ABSTRACT
We present results on sputtering and radiation chemistry of CO2 films induced by 100 keV H+ at 25 and 50 K. Using a quartz crystal microbalance, we measure a sputtering yield (SY) between ∼10 and 20 CO2 equivalent per ion at 25 K. The yield at 50 K is similar to that at 25 K at low fluences, but increases to ∼2400 by mid-1014 H+ cm−2 and declines at higher fluence. Irradiation to 1 × 1015 H+ cm−2 depletes ∼85%–90% of the initial film mass at 50 K, compared to 3% at 25 K. In both cases, mass spectrometry shows that CO is the dominant constituent in the sputtered flux, followed by O2, O, and CO2. Using infrared spectroscopy, we monitor the depletion of CO2 and the accumulation of CO and O2 and minor species as O3 and CO3. We determine G(−CO2) = 2.6 ± 0.3, the number of CO2 destroyed per 100 eV at 25 K. A significant fraction of the radiolyzed CO and O2 are retained in the film at 25 K; only those near the surface are removed during irradiation, contributing to a smaller SY. At 50 K, CO and O2 are unstable along the "hot" ion track and are expelled possibly from the entire depth of the film. Our results, and the lack of detection of CO in the exospheres around Rhea and Dione, show that the CO2 does not originate from sputtering, since otherwise the exosphere would be dominated by CO, the main molecule in the sputtered flux. We suggest that the exospheric CO2 is thermally released from an endogenic source.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Solid CO2 was first discovered in the interstellar medium (ISM) by D'Hendecourt & Jourdain de Muizon (1989). From observations of the Infrared Space Observatory and the Spitzer telescopes, we now know that it is one of the major constituents of icy mantles coating the micrometer-sized dust grains with abundance ranging from ∼10% to 40% relative to water ice toward various lines of sight (Boogert & et al. 2004; Gerakines et al. 1999; Gibb et al. 2004). The origin of CO2 in the solid phase in the ISM has been a long-standing problem, and we have tested several formation routes in the laboratory that would yield CO2 in the abundances observed in the ISM (Fulvio et al. 2012; Loeffler et al. 2005; Raut & Baragiola 2011; Raut et al. 2012) in addition to other efforts (Gomis & Strazzulla 2005; Ioppolo et al. 2011; Roser et al. 2001). While mechanisms like oxidation of CO by thermal atomic O and radiolysis at the carbon–water interface can occur in the ISM, contributing to the CO2 abundance, we have yet to identify a compelling CO2 synthesis mechanism relevant to the ISM. However, since the abundant CO2 is subject to processing by energetic cosmic rays and UV photons, it is also important to investigate the response of CO2 to radiation. For instance, it is known that energy deposited by ionizing radiation can eject molecules from frozen solids into the gas phase. Such a process, sputtering is critical to the stability of ice mantles and the gas phase abundance of various species in different regions of the ISM. Furthermore, ionizing radiation can break up molecules forming radicals that can react to yield more complex species in the ices.
Solid CO2 is also known to exist on the surfaces of satellites of the outer planets (Brown et al. 2006; Buratti et al. 2005; Carlson et al. 1996; Cruikshank et al. 2010; Hibbitts et al. 2000). In addition, CO2 has also been detected in the exosphere around Callisto (Carlson 1999). More recently, the Cassini spacecraft detected CO2 and O2 exosphere at Rhea (Teolis et al. 2010) and  in Dione's exosphere (Tokar et al. 2012). Impacts from solar wind, cosmic rays, magnetospheric ions, and solar photons can desorb frozen species, contributing to the surface-bound exospheres that surround icy satellites and chemically alter the surface of these satellites by creating new species.
in Dione's exosphere (Tokar et al. 2012). Impacts from solar wind, cosmic rays, magnetospheric ions, and solar photons can desorb frozen species, contributing to the surface-bound exospheres that surround icy satellites and chemically alter the surface of these satellites by creating new species.
In an effort to understand the effects of energetic radiation of solid CO2, we recently published a report on Lyα driven photodesorption of solid CO2 between 6 and 60 K (Bahr & Baragiola 2012). Here we present new results that characterize sputtering and synthesis of new molecules resulting from irradiation of thin CO2 films with 100 keV protons, using microbalance gravimetry, infrared spectroscopy, and mass spectrometry. We have studied the process at 25 and 50 K, temperatures relevant to the cold ISM and the outer solar system.
2. EXPERIMENTAL SETUP
All experiments were performed in a cryopumped ultrahigh vacuum chamber with a base pressure of 10−10 Torr. The CO2 films were deposited at normal incidence using a collimated microcapillary array doser onto an optically flat gold mirror electrode of a quartz crystal microbalance (QCM) at 50 K. The areal mass of the films was determined from the change in the resonant frequency of the microbalance, which can be converted into column density η (molecules cm−2) by dividing by the molecular mass. The CO2 films had initial column densities between 505 and 510 × 1015 CO2 cm−2 (about 510 monolayers). A density of 1.5 g cm−3 for solid CO2 grown at 50 K (Satorre et al. 2008) implies a film thickness of ∼0.25 μm.
Following deposition, the CO2 films were either cooled to 25 K or irradiated at 50 K with 100 keV protons at 9° incidence. The proton beam was produced by a mass-filtered ion accelerator and its flux (∼1012 H+ cm−2 s−1) was monitored by a thin wire placed in its path. Electrostatic deflection plates were used to raster the beam to ensure uniform irradiation of the films. The range of the 100 keV protons in pure CO2 film is ∼2700 × 1015 CO2 cm−2 (Ziegler & Biersack 2013), ∼5 times the column density of our films.
During irradiation, we monitored the mass loss from the sample using the QCM to obtain the sputtering yield (SY). As we will show, different molecules other than CO2 can be ejected from the ice to cause the mass loss; yet, as a simplification, we express the yield in units of CO2 equivalent (eq.) per ion, which is areal mass loss/CO2 mass. A Dycor mass spectrometer (MS) aimed at near-normal incidence to the sample was used to analyze the molecules sputtered during irradiation and later during thermal desorption of films, following irradiation.
The radiation-induced changes in the chemical composition of the films were studied using infrared spectroscopy. Specular reflectance of the films was obtained at 35° incidence using a Thermo Nicolet Nexus 670 Fourier transform infrared spectrometer at 2 cm−1 resolution. The spectra R of the ice film taken following film deposition and after incremental fluence steps during irradiation were divided by the spectra of the gold substrate Ro, obtained prior to film deposition. The ratios were then converted to optical depth, −ln (R/Ro). Band areas for different absorptions were obtained by integrating regions of the spectra after subtraction of baselines that matched the absorption continuum.
We convert the band areas to column densities only for a specific case where the film is irradiated at 25 K to a fluence F of ∼1 × 1015 H+ cm−2 since the mass loss due to sputtering is minimal, 3% of the initial CO2. Most of the new species formed from destruction of CO2 are still within the film. This allows us to estimate the amounts of CO and O2 using conservation arguments, which will be explained later. Such an approach would not be valid in cases where significant fraction of the film is lost in non-stoichiometric sputtering at higher F.
The CO2 column density is deduced from the ν1 + ν3 band in the following manner. We divide the band area following deposition by the initial column density measured with the QCM to obtain an initial effective band strength Ai* = (1.95 ± 0.05) × 10−18 cm molecule−1. The qualifier "effective" is used to emphasize that A* is not a constant property of a molecule, but rather depends on the thickness and the optical properties of the film (Teolis et al. 2007), which change due to sputtering and synthesis of new molecules. To obtain the band strength Af* for CO2 at the end of the irradiation, we thermally desorbed the film to estimate the amount of residual CO2 from the QCM measurement of desorption peak at ∼90 K. The band area at the end of the irradiation divided by final CO2 amount gives Af* = (2.35 ± 0.05) × 10−18 cm molecule−1, a 20% increase from Ai*. The CO2 column densities are obtained by dividing the areas of the ν1 + ν3 bands that change during irradiation with linearly interpolated values of A*.
3. RESULTS
3.1. Sputtering
Figure 1 shows the MS measurements of the flux of CO, O2, CO2, and O sputtered from CO2 films and their sum labeled ∑ at 25 and 50 K versus irradiation fluence F. Background signals for each mass were obtained with the proton beam blocked, and have been subtracted for each species. Further, we have corrected the flux for species-dependent MS sensitivity and fragmentation in the ion source (Bahr & Baragiola 2012). The accuracy of the fluxes is ∼25%.
Figure 1. Flux of CO2, CO, O2, and O sputtered from ∼500 ML thick CO2 films vs. fluence of 100 keV H+ at 50 and 25 K, measured by the mass spectrometer. The data has been corrected for mass fragmentation and sensitivity. The gray lines labeled ∑ are the sum of the fluxes. We did not detect CO3 or O3 above the noise level.
Download figure:
Standard image High-resolution imageFirst, it is interesting to note the small CO2 flux, compared to CO and O2 at both temperatures, despite the films being pure CO2 prior to irradiation. At 50 K, we observe the flux of CO and O2 to increase with F, peaking at ∼5 × 1014 H+ cm−2 and then decreasing at higher fluence. The CO2 signal does not change with fluence. The maximum CO signal is ∼500 times larger than that of CO2 at 50 K. At this temperature, we did not observe sputtered C and O atoms above the level from fragmentation of molecules in the MS.
At 25 K we could measure the direct O flux coming from the ice film at 25 K. Though the sum of the sputtered flux remained fairly constant at 25 K, variation in flux of individual species can be observed. For instance, the O/O2 decreases ∼75-fold by 5 × 1015 H+ cm−2, as the sputtered O2 signal increases as O2 builds up in the ice. Similarly, the O/CO signal also decreases by a factor of 15. The CO signal is ∼10 times larger than the CO2 signal at 25 K.
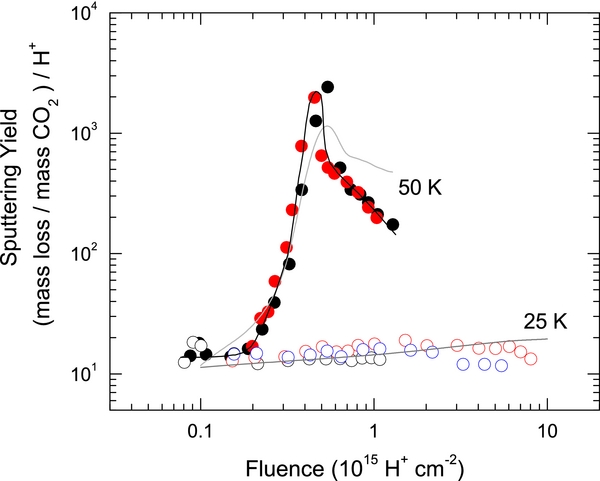
Figure 2 shows the SY of initially pure CO2 films with increasing fluence at 25 and 50 K, measured using the QCM. At 25 K, the yield varies over a small range, between 10 and 20 CO2 eq. per ion. In contrast, the yield shows a strong dependence on fluence at 50 K. The yield is similar to that at 25 K at low fluences. It sharply increases to peak at ∼2400 by 5 × 1014 H+ cm−2 and then decreases at higher fluence.
Figure 2. Sputtering yield vs. fluence during 100 keV H+ irradiation of initially pure CO2 films at 25 and 50 K. The gray lines are the sums of the sputtered fluxes of species, ∑ (Figure 1), scaled to match the sputtering yields. The black line is to guide the eye.
Download figure:
Standard image High-resolution imageThere is good agreement between the MS (Figure 1) and the QCM measurements (Figure 2) at 25 K, as indicated by the overlap of ∑ (scaled) and the SY measurements. At 50 K, a similar trend exists between the scaled ∑ and SY, though disagreements are present at higher F, as SY decreases faster than ∑ (scaled) above 5 × 1014 H+ cm−2. At the highest fluence, ∑ is nearly three times larger than SY. This may be due to a variation of the angular distribution of the sputtered flux with ion fluence and, therefore, of the fraction measured by the MS.
3.2. Infrared Measurements
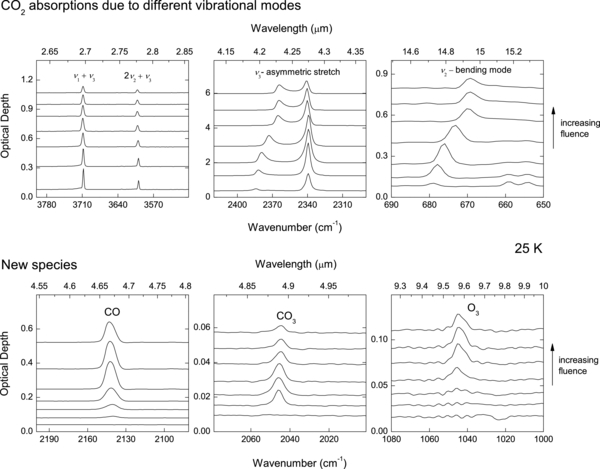
The top panel in Figure 3 shows changes in the CO2 absorptions assigned to various modes of vibration as a result of ion irradiation at 25 K. The weak absorptions at ∼2.75 μm correspond to the ν1 + ν3 (3708 cm−1) and 2ν2 + ν3 (3600 cm−1) combination modes. The stronger absorption at ∼4.25 μm is due to the asymmetric C–O stretch mode (ν3), while that at ∼15 μm is due to the bending mode (ν2) (Sandford & Allamandola 1990). The stronger ν2 and ν3 bands exhibit LO–TO splitting as previously observed for solid CO2 films (Baratta & Palumbo 1998).
Figure 3. Infrared absorption spectra for a ∼500 ML thick CO2 film and their change due to irradiation by 100 keV protons, showing the appearance of CO, CO3, and O3. Not shown is O2, which is infrared inactive, but is detected by mass spectrometry. The spectra are for the film irradiated to fluences of 0, 0.03, 0.07, 0.2, 0.7, 3.02, 8.04, in units of 1015 H+ cm−2. The spectra have been shifted vertically for clarity.
Download figure:
Standard image High-resolution imageIn the bottom panel, we show the appearance of new absorption features with increasing fluence due to synthesis of CO, CO3, and O3 during irradiation. The forbidden absorption band of O2 at 1550 cm−1 is not seen above the noise, but we detect O2 in the sputtered flux (Figure 2). The residual CO2 becomes diluted as more CO and O2 are formed with increasing fluence. Such mixing of CO2 in other species leads to the convergence of the LO–TO bands (Raut et al. 2012) as seen in top panel of Figure 3.
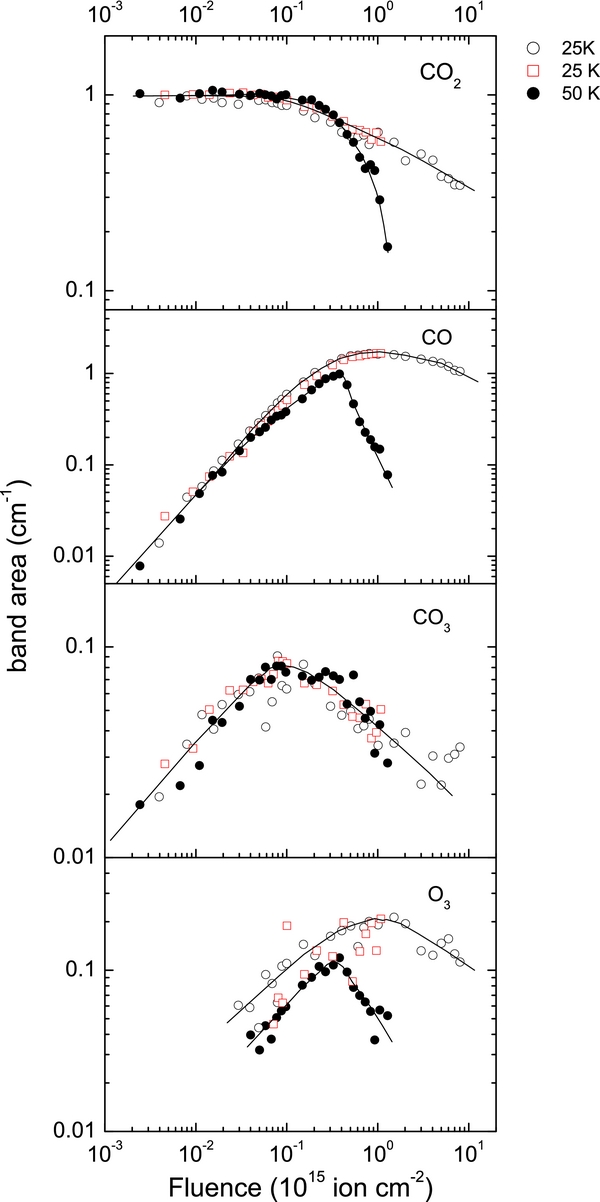
Figure 4 compares the fluence dependence of the band areas of four infrared-active species at 25 and 50 K. For CO2, we chose to integrate the ν1 + ν3 band, since the stronger absorptions (ν3, ν2) are complicated by their LO–TO splitting and the merging of these features as the film evolves from pure CO2 to a mixed one. These effects are absent in the weaker combination bands (see Figure 3) where the real part of the refractive index, n, in the absorption region >1 (Baratta & Palumbo 1998). We integrated the ν1 absorption band for CO and CO3 and the ν3 band for O3. The CO2 band area decreases while those of CO, O3, and CO3 initially increase at both temperatures and then decrease at higher fluence. At 50 K, the CO band area is smaller than at 25 K, beginning at ∼1014 H+ cm−2. Similarly, the O3 band area is smaller at 25 K. This is consistent with our observation of sputtering where CO and O2 are the dominant species. The band areas decrease faster at higher F due to high sputtering at 50 K.
Figure 4. The change in absorption band areas of different species with fluence during 100 keV irradiation of an initially pure CO2 film at 25 and 50 K. The absorptions used are the CO2 ν1 + ν3 at 3707 cm−1, ν1 absorption of CO and CO3 at 2140 and 2045 cm−1, respectively, and ν3 of O3 at 1040 cm−1. The lines are guides to the eye.
Download figure:
Standard image High-resolution image3.3. Thermal Desorption
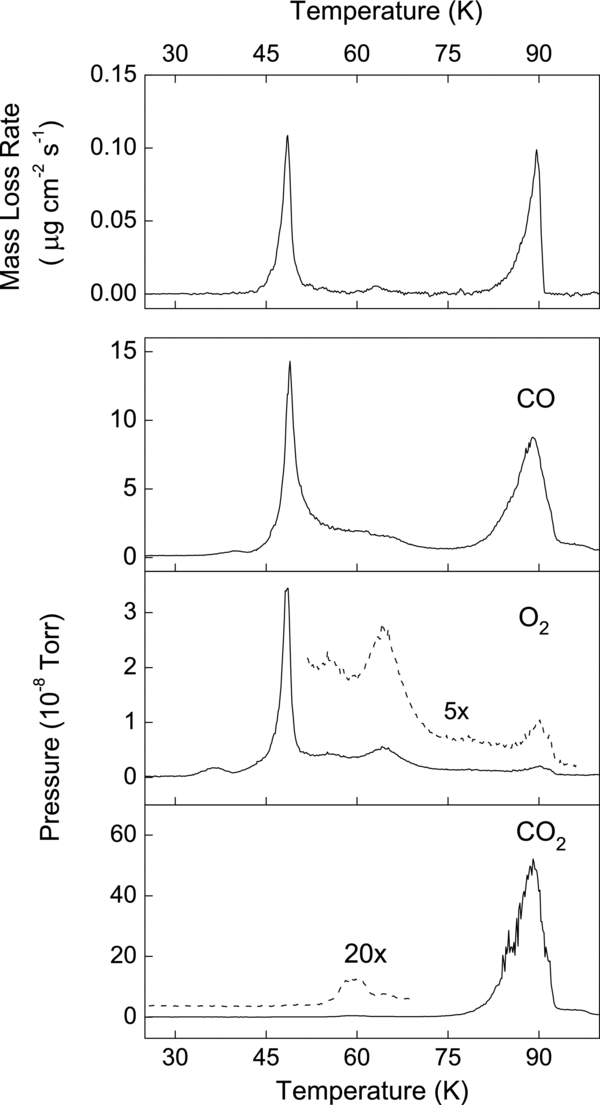
After irradiating to a fluence of ∼1 × 1015 H+ cm−2 at 25 K, we desorbed the film by heating at 1 K minute−1, while continuously monitoring the mass with the QCM and the ejected molecules with the MS (Figure 6). We do not show small C and O signals at this temperature since they are, within errors, due to fragmentation of CO and O2 in the ion source of the MS. The desorption peak at ∼50 K is mainly due to CO and O2. The second desorption peak at 90 K is due to residual CO2 in the film, although it is possible that CO2 on the unirradiated regions of the substrate contributes to the MS signal at 90 K. The absence of significant O2 at 90 K confirms that the dominant fragmentation route is CO2 → CO + O. We observe a small desorption peak between 60 and 70 K, which we attribute to the release of minor constituents of the film, O3 and CO3.
4. DISCUSSION
4.1. Radiation-induced Reactions
The inelastic energy is deposited by the projectiles in a track of ionizations and electronic excitations. It is then mostly transferred to motion of the CO2 molecule as a whole or to dissociation fragments. After electron–ion recombination, the outcome of the energy deposition by an ion is a distribution of neutral dissociation fragments, the main initial products being CO, C, O, and O2. The main dissociation channel for CO2 is
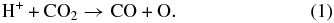
In a dense track, the radicals and molecules can react. An example is the recombination reaction:
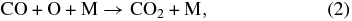
where M represents the matrix. A fraction of the dissociated fragments can escape the matrix cage and be trapped away from the ion track. In the bulk, the O atoms can react with additional CO2 to form CO3 (Moll et al. 1966) or react with another O to form O2. Ozone synthesis can proceed via additive reactions, O + O2 → O3. Within a few monolayers from the surface, electronic excitations leading to atomic and molecular motion can result in sputtering, i.e., the ejection of the atomic and molecular species from the ice (Johnson & Schou 1993). The fluxes of these sputtered fragments are shown in Figure 1.
As irradiation progresses, the film composition changes with new species appearing. These can be converted by an incoming ion:
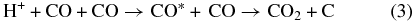

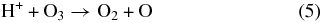
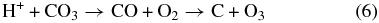
These reactions account for the species observed via infrared (Figure 3) and mass spectroscopy (Figure 1).
4.2. Sputtering: Effect of Fluence at Two Temperatures
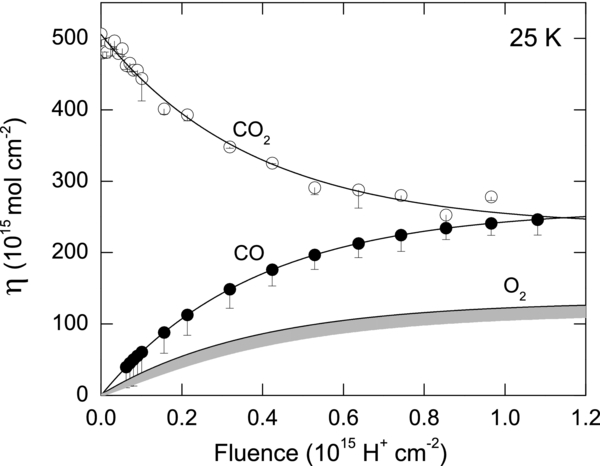
We observed a dramatic change in behavior of the SY with fluence and temperature, as shown in Figure 5. SY changes by <50% from the average of 15 per ion with fluence at 25 K. CO2 is converted mainly into CO and O2, which accumulate in the film with increasing F. These new species, though more volatile than CO2, are stable within the film. The erosion of the film at 25 K is due to loss of molecules only a few monolayers from the ice–vacuum interface.
Figure 5. Change in the column densities of CO2, CO, and O2 with fluence during 100 keV H+ irradiation of an initially pure, 500 ML CO2 film at 25 K. The decrease in CO2 column density is estimated from the ν1 + ν3 absorption band at 3707 cm−1, shown in Figure 1. Estimates of the upper limits of CO and O2 are deduced from conservation of C and O atoms, neglecting mass loss from sputtering, ∼3% of the initial CO2. The error on CO2 and O2 (shaded gray area) is due to the presence of minor species such as CO3 and O3. The lines are guides to the eye.
Download figure:
Standard image High-resolution imageIn contrast, at 50 K, the remarkable increase of the SY with F is due to the escape of the CO and O2 from the penetration depth of the ion, which in our case exceeds the film thickness. As seen in Figure 6, CO and O2 thermally desorb when the temperature exceeds 50 K. The energy deposited by the ion first converts CO2 into CO and O2. These molecules can diffuse along the "hot" ion track, across the thickness of the film, and escape the ice, causing substantial mass loss that depletes 85%–90% of the initial film at the highest F.
Figure 6. Top panel shows the rate of mass loss measured with the microbalance during heating of an initially pure CO2 film at 1 K minute−1, following irradiation to ∼1 × 1015 H+ cm−2 at 25 K. The bottom panel shows mass spectrometer readings of multiple species desorbed that contribute to the mass loss at different temperatures. The first mass loss at ∼50 K is mainly due to CO and O2, while the second peak at 90 K is due to the residual CO2. The small loss at ∼60 K is due to O3 and CO3.
Download figure:
Standard image High-resolution image4.3. Fluence Dependence at 25 K: CO2, CO, and O2
We estimate column densities of CO2, CO, and O2 and their fluence dependence for the case where the film is irradiated at 25 K (Figure 5). We find that the amount of CO2 drops by half at a fluence of ∼1 × 1015 H+ cm−2. The initial slope of the fit to the CO2 data gives a destruction yield of 600 ± 55 CO2 per H+. Each 100 keV proton deposits, on average, 22.7 keV as it traverses the 0.25 μm thick CO2 film (Ziegler & Biersack 2013). Using this energy, we obtain the radiation yield G(−CO2) = 2.6 ± 0.3, defined as the number of CO2 destroyed per 100 eV of energy absorbed. This value is nearly five times larger than those for destruction of CO2 mixed in water ice (1:1) and irradiated with 0.8 MeV H+ and Lyα at 18 K (Gerakines et al. 2000), which suggests that the matrix plays an important role in radiation stability of the CO2.
The decomposition of CO2 leads to the formation of CO and O2. We derive the CO and O2 column densities at 25 K using conservation arguments. At an average SY of 15 (Figure 2), only 3% of the initial CO2 amount is removed by ∼1 × 1015 H+ cm−2. Ignoring this small mass loss and the presence of minor radiolytic products such as CO3 and O3, we estimate the amount of CO and O2 based on conservation of the number of C and O atoms in the film. In a previous study of CO2 film irradiated with 5 keV electrons, only 2% of the O atoms generated from CO2 destruction was estimated to react with additional CO2 to form CO3 (Bennett et al. 2004). The destruction of CO2 should create CO and O2 as per 2CO2 → 2CO + O2. Therefore, CO (F) = CO2 (0) − CO2 (F), and therefore, O2 (F) = 1/2 CO (F). For both CO and O2, these are upper-limit estimates. The errors on the η of CO and O3 are due to the fluence-dependent amounts of CO3 and O3, which is estimated from the small desorption peak between 60 and 65 K in Figure 6.
4.4. Comparison with Previous Studies
Condensed CO2 has been irradiated previously with 1.5 MeV He+ ions at 10 and 70 K (Brown 1982; Johnson et al. 1983). While they reported ejection of CO, O2, and CO2 that increased in steps with temperature, they did not quantitatively determine the contribution of each species to the total flux. As we will show in the discussion, this is important in astronomical applications. Also, while they observed an increase of the SY with fluence at 25 K similar to ours, their 50% increase at 50 K is much lower than the tenfold increase we measured at this temperature.
A recent study where C18O2 films were irradiated with 46 MeV Ni11+ reports a SY of 40,000 molecules per ion at 13 K (Seperuelo Duarte et al. 2009). In addition to CO, CO3, and O3, this study also identified the formation of C3 via infrared absorption at 2039 cm−1. Irradiation of solid CO2 with 5 keV D2+ ions also forms the same species, with the exception of C3 (Ennis et al. 2011).
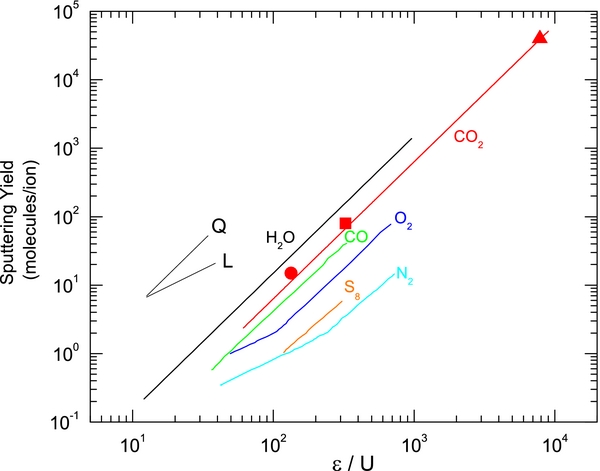
We also observed a previously unknown quadratic dependence of the SY for solid CO2 at low temperatures (<25 K) on ε, the energy deposited by the projectile per monolayer in electronic excitations and ionizations. To place the data in context, we compare it to a compilation of SYs from different reports (Brown 1982; Seperuelo Duarte et al. 2009) using ε from the tables in SRIM 2013 (Ziegler & Biersack 2013). Figure 7 shows the dependence of the CO2 yield on a dimensionless parameter, ε/U, where U is the sublimation energy, along with measurements for other condensed gases (Johnson 1996). We note that the quadratic dependence for CO2 extends to the energies of cosmic rays (Seperuelo Duarte et al. 2009).
Figure 7. The quadratic dependence of sputtering yield for several condensed gases on energy loss per monolayer, ε, normalized to the sublimation energy U. The graph is adapted from Johnson (1996), to which we added the CO2 sputtering below 25 K from this work, circle; Brown (1982), square; and Seperuelo Duarte et al. (2009), triangle. In the regime of dilute excitations that would occur at small dE/dx, linear dependences are observed for O2 and N2. The lines labeled Q and L indicate quadratic and linear dependence.
Download figure:
Standard image High-resolution image5. CONCLUSIONS
Irradiation of pure CO2 films by 100 keV protons leads to destruction of CO2 and synthesis of CO, O2, CO3, and O3. We estimate that ∼2.6 CO2 molecules are destroyed per 100 eV of deposited energy at 25 K. Irradiation can also eject these species from the ice into the gas phase. Our MS analysis shows that CO dominates the sputtered flux, followed by O2, O, and CO2. At 25 K, we measured a SY of ∼15 ± 5. The yield showed a remarkable ∼150-fold increase with fluence at 50 K. We posit that the CO and O2 formed during irradiation escape for the entire thickness of the film at 50 K. In contrast, CO and O2 are retained in the bulk at 25 K. Only the molecules/atoms few monolayers from the surface are ejected, as they are set into motion from the electronic energy deposited by the ions. The CO2 yield also exhibits a quadratic dependence on the electronic energy deposited per unit path length, as previously observed for other condensed gases.
5.1. Astrophysical Implications
CO2 and O2 have recently been detected in the exospheres of Saturnian moons Rhea and Dione. The abundance of O2 is roughly twice that of CO2 at Rhea (Teolis et al. 2010), while they exist in nearly equal proportions at Dione (Teolis & Waite 2012). Previously, a CO2 exosphere was detected at Callisto (Carlson 1999) and O2 exospheres at Europa and Ganymede (Hall et al. 1998). Teolis et al. (2010, and online supplementary material) explained the O2 abundance in Rhea's exosphere using laboratory measurements of radiolysis of water ice (Teolis et al. 2009) and measurements of ion and electron fluxes taken by Cassini (Paranicas et al. 2012; Teolis et al. 2010).
In contrast, the source of the observed CO2 is uncertain. Cruikshank et al. (2010) and Stephan et al. (2012) have suggested that, in the absence of a known endogenic source, the exospheric CO2 is generated by sputtering of surficial CO2 by impact from magnetospheric ions. While Cassini's infrared spectrometer indicates that Rhea's surface is pure water ice (99%) with only trace amounts of CO2 (Stephan et al. 2012), it is possible that surface concentrations are much higher due to segregation, since CO2 is more volatile than water. However, our results show that sputtering should produce about a thousand times more CO than CO2, while CO has not been detected by Teolis et al. (2010). We thus conclude that other processes, such as outgassing of endogenic CO2 or seasonal sublimation of condensed CO2, rather than sputtering, are the sources of the tenuous CO2 exosphere.
This work was supported by NASA's Origins of the Solar System program.