table 1.
Table 1. Oxidation parameters (partial oxygen pressure ( ) and oxidation time (
) and oxidation time ( )) and barrier thickness measurement results (average barrier thickness (〈l〉) and standard deviation (
)) and barrier thickness measurement results (average barrier thickness (〈l〉) and standard deviation ( ) for the three samples analysed in this study.
) for the three samples analysed in this study.
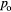 (mbar) (mbar) |
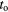 (min) (min) |
〈l〉 (nm) | 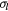 (nm) (nm) |
|
|---|---|---|---|---|
| Sample 1 | 0.1 | 3 | 1.66 | 0.35 |
| Sample 2 | 0.1 | 30 | 1.88 | 0.32 |
| Sample 3 | 1 | 3 | 1.73 | 0.37 |
Cross-section TEM specimens were prepared by grinding and polishing the specimen down to ~20 μm, followed by Ar ion milling. The specimens were kept at about −80 °C during milling to minimize the damage from the ion beam. An FEI Titan 80–300 TEM/scanning TEM (STEM) was used for high resolution imaging. Annular dark field (ADF) STEM images were acquired with a 19.7 mrad beam convergence angle and 54–270 mrad detector collection angle. The spatial resolution of the microscope in ADF STEM mode is determined to be ~1 Å.
3. Results and discussion
Figures 1(a) and (b) show typical cross-section STEM ADF images of the junction (sample 1). Different layers (SiO2 substrate, bottom Al electrode, AlOx barrier, and top Al electrode) in the junction are indicated in the images and the film growth direction is from bottom to top. In figure 1(a) we can clearly see the polycrystalline and columnar nature of both top and bottom Al electrodes. Because of the channelling effect, there is a higher intensity in the ADF image when the electron beam travels along the zone axis of the Al grains compared to when the incident beam direction is different from the zone axis. Thus, the varying intensity in the Al layers in the images is due to Al grains with different orientations. The grain size of the Al in the junction can therefore be measured from the images. The Al grain size measured along the direction perpendicular to the film growth direction varies from 20 nm to 50 nm for the bottom Al electrode, and from 80 to 200 nm for the top Al electrode. There is a dependence of the grain size of the top layer Al on the oxidation time. Along the film growth direction, each of the Al grains in both the bottom and top Al layers extend throughout the film thickness as a consequence of the columnar growth. The AlOx barrier layer follows the top surface of the bottom Al layer, where the surface roughness differs from area to area. No voids or pinholes were observed in any of the samples we have investigated. Figure 1(b) shows a high magnification STEM image taken from the area indicated by the dashed frame in figure 1(a). The atomic columns and lattice fringes of the Al grains and the Al/AlOx interface structure can be clearly seen in the image.
Figure 1. ADF STEM images of an Al/AlOx/Al tunnel junction. (a) An overview of the cross-section of the junction showing the Al electrodes, AlOx barrier and SiO2 substrate layer. (b) An ADF image acquired from the dashed area in (a).
Download figure:
Standard image High-resolution imageBecause of the incoherent nature of STEM ADF imaging, Fresnel fringes and other interference phenomena will not appear in the ADF image and degrade the accuracy of the barrier thickness measurement [24]. STEM ADF images were thus used for barrier thickness measurements.
Figure 2(a) shows a high resolution STEM ADF image of the junction from sample 3. The atomic columns and lattice fringes from Al grains on both sides of the amorphous barrier are clearly visible in figure 2(a). In such an ADF STEM image, brighter contrast normally corresponds to atomic columns and lattice fringes in the material because in STEM ADF mode we are collecting electrons that are scattered by the atomic columns to a certain angle. The space between the atomic columns contributes much less to the scattering of incoming electrons so in the image it appears dark. From figure 2(a), we can also examine the detailed interface structure at the Al/AlOx interface, e.g. there are atomic steps of Al at the interface, which can contribute to the variation of barrier thickness at the atomic scale. Intensity profiles at three positions (A, B, and C) in the image were measured to demonstrate how the barrier thickness measurement is done and how the barrier thickness varies. At each position, the intensity profile across the barrier was measured and integrated along the direction parallel to the Al/AlOx interface. Each measurement was done using a window with the size of ~4 nm × 0.5 nm as shown in figure 2(a). The corresponding intensity profiles are shown in figure 2(b). The intensity profiles from Al show sharp peaks corresponding to the positions of Al atomic planes. The distance between two neighbouring peaks is the atomic plane distance, which is indexed and marked in figure 2(a). In contrast, the intensity profile from the AlOx barrier has a relatively random intensity distribution. Thus, we can determine the interface between the crystalline Al layers and the amorphous AlOx barrier from the intensity profiles and consequently measure the barrier thicknesses. The pixel size of figure 2(a) is 0.019 nm. We estimate that the uncertainty in the measurement is around 0.04 nm. As shown in figure 2(b), the barrier thickness (l) was measured to be 1.78 (±0.04), 1.51 (±0.04), and 2.20 (±0.04) nm at position A, B, and C, respectively.
Figure 2. (a) A high resolution ADF STEM image of the junction from sample 3. The Al (1 1 1) atomic plane and the plane distance are indicated. Windows A, B and C with size of ~4 nm × 0.5 nm in the image show the areas used to measure the intensity profile of the image. Intensity profiles are measured across the barrier and integrated along the Al/AlOx interface. (b) Image intensity profiles acquired from area A, B and C that are marked in (a). Barrier thicknesses measured from the intensity profiles are also shown.
Download figure:
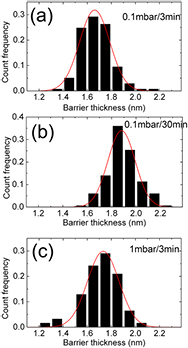
Standard image High-resolution imageWe measured the thickness of the barrier at over 300 different positions for each sample in order to measure the barrier thickness distribution. For each sample, we used around 50 STEM images from approximately 20 different grains for the thickness measurement. In each high resolution STEM image we measured the barrier thicknesses at positions at a distance around 2 nm away from each other. The resulting barrier thickness distributions for the three samples are shown in figure 3. As shown in figure 3(a), for the barrier oxidized with  mbar and
mbar and  min (sample 1) the barrier thickness varies from 1.2 to 2.2 nm. More than 60% of the barrier thickness is in the range from 1.5 to 1.8 nm, and the mean barrier thickness is 1.66 nm. A comparison between the barriers in sample 1 and sample 3 shows that the mean barrier thickness increases from 1.66 nm to 1.73 nm when
min (sample 1) the barrier thickness varies from 1.2 to 2.2 nm. More than 60% of the barrier thickness is in the range from 1.5 to 1.8 nm, and the mean barrier thickness is 1.66 nm. A comparison between the barriers in sample 1 and sample 3 shows that the mean barrier thickness increases from 1.66 nm to 1.73 nm when  was increased by a factor of 10. There is a relatively larger change of mean barrier thickness when the
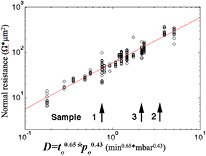
was increased by a factor of 10. There is a relatively larger change of mean barrier thickness when the  is increased from 3 min for sample 1 to 30 min for sample 2, even though the change is also rather small (from 1.66 nm to 1.88 nm). We can compare this with statistics on how the junction resistances depend on oxidation parameters for 266 samples made in the same way as the three samples presented here. We present this data in figure 4. Defining an effective oxidation dose
is increased from 3 min for sample 1 to 30 min for sample 2, even though the change is also rather small (from 1.66 nm to 1.88 nm). We can compare this with statistics on how the junction resistances depend on oxidation parameters for 266 samples made in the same way as the three samples presented here. We present this data in figure 4. Defining an effective oxidation dose  with a and b being variable parameters, we find that for a large number of samples, the conductance per unit area decreases linearly with D. The two exponents have been optimized to minimize the spread of the data for the linear fit, giving rise to a being 0.65 and b is 0.43, as shown in figure 4. The measured average thicknesses presented in table 1 agree with this and confirms that the oxidation time is more important than oxidation pressure, that is, a tenfold increase in
with a and b being variable parameters, we find that for a large number of samples, the conductance per unit area decreases linearly with D. The two exponents have been optimized to minimize the spread of the data for the linear fit, giving rise to a being 0.65 and b is 0.43, as shown in figure 4. The measured average thicknesses presented in table 1 agree with this and confirms that the oxidation time is more important than oxidation pressure, that is, a tenfold increase in  gives a thicker barrier than a tenfold increase of
gives a thicker barrier than a tenfold increase of  .
.
Figure 3. Barrier thickness distributions measured from ADF STEM images of three AlOx barriers made at different oxidation conditions: (a)  /
/ ~0.1 mbar/3 min (sample 1), (b)
~0.1 mbar/3 min (sample 1), (b)  /
/ ~0.1 mbar/30 min (sample 2), (c)
~0.1 mbar/30 min (sample 2), (c)  /
/ ~1 mbar/3 min (sample 3). Experimental thickness distributions are also fitted with Gaussian functions, shown as the red curves superimposed on the experimental data.
~1 mbar/3 min (sample 3). Experimental thickness distributions are also fitted with Gaussian functions, shown as the red curves superimposed on the experimental data.
Download figure:
Standard image High-resolution imageFigure 4. Dependence of normal resistance on oxidation parameters. The x-axis represents an effective oxygen dose defined as  (see main text for more details about D). Black circles show the experimental measurement results of normal resistance (Rn times junction area) of Al/AlOx/Al junctions prepared at different oxidation conditions. The red line shows the linear fit to the experimental data.
(see main text for more details about D). Black circles show the experimental measurement results of normal resistance (Rn times junction area) of Al/AlOx/Al junctions prepared at different oxidation conditions. The red line shows the linear fit to the experimental data.
Download figure:
Standard image High-resolution imageThe variation of the mean barrier thickness shown here is, however, different from the result of previous XPS measurements [22], in which the thickness of AlOx thin film increased more rapidly with  . Moreover, the inhomogeneity of the barrier thickness of the junction, ranging from ~1 to ~2 nm, was observed in all the samples we studied, as shown in figure 3. The thickness inhomogeneity has also been derived from CAFM and STM measurements [19–21]. In contrast, we have directly measured the barrier thickness with atomic resolution in TEM. The tunnelling probability of charge carriers across the barrier is an exponential function of the barrier thickness. It has been shown that a 0.2 nm decrease of barrier thickness could result in one order of magnitude increase in tunnelling current [21]. Thus, the thinnest region in the barrier may act as an active region or 'hot spot' for tunnelling. The distribution of the barrier thickness is also fitted with a Gaussian distribution for each sample. The fitting results are shown by the curves superimposed on the histograms in figure 3. The fitting gives the average thickness
. Moreover, the inhomogeneity of the barrier thickness of the junction, ranging from ~1 to ~2 nm, was observed in all the samples we studied, as shown in figure 3. The thickness inhomogeneity has also been derived from CAFM and STM measurements [19–21]. In contrast, we have directly measured the barrier thickness with atomic resolution in TEM. The tunnelling probability of charge carriers across the barrier is an exponential function of the barrier thickness. It has been shown that a 0.2 nm decrease of barrier thickness could result in one order of magnitude increase in tunnelling current [21]. Thus, the thinnest region in the barrier may act as an active region or 'hot spot' for tunnelling. The distribution of the barrier thickness is also fitted with a Gaussian distribution for each sample. The fitting results are shown by the curves superimposed on the histograms in figure 3. The fitting gives the average thickness  for each sample and the standard deviation
for each sample and the standard deviation  of the barrier thickness.
of the barrier thickness.  is 1.66 nm, 1.88 nm and 1.73 nm and
is 1.66 nm, 1.88 nm and 1.73 nm and  is 0.35 nm, 0.32 nm and 0.37 nm for sample 1, 2 and 3, respectively. A recent study of Cu/AlOx/Al junctions also shows a similar barrier thickness distribution [25].
is 0.35 nm, 0.32 nm and 0.37 nm for sample 1, 2 and 3, respectively. A recent study of Cu/AlOx/Al junctions also shows a similar barrier thickness distribution [25].
We note that though no pinhole-like microstructural defects were observed, the strong fluctuation of barrier thickness in the junction is likely to give rise to a variation in transmittance in the barrier. It, thus, could contribute to the excess subgap current in Al/AlOx/Al tunnel junctions via a multiple-particle tunnelling process in the thin area [13] e.g. Andreev transport [10].
Based on the barrier thickness distribution measurements, we further calculated the distribution of the tunnel current as a function of the barrier thickness. The specific conductance (conductance per unit area) of a rectangular potential barrier [26] of height  and thickness l can be expressed as
and thickness l can be expressed as
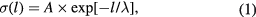
where  is the attenuation length in the barrier
is the attenuation length in the barrier

and  is expressed as
is expressed as
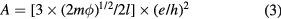
m is the mass of electron and  is chosen to be ~2 eV, which is a typical value for AlOx barriers [20, 21, 27]. This corresponds to
is chosen to be ~2 eV, which is a typical value for AlOx barriers [20, 21, 27]. This corresponds to  = 0.069 nm.
= 0.069 nm.
Thus the distribution function for the conductance in a tunnel junction is given by
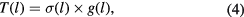
where  is the thickness distribution function obtained by fitting the experimental data to a Gaussian distribution as shown in figure 3. In order to investigate the proportion of the most active area in terms of electron tunnelling in the junctions, we define
is the thickness distribution function obtained by fitting the experimental data to a Gaussian distribution as shown in figure 3. In order to investigate the proportion of the most active area in terms of electron tunnelling in the junctions, we define
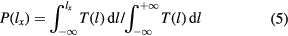
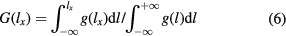
to calculate the proportion of the barrier area with the thickness smaller than  with respect to the whole barrier area in the junction.
with respect to the whole barrier area in the junction.
 as a function of
as a function of  for the three samples we investigated are calculated. We find that in sample 1,
for the three samples we investigated are calculated. We find that in sample 1,  is around 0.073 when
is around 0.073 when  . It means 90% of the tunnel current in the whole junction flows in about 7.3% of the junction area. Similarly, in sample 2, 90% of the total tunnel current is from 9.2% of the junction area. In sample 3, 90% of the total tunnel current comes from around 6.9% of the junction area. Under this criterion, based on our measurements and calculations shown in figure 3, less than 10% of the barrier area is active in each barrier. This finding is consistent with the assumptions made for theoretical modelling of superconductor–insulator–superconductor and normal metal–insulator–superconductor junctions [8, 10].
. It means 90% of the tunnel current in the whole junction flows in about 7.3% of the junction area. Similarly, in sample 2, 90% of the total tunnel current is from 9.2% of the junction area. In sample 3, 90% of the total tunnel current comes from around 6.9% of the junction area. Under this criterion, based on our measurements and calculations shown in figure 3, less than 10% of the barrier area is active in each barrier. This finding is consistent with the assumptions made for theoretical modelling of superconductor–insulator–superconductor and normal metal–insulator–superconductor junctions [8, 10].
The total junction conductance is given by the full integral, which for a Gaussian distribution becomes:  . We see that for a homogeneous distribution (
. We see that for a homogeneous distribution ( ) we recover the original exponential dependence, but for finite
) we recover the original exponential dependence, but for finite  we get an increased conductance.
we get an increased conductance.
There are several factors that may contribute to the variation of the thickness of the AlOx barrier oxidized directly on the Al bottom layer: (i) normally, the oxide barrier closely follows the morphology of the top surface of the bottom Al layer. However, the barrier tends to be thicker at some Al grain boundaries because of the grain boundary grooving [28]; (ii) the bottom Al layer is polycrystalline, so the Al grains have different crystallographic orientations along the film growth direction. Even on one single grain, the curvature of the top surface of the Al grain indicates that there might be local variation in the crystallographic orientation of Al. Al grains with different crystallographic orientations have different surface energy and also different atomic plane distance along the top surface and perpendicular to the surface. When oxidized, it will thus give rise to a variation in oxide thickness [22, 29]; (iii) atomic steps of Al at the Al/AlOx interfaces result in local change of the barrier thickness.
It is also worth noting that the tunnel barrier is not necessarily stoichiometric with a homogeneous structure. The structure and chemical composition of aluminium oxide may be different with different oxidation parameters [22] and even different from area to area within one oxide layer. AlOx regions with different atomic structure have different dielectric constants and also band gaps, so there can be a barrier height variation within the junction and between different junctions [26]. It is thus also important to examine the structure of the oxide in the barrier in order to fully understand the microstructure of the ultrathin AlOx barrier in tunnel junctions and its effect on the tunnelling properties.
4. Conclusions
In summary, the barrier thickness distribution of the AlOx barrier in state-of-the-art Al/AlOx/Al tunnel junctions has been investigated by transmission electron microscopy at atomic resolution. There is a small change of the mean barrier thickness for the barriers made with  /
/ of 0.1 mbar/3 min, 0.1 mbar/30 min, and 1 mbar/3 min. STEM imaging shows that the barrier thickness has a distribution, ranging from ~1 nm to ~2 nm, in every aluminium oxide barrier we have studied. The average barrier thickness confirms previous experimental results that oxidation time is a more important parameter than pressure during the oxidation. According to the barrier thickness distribution measurements and tunnel probability calculation, less than 10% of the total barrier area is active in the tunnelling process in all three junctions studied. The results show the strong inhomogeneity of tunnelling current resulting from the barrier thickness distribution.
of 0.1 mbar/3 min, 0.1 mbar/30 min, and 1 mbar/3 min. STEM imaging shows that the barrier thickness has a distribution, ranging from ~1 nm to ~2 nm, in every aluminium oxide barrier we have studied. The average barrier thickness confirms previous experimental results that oxidation time is a more important parameter than pressure during the oxidation. According to the barrier thickness distribution measurements and tunnel probability calculation, less than 10% of the total barrier area is active in the tunnelling process in all three junctions studied. The results show the strong inhomogeneity of tunnelling current resulting from the barrier thickness distribution.
Acknowledgments
We thank the Swedish Foundation for Strategic Research, the Swedish Research Council, the Knut and Alice Wallenberg Foundation for financial support.