ABSTRACT
HNCS and NCSH molecules, recently discovered in the interstellar medium, are likely formed via the dissociative recombination of H2NCS+ or HNCSH+ isomeric ions. Interstellar synthesis of the latter is discussed on theoretical grounds. The analysis of relevant potential energy surfaces suggests a key role for chemical processes in which CSH+ or HCS+ cations (most likely formed in  collisions) react with NH2 or NH3. The astrochemical kinetic database (kida.uva.2011), appended with 7 sulfur-bearing molecules and 48 corresponding reactions, has been applied to model the evolution of HNCS, NCSH, and their cationic precursors in a quiescent molecular cloud. Based on the model and on spectroscopic predictions, for an object like TMC-1, we expect the total intensity of H2NCS+ microwave lines to be comparable to that observed for HSCN. Theoretically derived molecular parameters, of interest for radio spectroscopy, are given for the most stable cations sharing the H2NCS+ stoichiometry.
collisions) react with NH2 or NH3. The astrochemical kinetic database (kida.uva.2011), appended with 7 sulfur-bearing molecules and 48 corresponding reactions, has been applied to model the evolution of HNCS, NCSH, and their cationic precursors in a quiescent molecular cloud. Based on the model and on spectroscopic predictions, for an object like TMC-1, we expect the total intensity of H2NCS+ microwave lines to be comparable to that observed for HSCN. Theoretically derived molecular parameters, of interest for radio spectroscopy, are given for the most stable cations sharing the H2NCS+ stoichiometry.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
An adequate description of the interstellar synthesis of sulfur-containing compounds remains a challenge. As pointed out by Adande et al. (2010), the chemistry of these species differs considerably from that of their oxygen-bearing analogs. An example is given by the reactions
S+ + H2 → SH+ + H,
O+ + H2 → OH+ + H,
only the second of which is exothermic (Oppenheimer & Dalgarno 1974). Also, the interstellar abundance ratio HNCS/NCSH, found to be 2–7 in Sgr B2 (Adande et al. 2010; Halfen et al. 2009) and close to unity in TMC-1 (Adande et al. 2010), is completely different from the value of approximately 200 observed for HNCO/HOCN (Brünken et al. 2009a; Marcelino et al. 2010; Adande et al. 2010). HSCN is, however, as much as 26 kJ mol−1 less stable than HNCS (Wierzejewska & Moc 2003). Adande et al. (2010) proposed that these findings be rationalized in terms of both HNCS and NCSH arising from a common precursor species HNCSH+—which would resemble the commonly accepted scheme of HCN/CNH synthesis from HCNH+ (Ziurys & Turner 1986). For the cationic precursor HNCSH+, Adande et al. (2010) proposed—based on analogy to relevant oxygen-bearing molecules—a pathway starting with NCS+, namely,
NCS+ + H2 → HNCS+ + H,
HNCS+ + H2 → H2NCS+/HNCSH+ + H,
HNCSH+ + e− → HNCS/NCSH + H,
H2NCS+ + e− → HNCS + H.
HNCSH+ appears here in tandem with its isomer H2NCS+. In general, positively charged species of this atomic composition can be regarded as precursors of a wealth of sulfur-containing molecules. The identification of such interstellar ions would be important for the HNCS/NCSH issue and for sulfur astrochemistry in general.
Here, we describe the possible cold-chemistry routes leading to HNCSH+ and to its structural isomer H2NCS+, and we start by revisiting the scheme proposed by Adande et al. (2010). The quantum mechanical considerations are followed by modeling of the relevant chemical reaction network. We also address the problem of possible microwave detections.
2. THEORETICAL METHODS
2.1. Quantum Chemistry
In quantum chemical calculations, we have combined the ab initio coupled cluster (CC) theory (Pople et al. 1987) and the density functional theory (DFT; Becke 1993; Lee et al. 1988). The CC variants CCSD (single and double excitations), CCSD(T) (perturbative triple excitations), and EOM-CCSD (equation-of-motion CC; Purvis III & Bartlett 1986; Rittby & Bartlett 1988; Stanton & Bartlett 1993) were employed; the B3LYP approach to DFT was used; standard aug-cc-pVDZ and cc-pVTZ (Kendall et al. 1992) basis sets were applied.
Preliminary structures of CH2NS+ isomers were indicated with DFT computations, B3LYP/aug-cc-pVTZ. Singlet and triplet potential energy surfaces (PESs) have been analyzed. Consecutive advanced calculations were performed in several steps. First, the molecular geometries were optimized at the CCSD/cc-pVTZ level, taking DFT-derived structures as starting points. Equilibrium permanent electric dipole moments (μD) were thus obtained. Next, CCSD(T)/cc-pVTZ geometries were found. Independently, calculations of vibrational frequencies (VPT2, developed by Barone 2005 at the B3LYP/aug-cc-pVTZ level), involving numerical differentiations along normal modes, supplied vibration–rotation coupling constants ( ,
,  , and
, and  ).
).
The reaction path analysis, involving the search for transition states via PES scans, was accomplished using CCSD/aug-cc-pVDZ calculations. All thermodynamic considerations involved zero-point vibrational energy corrections.
Gaussian 03 (Frisch et al. 2004) and 09 (Frisch et al. 2009) software packages were used throughout the study.
2.2. Reaction Rate Constants
To estimate the total reaction rate constants for selected ion–neutral reactions, we made use of the formula (Su & Chesnavich 1982; Wakelam et al. 2010)
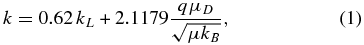
with kL (a "Langevin expression") given by

where q denotes the ionic charge, and μ the reduced mass of reactants; the permanent electric dipole moment μD and polarizability α were calculated at the CCSD/cc-pVTZ level. In order to estimate the branching ratios, we adopted the approach formulated by Kassel (1928). Namely, it is assumed that a transient product with s vibrational degrees of freedom may dissociate along a path i with the probability

where E0, the excess energy of a transient species, should be higher than the dissociation barrier E0, i, otherwise the reaction does not occur. The condition to be fulfilled by the normalization factor A is
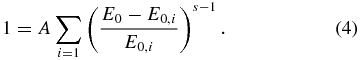
The total dissociative recombination rate constant is assumed here to equal the ion–electron collision rate, approximated by
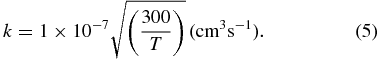
The branching ratios for dissociative recombination are estimated with the formulae (3) and (4); however, the expected accuracy of this approach, which neglects the existence of excited electronic states, is lower than in the case of ion–neutral reactions.
2.3. Chemical Modeling
The reaction network database for interstellar gas-phase chemical modeling (kida.uva.2011, hereafter KIDA; Wakelam et al. 2012) has been obtained, together with the Nahoon software (Wakelam et al. 2012)1. The public code Nahoon, which is compatible with the KIDA format, computes the temporal evolution of the chemical species in a volume of gas. Grains and electrons are treated as chemical objects, their abundances being computed in parallel to those of the other species. Fixed temperature and density conditions have been chosen (a "zero-dimension" variant of Nahoon computations); just as in the Adande et al. (2010) study, we have assumed nH = 2 × 104 cm−1, T = 10 K, and other values characteristic for the TMC-1 cloud. Initially, hydrogen is in its molecular form; helium, nitrogen, oxygen, and fluorine are present as neutral atoms while the remaining elements are ionized. Modeling has been carried out for two distinctly different sets of elemental abundances (Table 1). The low-metal set (hereafter LM) originates in the observations of the diffuse region ζ Oph with the respective abundances of elements heavier than oxygen being decreased by two orders of magnitude to account for depletion. The LM set is similar to that used by Graedel et al. (1982), and it is equivalent to the set referred to as EA1 in the paper by Wakelam & Herbst (2008), with the exception of helium, for which a relative (to hydrogen) abundance of 0.09 has been assumed (based on Orion Nebula observations; Baldwin et al. 1991; Osterbrock et al. 1992). The other set (HM) corresponds exactly to the high-metal elemental composition introduced by Wakelam & Herbst (2008), and designated EA3 in their paper. It is generally based on recent ζ Oph observations (Meyer et al. 1998; Federman et al. 2005, 1993; Savage & Sembach 1996; Savage et al. 1992; Cardelli et al. 1994, 1993)—with the assumption, however, that all silicon and magnesium, as well as a significant part of iron, is depleted in the form of olivines (Flower & Pineau des Forêts 2003).
Table 1. Elemental Abundances Relative to Hydrogen
| Element | Abundance | |
|---|---|---|
| LMa | HMb | |
| H2 | 5.00 × 10−1 | 5.00 × 10−1 |
| He | 9.00 × 10−2 | 9 × 10−2 |
| N | 2.14 × 10−5 | 7.60 × 10−5 |
| O | 1.76 × 10−5 | 2.56 × 10−4 |
| F | 6.68 × 10−9 | 6.68 × 10−9 |
| C+ | 7.30 × 10−5 | 1.20 × 10−4 |
| Si+ | 8.00 × 10−9 | 0 |
| S+ | 8.00 × 10−8 | 1.50 × 10−5 |
| Fe+ | 3.00 × 10−9 | 1.50 × 10−8 |
| Na+ | 2.00 × 10−9 | 2.00 × 10−7 |
| Mg+ | 7.00 × 10−9 | 0 |
| Cl+ | 1.00 × 10−9 | 1.80 × 10−8 |
| P+ | 0.21 × 10−9 | 1.17 × 10−7 |
Notes. aLow-metal (LM) abundances, after Wakelam & Herbst (2008), with the exception of He; see the text. bHigh-metal (HM) abundances, corrected for high Si, Mg, and Fe depletion, equivalent to the EA3 set of Wakelam & Herbst (2008).
Download table as: ASCIITypeset image
3. RESULTS AND DISCUSSION
3.1. Family of Five Atomic [C, H, H, N, S] Cations
3.1.1. Thermodynamic Stability
More than 20 cationic isomers containing differently linked atoms C, H, H, N, and S were initially proposed and investigated at the B3LYP/aug-cc-pVTZ level of theory. Structures corresponding to the deepest minima on the singlet PES, numbered in order of increasing electronic energy, are shown in Figure 1. Geometry optimizations always departed from several qualitatively dissimilar starting geometries. Vibrational frequency calculations allowed for a distinction between true minima and saddle points. The energetic separation of the most stable isomers H2NCS+ and HNCSH+ (38 kJ mol−1 at the B3LYP level; see Figure 1) is by far smaller than in an otherwise analogous oxygen-bearing pair H2NCO+/HNCOH+, for which Green (1981) calculated the difference of 75 kJ mol−1. Coupled cluster calculations confirmed the rather high electronic energies of the more exotic isomers HSNCH+ and H2CNS+ preliminarily predicted with DFT. Such high-energy values do not necessarily rule out interstellar detection, as exemplified by the isomeric species HC2NC and C3NH—less stable than HC3N (cyanoacetylene) by 111 kJ mol−1 and 213 kJ mol−1, respectively (Kołos & Dobrowolski 2003)—and yet observed in space (Kawaguchi et al. 1992a, 1992b) with abundances that are two orders of magnitude smaller than that of cyanoacetylene. Here, however, we have decided to focus on the chemistry and reactivity of H2NCS+ and HNCSH+. Energies of the most stable triplet isomers, corresponding to the a3A' PES, were systematically higher than those of their respective (i.e., similarly linked) singlet counterparts. The smallest singlet–triplet splitting (0.8 eV at the B3LYP/aug-cc-pVTZ level of theory) was found for H2CNS+. We have not investigated the triplet species any further.
Figure 1. CH2NS+ structures corresponding to the lowest energy minima of the singlet PES. Isomer labels and symmetry designations are followed by B3LYP/aug-cc-pVTZ relative energies (kJ mol−1), calculated with respect to species 1. Equilibrium ground state CCSD(T)/cc-pVTZ geometries (Å, degrees) and energy values corrected for the zero-point vibrational energy (underlined, in parentheses) are supplied for the most stable cations.
Download figure:
Standard image High-resolution image3.1.2. Microwave Spectroscopy
Equilibrium rotational constants Ae, Be, and Ce of the cationic isomers H2NCS+ and HNCSH+ were obtained following the geometry correction recipe, which has been shown to produce reliable results in our former studies (Kołos et al. 2009; Gronowski & Kołos 2007; Gronowski et al. 2013). Namely, the interatomic distances derived with CCSD(T)/cc-pVTZ, downscaled by a factor of 0.9949, were used for the C–N and C–S bonds, while the unscaled B3LYP/aug-cc-pVTZ results served for C–H, N–H, and S–H. Rotational constants in the ground vibrational state (A0, B0, and C0; Table 2) were given by
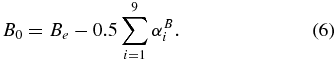
To check the adequacy of this procedure, we have applied it to quasi-linear NCSH and HNCS molecules of known rotational spectroscopy (Brünken et al. 2009b; Beard & Dailey 1950; Kewley et al. 1963). The resultant molecular constants were acceptably close to experimental values with B0 and C0 deviations of −0.6% for NCSH and −0.3% for HNCS. These errors, slightly larger than in our previous studies, stem primarily from C–S bond lengths. The isomers H2NCS+ and HNCSH+ are prolate symmetric tops. Of importance to microwave spectroscopy, all of these molecules bear substantial equilibrium electric dipole moments (Table 2), similar to NCSH or HNCS.
Table 2. Predicted Rotational Constants (MHz) and Equilibrium Electric Dipole Moments (debye) for Selected H2NCS+ Isomers
| Parameter | Theoretical Method | Isomer | |||
|---|---|---|---|---|---|
| H2NCS+ | HNCSH+ | HSNCH+ | H2CNS+ | ||
| A0 | CCSD(T)+B3LYP | 324 × 103 | 278 × 103 | 278 × 103 | 273 × 103 |
| B0 | CCSD(T)+B3LYP | 5581.2 | 5441.7 | 5738.0 | 6043.5 |
| C0 | CCSD(T)+B3LYP | 5483.2 | 5333.5 | 5616.4 | 5906.8 |
| μD | CCSD | 3.02 | 3.03 | 3.65 | 1.94 |
Download table as: ASCIITypeset image
3.2. Interstellar Reactions
3.2.1. NCS Routes
Electronic energy values for diverse chemical species possibly involved in the creation of H2NCS+ or of its isomers are listed in Table 3 (calculations on several [N, C, S] triatomics, including NCS, NCS−, and NCS+, were already carried out by Fitzgerald & Bowie 2004 at a theory level lower than that applied here).
Table 3. Theoretical (UCCSD(T)/cc-pVTZ) Energies (Hartree) for Molecules Relevant to the Synthesis of CH2NS+ Isomers
| Molecules | State | E |
|---|---|---|
| NCS+ | a1Σ | −489.944732 |
| NCS+ | X3Π | −489.982554 |
| NCS | X2Σ | −490.368970 |
| HNCS+ | X2Π | −490.648683 |
| NCSH+ | X2A'' | −490.600933 |
| HNCS | X1A' | −491.006385 |
| NCSH | X1A' | −490.996757 |
| CS | X1Σ | −435.668390 |
| CS+ | X2Σ | −435.274742 |
| CSH+ | X1A' | −435.878239 |
| HCS+ | X1Σ | −435.991341 |
| SH | X2Π | −398.280950 |
| SH+ | a1Π | −397.850392 |
| SH+ | X3Σ | −397.906742 |
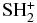 |
X2B1 | −435.991342 |
| SH2 | X1A' | −398.895543 |
| HNC | X1Σ | −93.235892 |
| HNC+ | X2Σ | −92.797605 |
| H2NC | X2B2 | −93.748824 |
| H | X2S1/2 | −0.499810 |
| H2 | X1Σg | −1.162290 |
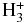 |
X1A' | −1.321137 |
| H2NCS+ | X1A1 | −491.302137 |
| HNCSH+ | X1A' | −491.288083 |
| HNSCH+ | X1A | −491.116465 |
| H2NCSH+ | X2A1 | −491.842936 |
| H2NC(H)S+ | X2A | −491.881119 |
| H2NSCH+ | X2A | −491.787160 |
| H3NCSH+ | X1A' | −492.418957 |
| H3NC(H)S+ | X1A' | −492.490735 |
| NH | X3Σ | −55.133245 |
| NH2 | X2B1 | −55.774534 |
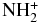 |
X3B1 | −55.371741 |
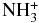 |
X2A' | −56.072518 |
| NH3 | X1A' | −56.438679 |
| NH4 | X2A1 | −56.917631 |
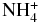 |
X1A1 | −56.765475 |
Note. Values corrected for the zero-point vibrational energy.
Download table as: ASCIITypeset image
Chemical pathways toward H2NCS+ and HNCSH+, involving NCS+ cations, have been proposed (Adande et al. 2010; see the Introduction). Our present study indicates the reaction between NCS+ and H2, barrierless and exothermic, as a highly probable source of interstellar HNCS+, but not of the more (by 125 kJ mol−1) energetic isomer NCSH+. The next step postulated by Adande et al. (2010) was expected to yield H2NCS+ or HNCSH+ via HNCS+ colliding with H2. Our calculations, however, indicate endothermicity of as much as 23.7 kJ mol−1 (2900 K) and 60.6 kJ mol−1 (7300 K) for the respective H2NCS+ and HNCSH+ branches. These processes do not therefore seem to be of importance within the cold interstellar clouds.
As pointed out by Milligan et al. (2002), proton transfer from highly energetic species, e.g.,  or H3O+, may efficiently produce bonding between hydrogen and a heavy atom. This process, involving the ubiquitous
or H3O+, may efficiently produce bonding between hydrogen and a heavy atom. This process, involving the ubiquitous  cation, has in fact been postulated for the creation of S–H bonds (Prasad & Huntress 1982). Indeed, according to our prediction, not only HNCS+ (a species of no direct interest to H2NCS+ or HNCSH+ synthesis, as discussed above) can be produced that way from neutral NCS molecules:
cation, has in fact been postulated for the creation of S–H bonds (Prasad & Huntress 1982). Indeed, according to our prediction, not only HNCS+ (a species of no direct interest to H2NCS+ or HNCSH+ synthesis, as discussed above) can be produced that way from neutral NCS molecules:
 + 362 kJ mol−1,
+ 362 kJ mol−1,
but also the HSCN+ cation:
 + 244 kJ mol−1,
+ 244 kJ mol−1,
since both reactions are exothermic and (at the CCSD/aug-cc-pVDZ level of theory) barrierless. The route to HNCSH+, making use of HSCN+,
NCSH+ + H2 → HNCSH+ + H + 65 kJ mol−1,
is also exothermic, but requires activation energy (36 kJ mol−1 or 4300 K), which makes it improbable for the cold interstellar medium.
On the other hand, another set of exothermic and barrierless reactions,
 /H2NCS+ + H2 + 323/359 kJ mol−1
/H2NCS+ + H2 + 323/359 kJ mol−1
 + 348 kJ mol−1,
+ 348 kJ mol−1,
seems to be an important factor shaping the balance between HNCS isomers and H2NCS+ isomers (keeping in mind that the latter are the source of the former via dissociative recombination).
3.2.2. Routes Without NCS Precursors
As an alternative to the reactions involving NCS or NCS+, it is of interest to consider whether the N–C–S backbone of the H2NCS+ or HNCSH+ cations could be assembled either via the collision of an N-containing reactant with a CS-containing one, or from colliding S- and NC-containing reactants. In this context, investigating the reactivity of the HCS+ and CSH+ cations seems to be promising. These can be regarded as analogous to HCO+ and COH+ (the interstellar abundance of HCO+ is higher by two orders of magnitude than that of COH+ (Fuente et al. 2003; Apponi & Ziurys 1997).
HCS+ was detected in Sgr B2 and in Ori A (Thaddeus et al. 1981) in several translucent molecular clouds (Turner 1996), and also beyond the Galaxy (Muller et al. 2013). CSH+ has not as yet been discovered in space. For the HCS+ and CSH+ cations, generally much less studied than the HCO+/COH+ pair, the theoretical analysis of PESs has been reported (Bruna 1978; Puzzarini 2005). HCS+ is linear, unlike CSH+, with the latter being significantly bent and less stable than the former by 302 kJ mol−1. The CSH+ → HCS+ isomerization barrier amounts to 5.4 kJ mol−1 (Puzzarini 2005).
CSH+ and HCS+ may arise in collisions between relatively abundant  and CS species,
and CS species,
 / CSH+ + H2 + 78/375 kJ mol−1,
/ CSH+ + H2 + 78/375 kJ mol−1,
in a process analogous to that proposed by Le et al. (2010) for HCO+/COH+ synthesis. Our calculations indicate that both HCS+ and CSH+ can thus be formed without a barrier. The CCSD/cc-pVTZ-derived polarizability (3.5 Å3) and the experimental value of the electric dipole moment, 1.958 D, as measured by Winnewisser & Cook (1968), were used to predict the reaction rate constants. According to Harrison (2006) and our present CCSD/cc-pVTZ calculations, the carbon atom of CS bears a fractional negative charge, pointing to HCS+ as the main reaction product (which is also predicted by the modeling described in Section 3.2.3).
Next, we analyze the possible elongation of HCS+ or CSH+ chains with a nitrogen atom. To the best of our knowledge, reactions involving any of these cations and nitrogen hydrides, NHn, have not been studied. We have carried out CCs calculations for n equal from one to three, as all three hydrids have quite similar abundances in the interstellar medium (Persson et al. 2010).
According to our predictions, CSH+ + NHn reactions lead without barriers to intermediate products of the general formula HnNCSH+. Once formed, the latter bear excess energy (726, 540, 268 kJ mol−1, for n equal 1, 2, and 3, respectively), which they cannot easily get rid of—the radiative relaxation is slow, and collision-induced energy exchange processes can be neglected. The fate of this cation is determined by energetically available unimolecular rearrangements that eventually result in its disintegration, accompanied by the transformation of chemical energy into kinetic energy. The resultant products are diverse; we focus here on the formation of doubly hydrogenated NCS cationic chains.
The synthesis of HNCSH+ through the association of CSH+ and NH is formally possible, requiring no activation energy and being exothermic. This is not, however, an efficient pathway toward HNCSH+, since the newly formed, energy-rich cation is a short-lived intermediate species, prone to spontaneous dissociation in processes that occur at the singlet PES:
CSH+ + NH → [HNCSH+] → HNCS+ + H + 360 kJ mol−1
→ NCSH+ + H + 234 kJ mol−1
→ HNC + SH+ + 344 kJ mol−1.
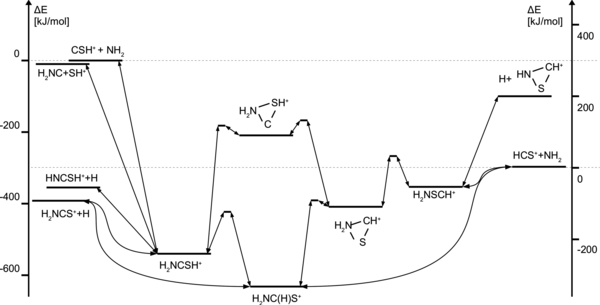
More promising are the reactions involving NH2 and CSH+ (Figure 2). The intermediate product H2NCSH+ may serve as an efficient precursor for CH2NS+ isomers (Figure 2). The following processes are possible even within cold interstellar regions, requiring no external energy:
Figure 2. Energy diagram illustrating the reactions of NH2 with CSH+ or HCS+.
Download figure:
Standard image High-resolution image![$\mathrm{CSH^{+} \,{+}\, NH_2 \!\rightarrow\! [H_2NCSH^{+}] \rightarrow H_2NCS^{+} \,{+}\, H}$](https://content.cld.iop.org/journals/0004-637X/792/2/89/revision1/apj498572ieqn18.gif) + 392 kJ mol−1
+ 392 kJ mol−1
→ HNCSH+ + H + 355 kJ mol−1.
One can expect H2NCS+ and HNCSH+ to be the most important products. Competing paths would include
CSH+ + NH2 → [H2NCSH+] → H2NC + SH+ + 7 kJ mol−1.
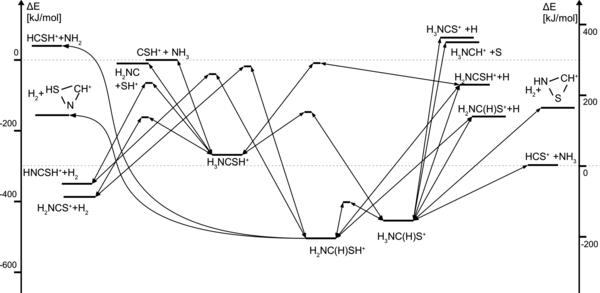
Finally, CSH+ may react with NH3, creating a variety of products (see Figure 3); only the processes directly relevant to the synthesis of HNCSH+ or H2NCS+ are discussed here. The involved activation barriers can be crossed for
Figure 3. Energy diagram illustrating the reactions of NH3 with CSH+ or HCS+.
Download figure:
Standard image High-resolution imageCSH+ + NH3 → [H3NCSH+] → HNCSH+ + H2 + 350 kJ mol−1
→ H2NCS+ + H2 + 387 kJ mol−1.
We note that H2NCSH+ can also be formed:
CSH+ + NH3 → [H3NCSH+] → H2NCSH+ + H + 68 kJ mol−1.
The dissociative recombination of the latter triply hydrogenated species can presumably be regarded as an additional source of HNCS and NCSH. In what follows, we have not, however, included that possibility.
Important reactions competing with the formation of H2NCS+ or HNCSH+ involve proton transfer processes leading from CSH+ to HCS+:
CSH+ + NH2 → [H2NCSH+] → HCS+ + NH2 + 297 kJ mol−1
CSH+ + NH3 → [H3NCSH+] → [H3NC(H)S+] → HCS+ + NH3 + 297 kJ mol−1.
As mentioned above, the HCS+ cation is more stable by far than CSH+, which makes it much less reactive. Collisions involving HCS+ and NHn may produce, for n = 1, the HNSCH+ cation, which quickly breaks apart. The other expected short-lived products are
- 1.for n = 2: H2NSCH+(with excess energy of 56 kJ mol−1) and H2NC(H)S+ (excess energy 303 kJ mol−1);
- 2.for n = 3: H3NC(H)S+ (excess energy 159 kJ mol−1),
of which, as illustrated by Figures 2 and 3, only the H2NC(H)S+ cation possesses enough excess energy to follow the paths toward the hydrogen abstraction products HNCSH+ and H2NCS+:
HCS+ + NH2 → [H2NC(H)S+] → [H2NCSH+] → HNCSH+ + H + 58 kJ mol−1,
or to direct dissociation:
HCS+ + NH2 → [H2NC(H)S+] → H2NCS+ + H + 95 kJ mol−1.
Note that our analysis did not reveal any minimum corresponding to the H3NSCH+ cation (which could be viewed as a direct product of the HCS+ + NH3 reaction). Exothermic processes,
HCS+ + NH3 → HNCSH+ + H2 + 53 kJ mol−1
→ H2NCS+ + H2 + 90 kJ mol−1,
(shown in Figure 3) are not interesting in the discussed astrochemical context, as both require an external input of energy for the creation of intermediate products.
Important processes competing with the formation of H2NCS+ or HNCSH+, and occurring neither via bound, intermediate products nor with any other energetic barriers, involve proton transfers from CSH+ or HCS+ to NHn:
 + 99 kJ mol−1,
+ 99 kJ mol−1,
 + 231 kJ mol−1,
+ 231 kJ mol−1,
 + 307 kJ mol−1,
+ 307 kJ mol−1,
 + 10 kJ mol−1.
+ 10 kJ mol−1.
Next, it is of interest to discuss an a priori possible synthetic route toward H2NCS+ or HNCSH+, occurring via a molecule possessing an –NC (isocyano) group, which would collide with a sulfur-containing reaction partner. The present study indicates that HNC and  do not react with each other, forming only a weak intermolecular complex, with a C–S distance of about 2.5 Å. The creation, out of that complex, of a cationic [C, H, H, N, S] isomer would require some external energy (the same is true for the reaction of HCN with
do not react with each other, forming only a weak intermolecular complex, with a C–S distance of about 2.5 Å. The creation, out of that complex, of a cationic [C, H, H, N, S] isomer would require some external energy (the same is true for the reaction of HCN with  ). An analogous reaction of HNC+ and SH2 is less predisposed to theoretical treatment. The intermolecular complex formed by these reactants represents in fact an electronically excited state of the above-mentioned
). An analogous reaction of HNC+ and SH2 is less predisposed to theoretical treatment. The intermolecular complex formed by these reactants represents in fact an electronically excited state of the above-mentioned  system, and therefore its investigation necessitates an analysis of the corresponding excited state PES. Preliminary TD-B3LYP/aug-cc-pVDZ calculations indicate that the reaction involves a barrierless channel leading to HNCSH+, but no path toward H2NCS+ is predicted to exist. This result has to be verified with high-level ab initio calculations.
system, and therefore its investigation necessitates an analysis of the corresponding excited state PES. Preliminary TD-B3LYP/aug-cc-pVDZ calculations indicate that the reaction involves a barrierless channel leading to HNCSH+, but no path toward H2NCS+ is predicted to exist. This result has to be verified with high-level ab initio calculations.
3.2.3. Astrochemical Modeling
The search for interstellar H2NCS+ and/or HNCSH+ seems to be an important observational task; we have therefore calculated the basic molecular parameters relevant to the radio astronomical detection of these cations and have also tried to estimate their abundance. The chemical model employed here makes use of the KIDA database and the Nahoon code (Wakelam et al. 2012; see Section 2.3). The kida.uva.2011 network comprises 783 reactions of 44 sulfur-bearing species, but it is obviously not complete, just as any astrochemical database, and, importantly, it does not include any molecules simultaneously containing carbon, sulfur, and nitrogen. In tandem with Nahoon, KIDA proved to make satisfactory abundance predictions for some 79% of the molecules detected in TMC-1 (Wakelam et al. 2010). The best agreement between available observations and modeling has been found for the times 4.7 × 105 to 6.3 × 105 yr. KIDA is not successful, however, in modeling the abundance of HCS+ (a crucial precursor in the proposed synthetic pathway toward H2NCS+ and HNCSH+). The abundance sets LM and HM (see Section 2.3) lead to the respective HCS+ abundances of 2 × 10−12 and 9 × 10−12, relative to H2, while the value observed for TMC-1 is as high as 6 × 10−10 (Irvine et al. 1987).
We have assumed the dissociative recombination of HNCSH+ and H2NCS+ with electrons to be the most important decay mechanism for these cations. Such reactions have been computationally studied by Gronowski & Kołos (2012). The modeling presented here makes use of these previous findings and of our new theoretical results concerning the rearrangement of neutral H2NCS species. We have appended the database with 48 reactions (Table 4) and 7 additional molecules. The ionization of HNCS and NCSH by atomic cations has been included (note that HNCS cannot be ionized by S+, in contrast to NCSH).
Table 4. Gas-phase Reactions Related to the Synthesis and Destruction of HNCSH+ and H2NCS+
| Substrates | Products | α | β | γ | ||
|---|---|---|---|---|---|---|
| NH | CSH+ | H | HNCS+ | 0.579E00 | 0.779E-09 | 0.480E+01 |
| NH | CSH+ | H | NCSH+ | 0.185E-01 | 0.779E-09 | 0.480E+01 |
| NH | CSH+ | HNC | HS+ | 0.402E+00 | 0.779E-09 | 0.480E+01 |
| NH | CSH+ | 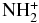 |
CS | 0.189E-04 | 0.779E-09 | 0.480E+01 |
| NH2 | CSH+ | H | H2NCS+ | 0.839E+00 | 0.899E-09 | 0.469E+01 |
| NH2 | CSH+ | H | HNCSH+ | 0.154E-00 | 0.899E-09 | 0.469E+01 |
| NH2 | CSH+ | 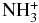 |
CS | 0.136E-02 | 0.899E-09 | 0.469E+01 |
| NH2 | CSH+ | HCS+ | NH2 | 0.625E-02 | 0.899E-09 | 0.469E+01 |
| NH3 | CSH+ | H2 | H2NCS+ | 0.342E+00 | 0.951E-09 | 0.377E+01 |
| NH3 | CSH+ | H2 | HNCSH+ | 0.960E-06 | 0.951E-09 | 0.377E+01 |
| NH3 | CSH+ | 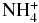 |
CS | 0.570E+00 | 0.951E-09 | 0.377E+01 |
| NH3 | CSH+ | HCS+ | NH3 | 0.879E-01 | 0.951E-09 | 0.377E+01 |
| NH2 | HCS+ | H | H2NCS+ | 0.504E-00 | 0.899E-09 | 0.469E+01 |
| NH2 | HCS+ | H | HNCSH+ | 0.183E-02 | 0.899E-09 | 0.469E+01 |
| NH2 | HCS+ | H | H2NCS+ | 0.484E-00 | 0.899E-09 | 0.469E+01 |
| NH3 | HCS+ | 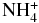 |
CS | 1.000E+00 | 0.951E-09 | 0.377E+01 |
| HNCSH+ | e − | HNC | HS | 0.526E-07 | 0.500E-01 | 0.000E+00 |
| HNCSH+ | e − | HNCS | H | 0.195E-07 | 0.500E-01 | 0.000E+00 |
| HNCSH+ | e − | NCSH | H | 0.194E-07 | 0.500E-01 | 0.000E+00 |
| HNCSH+ | e − | H2NC | S | 0.709E-10 | 0.500E-01 | 0.000E+00 |
| HNCSH+ | e − | CS | NH2 | 0.161E-09 | 0.500E-01 | 0.000E+00 |
| H2NCS+ | e − | HNCS | H | 0.718E-07 | 0.500E-01 | 0.000E+00 |
| H2NCS+ | e − | H2NC | S | 0.844E-09 | 0.500E-01 | 0.000E+00 |
| H2NCS+ | e − | CS | NH2 | 0.192E-08 | 0.500E-01 | 0.000E+00 |
| H2NCS+ | e − | HNC | HS | 0.155E-07 | 0.500E-01 | 0.000E+00 |
| H2NCS+ | e − | NCSH | H | 0.100E-07 | 0.500E-01 | 0.000E+00 |
| H3O+ | CS | H2O | CSH+ | 0.315E-07 | 0.134E-08 | 0.334E+01 |
| CS | HCO+ | CO | CSH+ | 0.300E-07 | 1.160e-09 | 3.280e+00 |
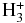 |
CS | H2 | CSH+ | 0.350E-01 | 0.285E-08 | 0.334E+01 |
| H2S | C+ | H | CSH+ | 0.262E+00 | 0.151E-08 | 0.184E+01 |
| HNCS | C+ | HNCS+ | C | 0.100E+01 | 0.185E-08 | 0.292E+01 |
| HNCS | F+ | HNCS+ | F | 0.100E+01 | 0.154E-08 | 0.292E+01 |
| HNCS | H3O+ | HNCSH+ | H2O | 0.100E+01 | 0.154E-08 | 0.292E+01 |
| HNCS | H3O+ | H2NCS+ | H2O | 0.100E+01 | 0.154E-08 | 0.292E+01 |
| HNCS | 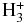 |
HNCSH+ | H2 | 0.100E+01 | 0.347E-08 | 0.292E+01 |
| HNCS | 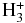 |
H2NCS+ | H2 | 0.100E+01 | 0.347E-08 | 0.292E+01 |
| HNCS | He+ | HNCS+ | He | 0.100E+01 | 0.303E-08 | 0.292E+01 |
| HNCS | H+ | HNCS+ | H | 0.100E+01 | 0.591E-08 | 0.292E+01 |
| HNCS | N2H+ | HNCSH+ | N2 | 0.100E+01 | 0.133E-08 | 0.292E+01 |
| HNCS | N2H+ | H2NCS+ | N2 | 0.100E+01 | 0.133E-08 | 0.292E+01 |
| HNCS | P+ | HNCS+ | P | 0.100E+01 | 0.130E-08 | 0.292E+01 |
| HNCS | S+ | HNCS+ | S | 0.100E+01 | 0.129E-08 | 0.292E+01 |
| NCSH | C+ | NCSH+ | C | 0.100E+01 | 0.171E-08 | 0.517E+01 |
| NCSH | F+ | NCSH+ | F | 0.100E+01 | 0.143E-08 | 0.517E+01 |
| NCSH | H3O+ | HNCSH+ | H2O | 0.100E+01 | 0.143E-08 | 0.517E+01 |
| NCSH | 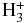 |
HNCSH+ | H2 | 0.100E+01 | 0.320E-08 | 0.517E+01 |
| NCSH | He+ | NCSH+ | He | 0.100E+01 | 0.280E-08 | 0.517E+01 |
| NCSH | H+ | NCSH+ | H | 0.100E+01 | 0.546E-08 | 0.517E+01 |
| NCSH | N2H+ | HNCSH+ | N2 | 0.100E+01 | 0.123E-08 | 0.517E+01 |
Note. Dissociative recombination reaction rate constants in units of cm3 s−1 can be calculated from tabulated coefficients according to α × (T/300)β × exp (− γ/T) while ion–neutral reaction rate constants can be calculated using α × β × (0.62 + 0.4767 × γ × (300/T)0.5).
Download table as: ASCIITypeset image
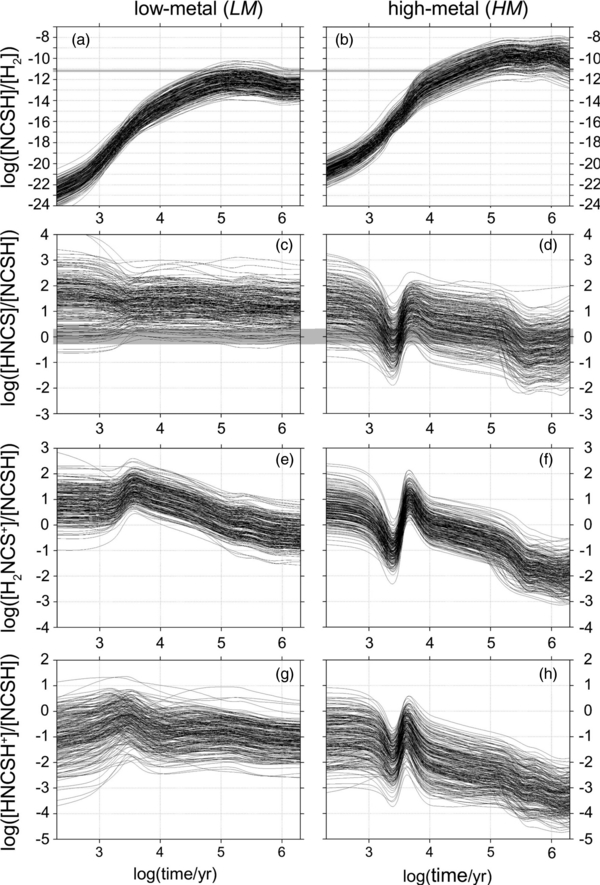
To investigate the importance of inaccuracies in the applied reaction rate constants, we randomly varied the ki values for newly added reactions. Consequently, 220 chemical models, differing by their ki sets, have been created. The rate constants used in the models came from log (ki) values given by log-normal distributions, with the maxima defined by the logarithms of theoretically predicted ki's (see Section 2) and with the half-widths arbitrarily set to log (5). This implies a standard deviation of approximately log(2) in any log (ki) distribution; formally, then, with a probability of ca. 70%, the ki's are expected to be known within a factor of two. A similar level of accuracy has, for example, been found by Pu & Truhlar (2002) using the transition state theory approach to the CH4 + H reaction. As expected, the predicted abundances of H2NCS+, HNCSH+, HNCS, and NCSH (Figure 4) were significantly dependent on the employed set of rate constants.
Figure 4. Relative NCSH ((a)–(b)), HNCS ((c)–(d)), H2NCS+ ((e)–(f)) and HNCSH+ ((g)–(h)) abundances for two different initial compositions of a cold, dense interstellar region; multiple kinetic traces represent different sets of reaction rates (see the text). Values observed in TMC-1 are marked in gray.
Download figure:
Standard image High-resolution imageThe relative abundance of NCSH (see Figure 4) is for the HM set of elemental composition higher than for LM (Figures 4(a) and (b)). In any case, the observed value of 6 × 10−12 (Adande et al. 2010) was, for t = 5 × 105 yr, within the specified inaccuracy range; the same applies to the comparison of the predicted (Figures 4(c) and (d)) HNCS/NCSH ratio to that measured in TMC-1: 1.4 ± 0.7 (Adande et al. 2010). The latter value was best reproduced with the HM abundance set.
The most important result of the chemical modeling presented here concerns the cationic species HNCSH+ and H2NCS+. The rapid increase of their abundance is predicted to occur for times from 1000 to 2000 yr; the abundance then continues to grow until approximately 2 × 105 yr, reaches a plateau, and slightly decreases for times longer than 5 × 105 yr. The modeled H2NCS+ abundance is one order of magnitude higher than that of HNCSH+ (see Figure 4). The H2NCS+/NCSH ratio (unlike HNCSH+/NCSH) significantly varies with time, which is consistent with the scheme that predicts H2NCS+ to be the earliest synthesized NCS-containing molecule. For t = 5 × 105 yr, H2NCS+/NCSH falls between 1 (for the LM elemental abundance set) and 0.02 (HM). The low relative abundance of H2NCS+ for a HM (i.e., high-sulfur) content set originates mainly from the corresponding high abundance of NCSH.
Based on the LM model and on the calculated electric dipole moment values (Section 3.1.2), the total intensity of rotational lines coming from H2NCS+ is expected to be of the same order of magnitude as that due to NCSH.
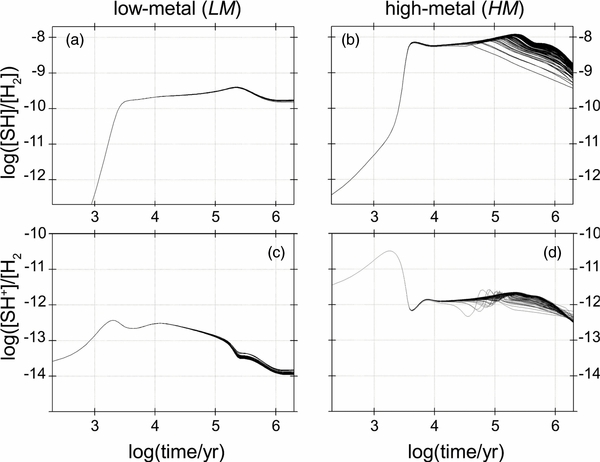
The influence of the newly introduced 48 sulfur-related reactions on the time evolution of molecular abundances—apart from NCS-bearing species—deserves comment. The effect was not, generally, notable with the LM set. In particular, the molecules NS, HCS+, S2,  , and H2S have not been affected. An important exception concerns the SH radical, the simplest sulfur-containing molecule. Neither SH nor SH+ have as of yet been detected in any cold interstellar region. Recently, however, SH was observed in absorption toward a submillimeter continuum source W49N (Neufeld et al. 2012), and SH+ toward Sgr B2 (Menten et al. 2011). Neutral SH is one of the main products of the HNCSH+ dissociative recombination, which increases the abundance of this diatomic compared to what was indicated with the original kida.uva.2011 reaction set. Our estimated SH/H2S and SH+/H2S values are about 0.1 (see Figure 5), which is close to the SH/H2S ratio of 0.13 measured for W49N and rather far from a lower limit of approximately 2 established for SH+/H2S in Sgr B2 observations. Each of these two regions, however, is characterized by qualitatively different physical conditions than TMC-1.
, and H2S have not been affected. An important exception concerns the SH radical, the simplest sulfur-containing molecule. Neither SH nor SH+ have as of yet been detected in any cold interstellar region. Recently, however, SH was observed in absorption toward a submillimeter continuum source W49N (Neufeld et al. 2012), and SH+ toward Sgr B2 (Menten et al. 2011). Neutral SH is one of the main products of the HNCSH+ dissociative recombination, which increases the abundance of this diatomic compared to what was indicated with the original kida.uva.2011 reaction set. Our estimated SH/H2S and SH+/H2S values are about 0.1 (see Figure 5), which is close to the SH/H2S ratio of 0.13 measured for W49N and rather far from a lower limit of approximately 2 established for SH+/H2S in Sgr B2 observations. Each of these two regions, however, is characterized by qualitatively different physical conditions than TMC-1.
Figure 5. SH ((a)–(b)) and SH+ ((c)–(d)) abundances for two different initial compositions of a cold, dense interstellar region; multiple kinetic traces represent different sets of reaction rates (see text).
Download figure:
Standard image High-resolution imageOn the other hand, the effect of added reactions was widely seen with the HM elemental abundance set. An example is offered by HC2n + 1N molecules, for which the abundance was altered at t = 5 × 105 yr by −4%, −10%, and −16% for n = 0, 1, and 2, respectively—or by the elements of the NHn series, where the analogous abundance changes are predicted as +2%, −5%, and −16%, for n = 1, 2, and 3.
4. CONCLUSIONS
For the interstellar synthesis of H2NCS+ and HNCSH+ (likely precursors to the already detected HNCS and HSCN neutrals), present theoretical arguments indicate several possible routes—starting with the ubiquitous  and relatively abundant CS and NH2 molecules. Namely, HCS+ and CSH+ can arise in the collisions of CS with
and relatively abundant CS and NH2 molecules. Namely, HCS+ and CSH+ can arise in the collisions of CS with  . Any of these cations is subsequently able to yield, when combined with NH2, the two products of interest, H2NCS+ and HNCSH+. Moreover, H2NCS+, together with HNCSH+, can be produced from CSH+ and ammonia, NH3. The hydrogenation of HNCS and NCSH is also important in shaping the HNCS/NCSH ratio. Bearing in mind the fairly limited accuracy of the reaction rate constants calculated in this study, we have constructed for a cold interstellar cloud like TMC-1 a family of chemical models with variable kinetic parameters. The influence of elemental abundances has also been investigated. The modeling suggests, for t = 5 × 105 yr, the abundances of H2NCS+ and HNCSH+ to be, respectively, similar within an order of magnitude and one order of magnitude lower than the abundance of already observed NCSH or HNCS molecules. This gives a good chance at least for the detection of H2NCS+, since the corresponding total absolute strength of microwave transitions, indicated by electric dipole moment, is expected to be similar as for the above mentioned neutrals.
. Any of these cations is subsequently able to yield, when combined with NH2, the two products of interest, H2NCS+ and HNCSH+. Moreover, H2NCS+, together with HNCSH+, can be produced from CSH+ and ammonia, NH3. The hydrogenation of HNCS and NCSH is also important in shaping the HNCS/NCSH ratio. Bearing in mind the fairly limited accuracy of the reaction rate constants calculated in this study, we have constructed for a cold interstellar cloud like TMC-1 a family of chemical models with variable kinetic parameters. The influence of elemental abundances has also been investigated. The modeling suggests, for t = 5 × 105 yr, the abundances of H2NCS+ and HNCSH+ to be, respectively, similar within an order of magnitude and one order of magnitude lower than the abundance of already observed NCSH or HNCS molecules. This gives a good chance at least for the detection of H2NCS+, since the corresponding total absolute strength of microwave transitions, indicated by electric dipole moment, is expected to be similar as for the above mentioned neutrals.
This work was mostly supported by the National Science Centre project No. 2011/01/D/ST4/04345. R.K. acknowledges the funding from the National Science Centre project 2011/03/B/ST4/02763 and Polish Ministry of Science & Higher Education research grant N203 012 32/1550. Quantum chemical calculations were partly accomplished at the Interdisciplinary Centre for Mathematical and Computational Modelling (grant No. G36-13). M.G. also acknowledges the START stipend granted by the Foundation for Polish Science.