-
PDF
- Split View
-
Views
-
Cite
Cite
Guy W. Neff, Norah J. Shire, Steven M. Rudich, Outcomes among Patients with End-Stage Liver Disease Who Are Coinfected with HIV and Hepatitis C Virus, Clinical Infectious Diseases, Volume 41, Issue Supplement_1, July 2005, Pages S50–S55, https://doi.org/10.1086/429496
Close - Share Icon Share
Abstract
In at-risk populations, shared routes of transmission lead to high rates of concordance between infection with human immunodeficiency virus (HIV) type 1 and hepatitis C virus (HCV). In the era of highly active antiretroviral therapy (HAART), end-stage liver disease (ESLD) has emerged as a leading cause of mortality in coinfected patients. HAART-related toxicities have been implicated, especially when given to patients with viral hepatitis. Rates of response to treatment for HCV infection in coinfected patients continue to lag behind those in monoinfected patients, even with the advent of pegylated interferons. Liver transplantation has been approached with caution in this population because of concern about the sequelae of immunosuppression and HAART-related hepatotoxicity, and results have been conflicting. Clinical and serological markers of ESLD in coinfected patients, management of cirrhosis, and the appropriateness of transplantation are discussed.
Coinfection with hepatitis C virus (HCV) and HIV is common in at-risk populations because of shared routes of transmission. In some high-risk populations, such as injection drug users, rates of concordance between HIV and HCV infection as high as 60%–80% have been observed [1, 2]. In the years before HAART was available, death from opportunistic infections caused by AIDS was the leading concern for HIV-infected patients. The advent of HAART, however, has allowed patients infected with HIV to restore and retain immune function, increasing life span and enabling the emergence of morbidity and mortality due to other causes. Bica et al. [3] demonstrated that, by the late 1990s, end-stage liver disease (ESLD) or cirrhosis was the underlying cause of nearly 50% of deaths observed in HIV-infected populations. Cirrhosis is a serious liver condition characterized by irreversible scarring of the liver that can lead to liver failure and death. Progression to ESLD in HCV-monoinfected patients typically takes 20–30 years after initial infection [4, 5]. With HIV coinfection, this process is accelerated [6, 7]. The mechanisms underlying this process are unclear, although HAART-related hepatotoxicity [8, 9], concomitant alcohol use [9], and immune reconstitution [10] have been implicated.
Rates of response to IFN-based treatment for HCV infection appear to be abrogated in coinfected patients, ranging from 27% to 40%, even with pegylated formulations [11–13]. Given their faster rates of progression and relatively poor rates of response to treatment for HCV infection, coinfected patients with ESLD are now increasingly being considered as candidates for orthotopic liver transplantation in several transplantation centers. Survival results in HIV-infected patients with ESLD, in general, appear promising, although improving survival in patients coinfected with HCV and HIV remains a challenge [14–16]. It has been predicted that the prevalence of coinfected patients with decompensated cirrhosis will increase over the next several years and further strain the limited resources of institutions that perform liver transplantation [14]. Here, we evaluate the medical and ethical aspects of liver transplantation, treatment of cirrhosis in coinfected patients, and the importance of a team approach with the management of liver disease in the population of coinfected patients.
Defining Cirrhosis
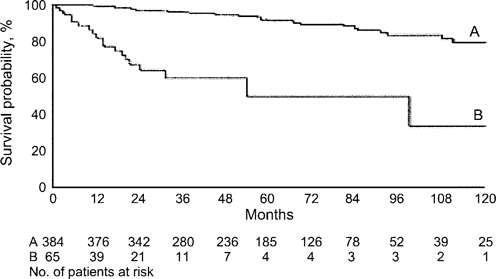
The liver is the largest organ in the body and performs many functions that are critical for survival, such as synthesis of blood clotting factors, creation of proteins necessary for growth and survival of the body, synthesis of bile components, and metabolic processing of most drugs and toxins. These functions are impaired in patients with chronic liver disease, critically so in patients with ESLD. Etiology of chronic liver disease includes hepatotropic viruses, such as HCV, repeated or prolonged exposure to certain drugs and toxins, steatosis, and excessive alcohol use or abuse. Chronic inflammation of the liver can lead to cirrhosis, a stage of organ dysfunction and damage in which scar tissue replaces normal functioning tissue and causes a dramatic, and potentially fatal, decline in liver function. Even in HCV-monoinfected patients, the probability of survival decreases quickly after decompensation (figure 1) [17]. Unattended liver disease, which currently is the 12th leading cause of death in the United States, often progresses without symptoms until end-organ failure, leaving liver transplantation as the only option for survival [18].
Probability of survival after diagnosis in all patients studied with compensated cirrhosis due to infection with hepatitis C virus (curve A) (the 5-year probability rate was 91%) and probability of survival after the appearance of the first major complication of the disease in 65 patients who developed decompensated cirrhosis (curve B) (the 5-year probability rate was 50%).
Despite the option of liver transplantation and the increasing public awareness of organ donation, nearly 50,000 persons die of complications of decompensated liver disease each year. The reasons for this are multifactorial, but the global effect of liver diseas—related morbidity can be expected to reach <$25 billion/year [19]. Clearly, the supply of cadaveric organs for candidates for transplantation with ESLD is insufficient and will not improve in the near future. In the United States, <5500 liver transplantations are performed annually, including those with use of live liver donors. However, the rapid decompensation and extreme muscle wasting seen in coinfected patients often leaves transplantation as their only option.
Detecting Cirrhosis in HIV-Infected Patients
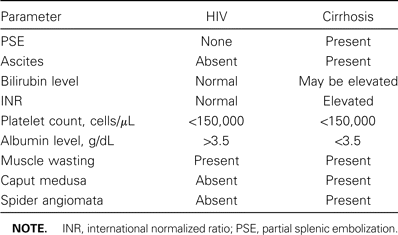
Findings of physical examination for cirrhosis and ESLD can vary widely, depending on the extent of liver disease and the degree of portal hypertension and nutritional depletion. Patients' complaints may vary from subtle findings, such as fatigue and malaise, to extreme muscle wasting and increased abdominal girth. Alternatively, some patients may present with the ominous finding of melena or hematemesis. However, because muscle wasting occurs in HIV-infected patients undergoing HAART, it is important to use laboratory values for the differential diagnosis of cirrhosis if there is reason to suspect liver disease.
Certain laboratory values that are helpful in the diagnosis of cirrhosis include the following: low platelet counts, hypoalbuminemia, increase in international normalized ratio, elevated bilirubin levels, and abnormal liver chemistry results (table 1). Radiographic testing with simple sonography can assist in detecting the nodular pattern typical of cirrhosis, as well as possible lesions. Some patients with cirrhosis have no overt physical signs or symptoms, especially early during the course of the disease. Some patients may have only subtle physical changes, such as red palms, red spots that blanch on their upper body (spider angiomata), muscle wasting, or gynecomastia. Some patients may have only slightly abnormal blood test results, and, in some cases, all blood test results may be normal. In other cases, the diagnosis of cirrhosis must be confirmed via liver biopsy.
Clinical and physical differences in patients with HIV infection versus those with cirrhosis.
The Progression of Cirrhosis
The duration of the advance of cirrhosis from compensated to decompensated is often asked by patients but remains very difficult to predict. Patients with chronic hepatitis C without coinfection tend to progress to ESLD over the course of 20–30 years, whereas coinfected patients have increased rates of progression [6, 7]. Time is of the essence when defining which coinfected patients should undergo transplantation quickly and which can be maintained with treatment. A systematic evaluation of published studies was undertaken to identify factors associated with accelerated progression of fibrosis in patients with chronic HCV infection [20]. Factors independently associated with disease progression included male sex (relative risk [RR], 1.08), elevated serum alanine aminotransferase levels (RR, 1.23), heavy alcohol consumption (RR, 1.61), and histologic analysis demonstrating high-grade necroinflammatory activity. Another review demonstrated a higher overall adjusted RR of histological cirrhosis or decompensated liver disease in subjects coinfected with HIV and HCV than in HCV-monoinfected subjects (figure 2) [28]. The key point is that a greater proportion of coinfected than monoinfected patients is likely to advance to ESLD and that this will occur more quickly in the coinfected population.
Meta-analysis of studies [21–27] examining the adjusted relative risk (RR) of decompensated liver disease or histological cirrhosis in patients with HIV and hepatitis C virus (HCV) coinfection, compared with patients with HCV infection alone. The RR for each study (squares) and 95% confidence index (CI) (bars) are displayed on a logarithmic scale, as well as the combined RR with 95% CI (diamond). The size of the squares is inversely proportional to the variance of the studies. Reprinted with permission from Graham et al. [18].
Management of Cirrhosis
Medical management. The medical management of patients with cirrhosis is based on preventive measures. Diuretics are used to treat ascites in hopes of decreasing the risk of spontaneous bacterial peritonitis. Beta-blockers are prescribed to treat portal hypertension, to decrease the risk of esophageal variceal bleeding. Treatment is begun with lactulose to promote bowel movements, ultimately decreasing the level of ammonia in serum and the risk of hepatic encephalopathy and coma. The medical management also includes the prevention of infection and recognition of cancer, while preparing the patient for the possibility of liver transplantation. Treatment of infection, in particular spontaneous bacterial peritonitis, is with broad-spectrum antibiotics. Spontaneous bacterial peritonitis may be prevented by prophylactic administration of antibiotics weekly.
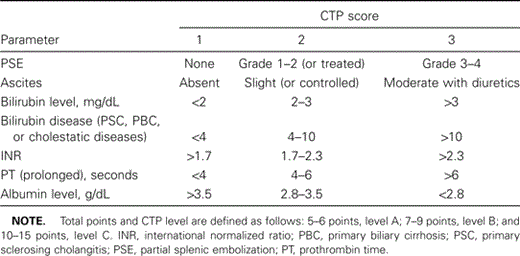
Making the determination of liver transplantation. Over the past 3 years, the United Network for Organ Sharing (UNOS) initiated a new system for the allocation of liver allografts for transplantation in the United States. The previous system was based on the Child-Turcotte-Pugh (CTP) scoring system, [29] initially developed to provide a quantifiable measure of prognosis in patients with cirrhosis undergoing portal shunt procedures. The system defines 3 levels of progressively deteriorating hepatic function as A, B, or C (table 2). The difference between each level is based largely on the ability of the liver to synthesize and metabolize proteins.
In addition, UNOS developed an additional means of prioritizing patients for liver transplantation. The patients in most urgent need of a liver transplantation were considered to be at status level 1. They were defined as those with fulminant hepatic failure with an expected survival of <7 days, or those who recently received a liver allograft in which the hepatic artery thrombosed. UNOS level 2A status was when the CTP score was ⩾10, the patient was located in the intensive care unit, and the patient had an expected survival of <10 days. Level 2B was defined as a CTP score of ⩾10 and mostly encompassed patients with acute or chronic liver disease, whereas level 3 was characterized as having a CTP score of ⩾7 but <9. The benefits of this system were aimed at the potential candidate with marked muscle wasting and subsequent limited reserve (e.g., coinfected or elderly patients). However, because there is a fair element of subjectivity in the determination of the CTP score (definition of ascites and encephalopathy), it was thought that a more robust and rigorous objective means of defining liver disease for patients in need of transplantation was warranted.
On 26 February 2002, a new mathematical model, the Model for End-Stage Liver Disease (MELD), replaced the CTP scoring system to prioritize patients for orthotopic liver transplantation [30]. The change in listing system was mandated by the Department of Health and Human Services. They declared that livers be allocated primarily by severity of liver disease and not by waiting time. Their rationale for abandoning the CTP system and waiting time was based on 2 studies. The first report appeared from the Institute of Medicine [31] and was later reinforced by a study by Freeman and Edwards [32]. Both studies concluded that waiting time was a poor predictor of mortality for patients on the liver transplant waiting list.
Kamath et al. [33] also studied the MELD scoring scheme as a possible prognostic model for mortality on the liver transplant waiting list. They demonstrated that MELD could predict the 3-month mortality in 4 diverse groups of patients with liver disease.
Despite the prognostic ability of the MELD scoring system, concerns remain with regard to coinfected patients. The model does not account for rate of progression of decompensated cirrhosis or muscle wasting, which may give HIV-infected patients lower scores than their condition merits. Moreover our experience in comparing patients with cirrhosis who are coinfected with those who are not is that muscle mass is lost at a faster rate in the coinfected patient population. The consequence is that most coinfected patients lose their muscle reserve while awaiting liver transplantation and either die while on the waiting list or require very a lengthy posttransplantation rehabilitative period. This issue stresses the importance of finding an appeal score for coinfected patients awaiting liver transplantation. The current system, MELD, neglects this cohort of critically ill patients.
Assessments of Liver Transplantation to Date in HIV-Infected Patients
There have been few reports, to date, on the outcomes of orthotopic liver transplantation in the coinfected population, and the results have been conflicting. The Pittsburgh group reported liver transplantation in coinfected patients in 1990 [34]. In that pre-HAART era, transplantation-related complications were numerous, and survival rates were poor. Many of the complications were attributed to the lack of potent immunosuppression, lack of monitoring for opportunistic diseases, and ineffective antiviral therapy against HIV. Several other centers around the world reported smaller series of cases with comparable results.
These initial results prompted the recommendation that coinfection with HCV and HIV in patients with ESLD be a contraindication for liver transplantation. Despite these recommendations, the UNOS registry reveals that 40 orthotopic liver transplantations were performed in HIV-positive recipients between 1998 and 2002. Reports from the University of Pittsburgh and the University of Miami in the HAART era reveal a marked improvement in survival, considerably fewer issues with posttransplantation morbidity, and a better appreciation of complex drug interactions with the HAART regimens [35]. These results suggested that HIV should no longer be considered an absolute contraindication for orthotopic liver transplantation in coinfected patients.
The Pittsburgh group has the most experience to date in orthotopic liver transplantation in coinfected patients, with 26 patients over an 8-year period [15]. They report that 1 survivor is nearly 6 years removed from time of transplantation and that the 1-year patient survival rate (85%) is very similar to that among non-HIV-infected patients. However, the King's College group reported very poor graft and patient survival among patients coinfected with HCV and HIV [36]. It should be noted that survival rates among HCV-monoinfected transplant recipients also differ between centers [14]. An important point of difference may be the different antiviral therapies used to treat recurrence of HCV among the various centers.
A multicenter, National Institutes of Healt—sponsored trial led by the University of California, San Francisco, transplantation team is investigating treatment outcomes and survival in coinfected patients undergoing orthotopic liver transplantation or kidney transplantation. This trial has several objectives, including proving safety and efficacy of liver transplantation for coinfected patients with ESLD. The study anticipates enrolling 150 kidney transplant recipients and 125 liver transplant recipients over the course of 3 years, with 2–5 years of follow-up. It is hoped that this ambitious study will offer a clearer perspective on the outcomes that should be expected when coinfected patients undergo transplantations and will give important insights as to how HAART regimens interact with immunosuppressive medications.
Arguments for and Against Liver Transplantation in Coinfected Patients
For nearly all forms of liver disease, orthotopic liver transplantation is an accepted mode of therapy. Nearly 5500 liver transplantations are performed annually in the United States, and 10,300 are performed worldwide. The increase in survival rates over the past 2 decades is attributed to success with the immunosuppressive protocols, much like the successes that have been seen in HIV disease, as a result of the potent antiretroviral regimens. One argument against liver transplantation in coinfected patients is that long-term survival has yet to be defined, and the outcome is uncertain. However, preliminary results suggest that this argument is not always true, because survival is comparable in all groups except patients infected with HCV [15, 35]. Another issue lies in the potential exacerbation of HIV, with the introduction of calcineurin inhibitors for immunosuppression after transplantation. Yet, in all of the reports to date, recurrence of HIV has had a minor role in survival outcomes. It is true that organs for donation are scarce, and the requests for liver transplantation are expected to increase by <500% over the next decade, making this resource even more scarce. However, this would be the case regardless of whether coinfected patients are in the recipient pool. The remaining challenge of reimbursement has recently been overcome in a legal challenge to Medicare. A request for approval for payment was granted in favor of HIV-infected patients, deeming liver transplantation as nonexperimental in this population.
From the surgical perspective, concerns arise as to the risk to the surgical team in doing complex surgical procedures for patients with known HIV infection. There are obvious risks to the surgeons, anesthesiologists, and the many operating room staff who participate in liver replacement therapy. However, it is well known that intraoperative transmission of HCV is far greater than that of HIV. There are also many more patients with HCV infection than with HIV infection who undergo surgery. In addition, those patients with HIV infection who undergo liver transplantation are known to have a nondetectable HIV load, as opposed to other at-risk populations, in whom the virus load is either unknown or much greater than undetectable. Overall, the risk of needlestick or other transmission of HIV during a liver transplantation is minimal and certainly less than the risk of transmission to health care workers in any busy trauma center.
In conclusion, as immunosuppression protocols, antiretroviral treatments, and diagnostic tests for cirrhosis improve, the outlook for transplantation in coinfected patients can only improve as well. A multidisciplinary approach involving hepatologists, HIV specialists, and surgeons in the clinical management of coinfected patients undergoing liver transplantation will help prevent unnecessary drug interactions. This setting revealed improved long-term posttransplantation survival, as has been shown at the University of Miami [14]. Future studies will be required to assess quality of life and long-term outcomes in coinfected transplant recipients.
Acknowledgments
Potential conflicts of interest. All authors: no conflicts.
References
- hiv
- antiretroviral therapy, highly active
- liver cirrhosis
- end stage liver disease
- hepatitis c
- interferons
- liver transplantation
- therapeutic immunosuppression
- natural immunosuppression
- infections
- mortality
- transplantation
- viral hepatitis
- hiv infections
- hepatotoxicity
- toxic effect
- hepatitis c virus
- appropriateness




![Meta-analysis of studies [21–27] examining the adjusted relative risk (RR) of decompensated liver disease or histological cirrhosis in patients with HIV and hepatitis C virus (HCV) coinfection, compared with patients with HCV infection alone. The RR for each study (squares) and 95% confidence index (CI) (bars) are displayed on a logarithmic scale, as well as the combined RR with 95% CI (diamond). The size of the squares is inversely proportional to the variance of the studies. Reprinted with permission from Graham et al. [18].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/41/Supplement_1/10.1086_429496/1/m_41-Supplement_1-S50-fig002.gif?Expires=1716491989&Signature=qZYqSgM-Jcx4uPAr3njOo2hlBEOqBLpmuDjBOifSkUb~Okw5iZYRNTjv6mVFmK4j2pBKYiD4X0RgrjrJA8sTf5yHnkXjDeNigJIjnsH0dG2OopDx7urgIYwPduCxPgRpBN4ptUFsaH1a8qyPapBslIOSRavujrAE5GCVn2ZI1A9lCJk8r7Hf12qS6OygxiXNgmP5IlCTETt-5BrKxB4PGgOYcgeqx3hiSlvYr1FCTu9AebGqx9JXzPVV5zQbxTtBvCEFhWIVZq-4uae6Ieu3ZNb1n4QdRed5S-2CwHGOwBLJj53W5xOnn-aK3pMe1ET6Wh6AnuJGZ5v0cMSe1fFpJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments