-
PDF
- Split View
-
Views
-
Cite
Cite
Elisabet Josefsson, Orla Hartford, Louise O’Brien, Joseph M. Patti, Timothy Foster, Protection against Experimental Staphylococcus aureus Arthritis by Vaccination with Clumping Factor A, a Novel Virulence Determinant, The Journal of Infectious Diseases, Volume 184, Issue 12, 15 December 2001, Pages 1572–1580, https://doi.org/10.1086/324430
Close - Share Icon Share
Abstract
The importance of the fibrinogen-binding adhesin clumping factor A (ClfA) in the pathogenesis of Staphylococcus aureus septic arthritis was examined in an animal model. The protective effect of active and passive immunization with ClfA also was investigated in S. aureus infection models. The severity of arthritis was markedly reduced in mice challenged intravenously with a clfA mutant, compared with mice infected with the wild-type strain. Mice immunized with recombinant ClfA and challenged with S. aureus developed less-severe arthritis than did mice immunized with a control antigen. Passive immunization of mice with rat and rabbit anti-ClfA antibodies protected against S. aureus arthritis and sepsis-induced death, indicating that the protection by active immunization is antibody mediated. Taken together, these data strongly suggest that ClfA is a crucial virulence determinant for septic arthritis and an excellent target for the generation of immune therapies directed against S. aureus
Staphylococcus aureus is an important pathogen that causes diverse infections, ranging from superficial skin infections to invasive and potentially life-threatening infections, such as endocarditis, osteomyelitis, and septic arthritis [1]. S. aureus is the predominant organism that causes septic arthritis, a dangerous condition that often causes persistent damage to the joints and has a high mortality rate [2]. Septic arthritis is triggered by the hematogenous spread of bacteria from an initial nidus to the joint spaces. We studied the pathogenesis of S. aureus septic arthritis in a murine model in which bacteria are injected intravenously (iv), spread hematogenously, seed to the joints, and within 24 h cause a disease that clinically resembles septic arthritis in humans [3]
S. aureus can express several cell wall–associated proteins that bind to host tissue or plasma components and promote colonization and evasion of defenses [4]. The collagen adhesin is implicated in the development of septic arthritis, but the mechanism has not been elucidated [5]. Clumping factor A (ClfA) is a surface protein that binds to fibrinogen and fibrin [6]. ClfA should not be confused with cell-bound coagulase [7]. Both ClfA and coagulase bind fibrinogen, but they are completely distinct molecules. ClfA promotes clumping of bacterial cells in plasma and adherence of bacteria to blood clots, to plasma-conditioned biomaterials, and to catheter-damaged heart valves in a rat model of endocarditis [6, 8, 9]. Thus, ClfA likely plays an important role in the ability of S. aureus to cause disease
The fibrinogen-binding domain of ClfA comprises 520 residues (the A domain, residues 40–559) [6]. This is followed by region R, which is composed mainly of serine-aspartate dipeptide repeats. This region is required for correct surface display of the ligand-binding A domain on the cell surface [10]. The C-terminus of the protein contains amino acids required for covalent linkage to the cell wall
Attempts to produce killed whole-cell vaccines for S. aureus infections have been unsuccessful [11]. In contrast, there are reports of successful protection of animals in experimental infections with vaccines containing purified surface components or inactivated toxins. Recently, a recombinant fragment of the collagen-binding protein protected against sepsis-induced mortality in mice [12]. The fibronectin-binding protein provided protection in models of mastitis and endocarditis [13, 14]. The in vivo–expressed surface polysaccharide product of the ica locus of Staphylococcus epidermidis and S.aureus protected mice from S. aureus–induced metastatic kidney infection and mortality [15]. A vaccine containingtype 5 polysaccharide protected against a homologous S. aureus challenge in a murine model [16]. A mutant form of enterotoxin A lacking superantigenicity protected against septic death in mice [17]. Similarly, passive immunization with antiserum to an inactivated form of the α-toxin protected from lethal infectionin mice [18]
In this study, we investigated the role of ClfA, which is expressed by virtually all S. aureus strains, as a virulence factor for inducing septic arthritis. We also investigated the potential of recombinant (r) ClfA A to act as a protective antigen in S. aureus infection models by both active and passive immunization
Materials and Methods
MiceFemale NMRI mice (obtained from B&K Universal) were maintained in the animal facility of the Department of Rheumatology, University of Göteborg. They were housed 10 to a cage on a 12-h light-dark cycle and were fed standard laboratory chow and water ad libitum. The animals were 7–9 weeks old at the start of the experiments. For the studies that used S. aureus strain 601, female BALB/c mice (5–6 weeks old) or Swiss webster mice were purchased from Taconic Quality Laboratory Animals and Services for Research
Bacterial strains.S. aureus strain Newman [19] and the clfA2::Tn917 mutant DU5876 [6], which carries a large deletion of the clfA gene adjacent to the transposon insertion, were studied along with strains DU5898 (clfA2::Tn917 geh::pCF16) and DU5899 (clfA2::Tn917 geh::pCL84), which carry a single copy of the clfA+ gene integrated into the geh gene and the empty vector pCL84, respectively [6], in infection studies. We used mouse isolate strain S. aureus LS-1 [3] in an immunization experiment. Bacteria were grown on blood agar plates for 48 h, were harvested, and were kept frozen at −20°C in PBS containing 5% bovine serum albumin (BSA) and 10% dimethyl sulfoxide
For some of the active and passive immunization studies, S.aureusstrain 601 [20] was obtained from M. S. Smeltzer (University of Arkansas for Medical Sciences). The strain was isolated from an intensive care unit and is resistant to cephalothin, ciprofloxacin, clindamycin, erythromycin, oxacillin (methicillin), penicillin G, and trimethoprim-sulfamethoxazole. Strain 601 cells were inoculated into a 10-mL brain-heart infusion (BHI) broth culture grown overnight at 37°C. Cells from the overnight culture were diluted 1:100 in BHI broth and were grown at 37°C until absorbance at 600 nm reached 1.8–2.0 OD units. The bacterial pellet was resuspended in 1:5 volume of freezing medium (as above) and was stored at −86°C until use
Before injection into animals, the bacterial suspensions were thawed, were washed in PBS, and were adjusted to appropriate cell concentrations. Mice were inoculated in the tail vein with 0.1–0.2mL of bacterial suspension. We measured the number of viable bacteria in conjunction with each challenge by dilution, spreading on blood agar dishes, and colony counting
Expression and purification of recombinant proteinsVector pCF40 expressed rClfA truncate rClfA40-559 [21]. The protein was expressed and purified as described elsewhere [21, 22]
Experimental protocols for clfA mutant infection studiesIn the first experiment, we injected 12–15 mice per group iv in the tail veinwith 1.4×107 cfu/mouse of Newman wild-type strain, 1.5×107 cfu/mouse of DU5876, 1.6×107 cfu/mouse of DU5898, and 1.9×107 cfu/mouse of DU5899 (all in 0.2 mL of PBS). The mice were weighed regularly and were examined for arthritis and generalappearance until death by cervical dislocation 14 days after challenge. In the second experiment, 15 mice per group received 1.0×107 cfu/mouse of wild-type Newman or 1.5×107 cfu/mouse of the clfA mutant DU5876. The experiment was terminated on day 22. One pair of limbs (fore and hind) was used for histopathologic examination; the other pair of paws and kidneys was examined for bacterial infection. In a third experiment, 10 mice per group were injected in the knee joint with either 3.6×104 cfu/mouse of Newman wild-type strain or 3.0×104 cfu/mouse of the clfA mutant DU5876 in 20 μL of PBS. The mice were killed 3 days after inoculation, and knees were collected for histopathologic examination
Active immunizationPurified rClfA region A, comprising residues 40–559 (rClfA40-559), and BSA (Sigma Chemical) were dissolved in physiologic saline and were emulsified 1:1 in Freund’s complete adjuvant (Difco Laboratories). In total, 200 μL of the emulsion containing 30 μg of protein was injected subcutaneously (sc) on day −21 (n=15/group). Booster immunization with 30 μg of protein in physiologic saline:Freund’s incomplete adjuvant 1:1 sc was done on day −10. On day 0, the mice were challenged iv with 1.6×107 cfu/mouse of wild-type Newman. In the secondactive immunization experiment, mice were immunized as described above with rClfA40-559 or BSA (n=15) and were challenged iv with 3.9×107 cfu/mouse of strain Newman. At the time of death, bacterial cultures from kidneys and a pair of joints were obtained, while the other pair of limbs was examined histopathologically. In the third and fourth active immunization experiments, mice immunized as above were challenged iv with 3.4×108 cfu/mouse of S. aureus strain 601 (n=9–10) or 4×106 cfu/mouse of strain LS-1 (n=15), respectively
Passive immunizationTo generate anti-ClfA antibodies and control antibodies, rats were immunized with rClfA40-559 and BSA (n=5). The rats were immunized as described above at day 0, were booster immunized at days 14 and 25, and were bled by heart puncture on day 38. Serum samples were pooled, and the IgG fraction was obtained by precipitation with a saturated ammonium sulfate solution followed by extensive dialysis against PBS. The concentration of immunoglobulins in the precipitate was determined by single radial immunodiffusion. Naive mice were passively immunized intraperitoneally (ip) on day −1 with 18 or 20 mg of the immunoglobulin fraction containing antibodies specific for rClfA40-559 and BSA, respectively (n=15). On day 0, the mice were challenged iv with 1.4×107 cfu/mouse of strain Newman. The development of arthritis was followed for 10 days
Naive mice were passively immunized ip with 10 mg of polyclonal rabbit anti-ClfA IgG or 10 mg of normal polyclonal rabbit IgGon day −1 (n=11). On day 0, the mice were infected with 3.4×108 cfu/mouse of S. aureus 601
ClfA40-559 antibody-enriched human IgG was purified from 7donors with elevated levels of IgG specific for the A domain (40–559) of the ClfA, as determined by ELISA (>5-fold increase in titer, compared with normal intravenous immunoglobulin; Polygam; Baxter Healthcare). Plasma was obtained from Serologicals, and the IgG was purified by Cangene (Winnipeg, Canada) as a sterile-filtered solution in 10% maltose and 0.03% polysorbate 80. By radial immunodiffusion, Cangene found that the material contained 47.55 mg/mL IgG
A sterile freeze-dried preparation of normal human IgG was used as a negative control. When reconstituted in sterile water, the material contained 45 mg/mL IgG. On day −1, mice were passively immunized ip with 20 mg of human IgG enriched for ClfA40-559 antibodies or normal human IgG (n=18–19). On day 0, 2.2 × 108 cfu/mouse of S. aureus 601 in 0.1 mL of PBS was administered in the tail vein
Clinical evaluation of arthritisClinical arthritis was evaluated blindly by a neutral observer. All mice were examined individually, and limbs were inspected visually. The inspection yielded a score of 0–3 points for each limb (0, normal appearance; 1, mild arthritis; 2, moderate arthritis; or 3, marked arthritis). Arthritis was defined as visible erythema and/or swelling of ⩾1 joint. The arthritic index was constructed by adding the scores from all 4 limbs for each animal [23]
Histopathologic examinationHistologic examination of joints was done after fixation, decalcification, and embedding in paraffin. Tissue sections from upper extremities (elbow, wrist, and carpal joints) and lower extremities (knee, ankle, and tarsal joints) were stained with hematoxylin-eosin. The joints were examined for synovitis (defined as synovial membrane thickness of >2 cell layers and infiltration of inflammatory cells to the extra-articular space) and cartilage and/or bone destruction. Microscopic inspection yielded a score for each joint with regard for synovitis as follows: 0, no synovitis; 1, mild synovial hypertrophy; 2, moderate synovial hypertrophy and infiltration of inflammatory cells; or 3, marked synovial hypertrophy and infiltration of inflammatory cells. Cartilage and/or bone destruction was scored as 0, no destruction; 1, mild destruction; or 2, marked destruction. The total histologic index was calculated by adding all scores for each animal. All evaluations were done on coded slides. The method was modified from one described elsewhere [24]
Bacteriologic examination of infected animalsOne pair of joints (ankle and wrist) was dissected, swabbed, and streaked on Staphylococcus medium 110 agar plates (BBL, Becton Dickinson). The joint area was considered to be positive for S. aureus when ⩾10 colonies grew. The kidneys were removed, homogenized, and diluted in PBS, and 100 μL was inoculated on blood agar plates. Colonies recovered from joints and kidneys were tested for catalase and coagulase activity. Colonies were scored for antibiotic resistance, to ensure stability of markers and to exclude contamination
Specific antibodiesSerum samples from actively immunized mice were obtained 10 days after the last immunization (before bacterial challenge) and after 13–14 days of infection. The serum level of specific antibodies against rClfA40-559 was measured by ELISA. Microplates (96-well; Nunc) were coated overnight at 4°C with rClfA40-559 (4.6 μg/mL) in physiologic saline. The plates were blocked with 0.5% ovalbumin (Sigma) in 0.05 M Tris (pH 7.4). Serum samples, biotinylated antibodies, and ExtrAvidin-peroxidasewere all diluted in 0.05 M Tris (pH 7.4) and 0.015 M NaCl. The plates were incubated with serum for 2 h, were washed, and were incubated stepwise with biotinylated goat anti–mouse IgG antibody (0.3 μg/mL; Jackson ImmunoResearch Laboratories), ExtrAvidine-peroxidase (0.5 μg/mL), and ABTS substrate (both from Sigma). Absorbance at 405 nm was measured in a Spectra Max Plus photometer (Molecular Devices). As a control antigen, we used influenza vaccine (Merieux Flu Vaccine) diluted 1:250. All serum samples were diluted 1:6000, and antibody response was monitored as absorbance
Statistical analysisStatistical evaluation was done by Mann-Whitney U test or Kaplan-Meier analysis. We used the Bonferroni method to adjust the significance level when multiple tests were performed in the same experiment. P<.05 was considered to be significant. Scores are reported as medians and quartiles or interquartile range, and numeric continuous data are reported as mean±SEM
Results
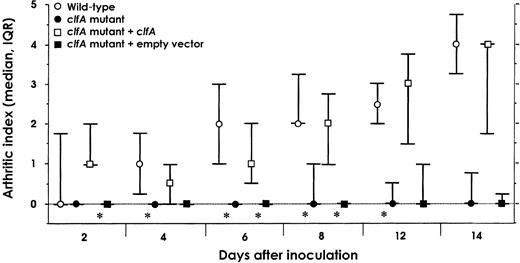
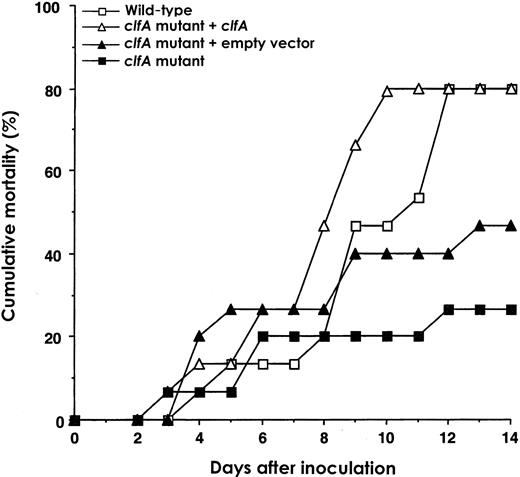
Infection with S. aureus Newman and with a clfA isogenic mutantTo determine whether ClfA is a virulence determinant in a mouse S. aureus–mediated septic arthritis model, we compared the arthrogenicity of S. aureus Newman and a clfA-negative mutant. Mice were inoculated with 1.4–1.9×107 cfu/mouse of S. aureus Newman wild-type strain and strains DU5876, DU5898, and DU5899. The wild-type strain gave rise to strikingly more severe arthritis than did the clfA mutant DU5876 strain. The differences in arthritis development in these groups were statistically significant at most time points during the experiment. The presence of a single copy of the wild-type clfA gene in the complemented clfA mutant DU5898 restored arthrogenicity to that of the wild type. The control clfA mutant DU5899 carries the empty vector plasmid pCL84. In mice injected with this empty vector control mutant, the degree of arthritis was similar to that in the mice challenged with clfA mutant DU5876. There were statistically significant differences in arthrogenicity between the groups that received the complemented mutant and the empty vector control mutant (figure 1). In addition, mortality rate was lower in mice infected with the clfA-negative mutants than in those infected with the clfA-positive strains (figure 2). In accordance with the mortality data, mice infected with the wild-type strain and the complemented mutant lost more weight than those inoculated with clfA-negative mutants (data not shown)
Development of arthritis in NMRI mice intravenously inoculated with 1.4–1.9×107 cfu/mouse of Staphylococcus aureus wild-type strain Newman, clfA mutant DU5876 (clfA), clfA complemented mutant strain DU5898 (clfA mutant + clfA) or clfA mutant with vector plasmid strain DU5899 (clfA mutant + empty vector) (experiment 1). Circles and squares medians; whiskers interquartile ranges (IQRs). At the start of the experiment, n=15/group, except for strain DU5899 (n=12). Data were analyzed by Mann-Whitney U test. Two sets of statistical tests were performed: wild-type– vs. DU5876-infected mice and DU5898- vs. DU5899-infected mice. For each test, significance was set at P=.004, by Bonferroni adjustment, with simultaneous significance set at P=.05. *P<.004
Cumulative mortality in NMRI mice of experiment 1 after intravenous inoculation with 1.4–1.9×107 cfu/mouse of Staphylococcus aureus wild-type strain Newman, strain DU5876 (clfA) strain DU5898 (clfA mutant + clfA) or strain DU5899 (clfA mutant + empty vector). At the start of the experiment, n=12–15
These results were repeated in the next experiment. Two groups of mice were injected with 1.0×107 cfu/mouse of wild-type S. aureus Newman or 1.5×107 cfu/mouse of the clfA mutant DU5876. The severity of arthritis was significantly lower in the clfA mutant group during the first 10 days of the experiment (data not shown); however, there were no obvious differences in mortality rates between mice challenged with the wild-type strain and clfA mutant (data not shown). The decrease in body weight was somewhat more pronounced in the clfA mutant group (data not shown). These 2 experiments strongly suggest that ClfA is an important virulence factor for septic arthritis
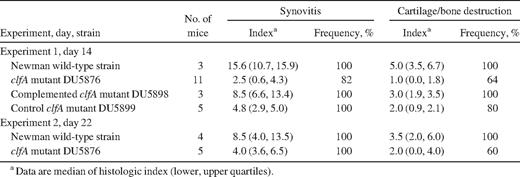
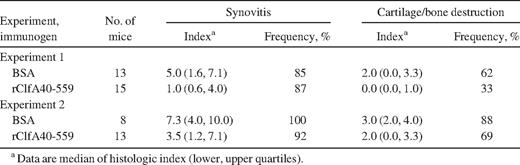
In addition to the clinically visual signs of virulence,histopathologic examinations of joints showed clfA-positive strains to be more virulent. The severity of synovitis was much higher, and the extent of cartilage and/or bone destruction was more severe in mice inoculated with clfA-positive strains than in those inoculated with the clfA-negative strains in both experiments (table 1). In general, the frequency of synovitis and joint destruction was lower in mice inoculated with clfA-negative strains than in mice infected with the clfA-positive strains, which all displayed synovitis and joint destruction. Consequently, the overall importance of clfA carriage for thedevelopment of septic arthritis is evident not only clinically but also histologically, because its presence aggravates synovitis and joint destruction
Severity and frequency of histologic joint affection in mice inoculated with wild-type, clfA mutant, or clfA complementing Staphylococcus aureus strains.
Joint areas and kidneys were assessed for the presence of S. aureus. In the second experiment, 100% of the mice injected with the wild-type strain had positive staphylococcal growth in the joint areas, whereas none of the mice injected with the clfA mutant showed positive growth (n=4 and 5, respectively). The same trend was seen in the first experiment with positive cultures obtained from the joint areas in 67% and 33% of mice infected with the clfA-positive wild type and strain DU5898, and in 18% and 20% of mice challenged with the clfA-negative mutants DU5876 and DU5899, respectively. However, there was no such correlation among the numbers of bacteria in the kidneys of the different groups in any experiments (data not shown). Accordingly, clfA-negative strains might be hampered in seeding to joints and in surviving there
Direct bacterial inoculation of jointsNext we wanted to learn whether ClfA-positive and -negative strains differ in their ability to induce an inflammatory response in a joint. To avoid the possibility that the clfA mutant might have a reduced ability to survive in the in bloodstream and/or to penetrate the wall of blood vessels, similar amounts of the wild-type strain Newman or the clfA mutant DU5876 were injected directly into the knee joints of mice. Three days later, the joints were examined histologically. In knee joints injected with the clfA mutant, there was either no inflammation or only mild signs of inflammation, whereas all joints injected with the wild-type strain displayed arthritis. The synovitis histologic index was 1.9±0.3 for joints injected with the wild type but only 0.6±0.3 for joints injected with the clfA mutant (P=.006). There were no signs of bone or cartilage destruction in any joints. Thus, the ClfA-positive strain was more inflammatory in the joint, supporting the notion that ClfA promotes joint colonization and/or survival
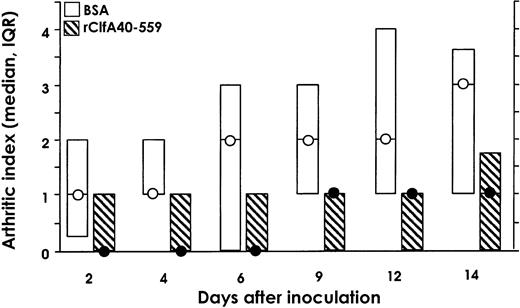
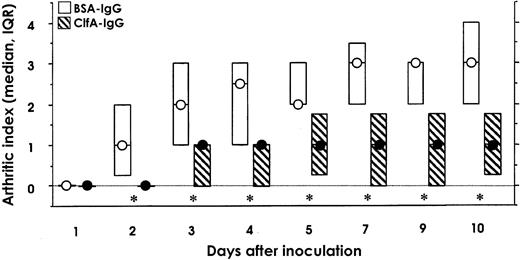
Active immunizationSince ClfA is an important virulence factor in septic arthritis, a ClfA-specific immune response might obstruct the development of this infection. To study this concept, mice were vaccinated sc twice with purified rClfA40-559 or BSA and were challenged with 1.6×107 cfu/mouse of S.aureus Newman. Mice immunized with rClfA40-559 had less severe arthritis than did the control BSA-immunized group throughout the experiment. Mice immunized with rClfA40-559 had a significantly lower level of arthritis (P=.016–.038), except at day 4 (P=.052), for tests of a single time point but not when all time points were tested. The latter requires P values of .008 (figure 3). The decrease in body weight was smaller in the group immunized with rClfA40-559 than in the BSA-immunized group (data not shown). We terminated the experiment at day 14, and paws were analyzed histologically. Both the severity of synovitis and the extent of joint destruction were markedly less pronounced in mice immunized with rClfA40-559 than in the BSA control group (table 2)
Development of arthritis in NMRI mice immunized with bovine serum albumin (BSA) or rClfA40-559 before intravenous inoculation with 1.6×107 cfu/mouse of wild-type strain Newman. Circles medians; boxes interquartile ranges (IQRs; n=15). Data were analyzed by Mann-Whitney U test. For each test, significance was set at P=.008, by Bonferroni adjustment, with simultaneous significance set at P=.05. If a single time point is tested, P=.033, P=.052, P=.038, P=.016, P=.033, and P=.022, respectively, for each time point on the X-axis
Severity and frequency of histologic joint affection after Staphylococcus aureus strain Newman inoculation of mice immunized with bovine serum albumin (BSA) or rClfA40-559.
To further investigate the vaccinating effect of rClfA40-559, the experiment was repeated with a larger bacterial inoculum. Mice were vaccinated with rClfA40-559 or BSA and were challenged with 3.9×107 cfu/mouse of S. aureus Newman. Compared with the first immunization experiment, the increased bacterial challenge produced a noticeable increase in the arthritic response in all infected animals. Of interest, arthritis remained suppressed in mice immunized with rClfA40-559 (data not shown). Histologic analysis of the paws supported the clinical findings. The severity of synovitis was ameliorated by immunization with rClfA40-559 (table 2). There was also less cartilage and bone destruction and lower mortality in the rClfA40-559–immunized mice. Thirteen days after challenge, 2 (13%) of 15 mice were dead in the rClfA40-559 group, compared with 7 (47%) of 15 in the control group. In addition, the control mice had significantly decreased body weight, compared with those immunized with rClfA40-559 (for each test, significance was set at P=.0056; P<.0056 throughout the experiment, except at day 9; data not shown). Taken together, these data show clearly that active immunization with the ClfA A region protects against septic arthritis and mitigates weight reduction during infection
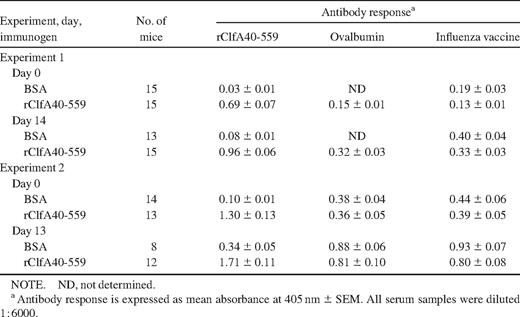
Specific antibodies to rClfA40-559 were detected in serum samples from rClfA40-559–immunized mice in the active immunization experiments on the day of bacterial challenge, and even higher levels were seen after 2 weeks of infection (table 3). The rClfA40-559 antibody response was much stronger than the antibody response to the blocking antigen ovalbumin or to the control antigen influenza vaccine. This shows that rClfA40-559 immunization evokes production of rClfA40-559–specific antibodies and that infection with S. aureus stimulates further production. However, BSA-immunized animals never responded with specific ClfA A region antibodies: the antibody response to rClfA40-559 did not exceed the antibody response to the blocking antigen or to the control antigen. Nevertheless, there was an increase in rClfA40-559 antibody titers from the day of bacterial inoculation to 13 days later, and a corresponding increase was seen in the antibody response to the blocking antigen and control antigen. This increase in antibody response was most likely due to a polyclonal B cell activation. In addition, wild-type–infected mice did not produce specific ClfA40-559 antibodies, nor did clfA mutant–challenged mice (experiments 1 and 2; data not shown). In summary, rClfA40-559 immunization induced rClfA40-559 antibody production, whereas S. aureus infection in mice without preceding rClfA immunization produced no specific antibody production to the ClfA A region. The bacterial load in kidneys and joints was measured in the 2 active immunization experiments, but there were no significant differences in the yields between the groups (data not shown)
Antibody responses to rClfA40-559, ovalbumin, and the control antigen influenza vaccine in serum samples of mice immunized with bovine serum albumin (BSA) or rClfA40-559, before and 2 weeks after infection with Staphylococcus aureus strain Newman.
To investigate whether vaccination with rClfA40-559 could also protect against infection by an S. aureus strain other than that from which the rClfA was derived, we used the methicillin-resistant S. aureus (MRSA) strain 601 and the arthritis-inducing mouse isolate LS-1. Mice vaccinated with rClfA40-559 and control mice were infected with 3.4×108 cfu of MRSA 601/mouse, and survival was studied over 5 days. The mortality was reduced by rClfA vaccination, when only 1 in 9 rClfA-vaccinated mice died, in contrast to 5 of 10 control vaccinated mice, although the difference was not statistically significant (P=.06, Kaplan-Meier survival analysis)
Finally, mice vaccinated with rClfA40-559 or BSA were infected with 4×106 cfu of strain LS-1/mouse (n=15/group). We followed arthritis and weight for 12 days. rClfA-vaccinated mice and control mice responded similarly to the LS-1 infection for both arthritis and weight development (data not shown). Accordingly, vaccination with rClfA40-559 from strain Newman was not able to significantly protect against infection induced by all other investigated S. aureus strains
Passive immunizationPassive immunization was performed to verify that antibodies recognizing ClfA mediate protection against arthritis. Rats were immunized with either rClfA40-559 or BSA, serum samples were pooled, and the IgG fraction from the pooled serum samples was administered ip to naive mice. The day after the passive immunization, the mice were challenged with 1.4×107 cfu/mouse of S. aureus Newman. The development of arthritis was significantly suppressed throughout the study in the group treated with anti–rClfA40-559 immunoglobulin, compared with the control anti-BSA immunoglobulin-treated group (P<.006; figure 4). The decrease in body weight was smaller in mice treated with anti–rClfA40-559 immunoglobulin (data not shown). The data clearly suggest that anti-ClfA antibodies, either transferred or elicited by active immunization, can protect mice against S. aureus–induced arthritis
Development of arthritis in NMRI mice passively immunized with rat antibodies specific for bovine serum albumin (BSA-IgG) or rClfA40-559 (ClfA-IgG) 1 day before intravenous inoculation with 1.4×107 cfu/mouse of wild-type strain Newman. Circles medians; boxes interquartile ranges (IQRs; n=15). Data were analyzed by Mann-Whitney U test. For each test, significance was set at P=.006, and simultaneous significance was set at P=.05. *P<.006
The next experiment assessed whether passive immunization with antibodies raised against rClfA40-559 derived from strain Newman also could protect against infection induced by another S. aureus strain. Mice were passively immunized with rabbit polyclonal anti–ClfA IgG or normal rabbit polyclonal IgG. The next day the mice were challenged with 3.4×108 cfu/mouse of MRSA strain 601. Administration of anti-ClfA antibodies manifestly protected from septic death: 1 of 11 mice died in 5 days, compared with 6 of 11 that were treated with normal IgG (P=.07, Kaplan-Meier survival analysis). This experiment strongly indicates that anti-ClfA antibodies are also protective against S. aureus strains other than that from which the rClfA was derived
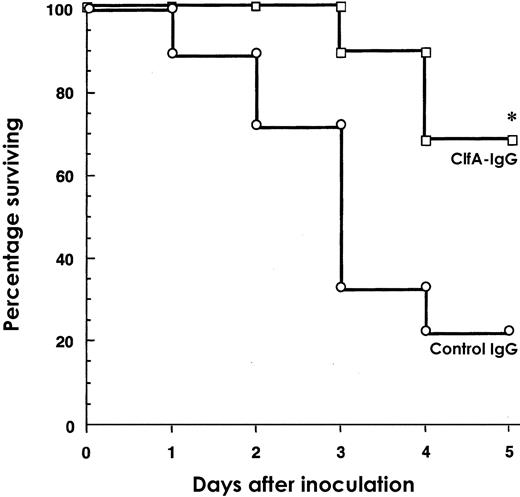
To evaluate whether human IgG containing elevated levels of IgG specific for ClfA could protect mice against MRSA septic death, mice were pretreated by ip injection with normal human IgG or a high titer S. aureus ClfA40-559–specific IgG. At 24 h after IgG administration, the mice were challenged with MRSA strain 601. The mice were followed-up for 5days. Significant differences in the relative survival times between treatment groups were detected. Of the mice given ClfA-specific IgG, 63% survived the bacterial challenge (P<.003; figure 5). In contrast, only 22% of the mice treated with the control IgG survived the study period. These results clearly indicate that prophylactic administration of a ClfA high-titer IgG provides a significant level of protection against lethal infection, compared with a commercial normal human IgG product
Survival in NMRI mice passively immunized with human antibodies specific for ClfA40-559 (ClfA-IgG) or control IgG 1 day before intravenous challenge with 2.2×108 cfu/mouse of Staphylococcus aureus 601(n=18 or 19). Statistical evaluation was done by Kaplan-Meier analysis. *P=.003
Discussion
In this study, we show that the clumping factor ClfA is an important virulence determinant in septic arthritis caused by S. aureus Newman. In addition, immunization with purified rClfA region A protected against staphylococcus-mediated arthritis, suggesting the possibility that this antigen could be used in the development of new immunotherapeutics. Furthermore, the protective effect of immunization with ClfA is at least partly antibody mediated. We believe that our study is the first to show that vaccination protects against S. aureus arthritis. Finally, passive immunization with anti-ClfA IgG protected against staphylococcal septic death
The intriguing question arises as to how surface-expressed ClfA is involved in initiating hematogenously spread S. aureus septic arthritis. There are several possibilities that require further investigation
First, ClfA could protect bacteria from phagocytosis in the bloodstream. This could be related to the formation of clumps of bacteria that might be more difficult to engulf. S. aureus also could be camouflaged from host phagocytes by being coated with plasma proteins such as fibrinogen. Then, in ClfA-negative mutant challenged mice, there are insufficient amounts of viablebacteria to exit the bloodstream and to establish a joint infection
Second, ClfA could promote passage of bacteria from the bloodstream to the joint. ClfA could mediate attachment of bacteria to endothelial cells and thus enhance penetration of the joint. S. aureus can adhere to cultured endothelial cells via deposited fibrinogen [25]. Indeed, the prevalence of viable bacteria in the joint area was diminished in clfA mutant–infected mice, compared with the wild-type controls. Thus, a ClfA-negative strain might be hampered in entering the joint
Third, ClfA could promote colonization and survival of the bacteria in the joints. ClfA might mediate attachment to the joint cavity [26] via fibrinogen or fibrin binding and/or block phagocytosis, thus promoting colonization and survival. This was indicated when mice were inoculated with the clfA mutant or wild-type staphylococci directly into the knee joint. Inoculation of ClfA-negative staphylococci evoked none or mild inflammation, compared with wild-type inoculation. Consequently, ClfA-negative bacteria might never colonize the joint or might be rapidly eliminated. In colonization, the fibrinogen-binding capacity of ClfA might be important for clustering the bacteria. Fibrinogen might also act as a bridge between the staphylococci and joint tissues and so facilitate colonization. Finally, ClfA expression might make the bacterium more resistant to phagocytosis. ClfA binding of fibrinogen or some other host molecule could prevent phagocytosis
ClfA seems also to be a virulence determinant for sepsis-triggered death. In the first clfA mutant experiment, there was an ameliorating effect, not only on arthritis but also in the mortality rate, with ClfA-negative strains. The size of the bacterial inoculum gives a hint as to why there is no difference in mortality between wild-type– and clfA mutant–infected mice in the second experiment. The clfA mutant–inoculated mice received 1.5 times more bacteria than did the wild-type controls, a difference that might have produced proportionally higher mortality among the clfA mutant–inoculated mice in the second experiment than in the first. ClfA could promote septic death by facilitating colonization and survival systemically in the same way as in the joint, via fibrinogen binding or interactions with other host factors. However, at the time of death, bacterial growth in the kidneys did not differ between wild-type– and clfA mutant–challenged mice. Differences might have evened out at that late stage of infection
rClfA works as a vaccine against septic arthritis. With a smaller bacterial inoculum the arthritic response was suppressed by rClfA vaccination. After a larger bacterial inoculum, the differences in arthritic response were not as clear between the rClfA- and control-immunized mice, but instead rClfA immunization protected against septic death and improved the mouse’s general status, measured as change in body weight. The protective effect of the vaccination was at least partly antibody mediated, as shown by the passive transfer experiment. Obviously, opsonization with a specific antibody could promote phagocytosis of the bacteria. Also, anti-ClfA antibodies could prevent both bacterial survival and colonization by blocking interactions between the staphylococci and its host ligand(s). The passage from venule to joint could be obstructed for an opsonized bacterium if the fibrinogen-ClfA interaction is crucial
Of interest, rClfA originating from strain Newman also acted as a protective antigen against infection induced by MRSA strain 601 in both active and passive immunization studies. In addition, administration of human serum containing high titers of anti-ClfA antibodies also prevented strain 601–induced mortality, which suggests that ClfA antibodies can protect against infection. Future studies will define the relative role of anti-ClfA antibodies, compared with other possible components of this human serum. However, vaccination with rClfA from strain Newman was unable to protect against arthritis induced by the mouse isolate LS-1, at least not with the vaccination procedure used in this study. This shows that there is a diversity between different S.aureus strains, either in ClfA sequence or in expression of other adhesins that would bind the same ligand(s) as ClfA. Indeed, in an adherence assay, all fibrinogen binding of strain Newman was blocked by polyclonal anti–ClfA40-559 (from Newman) antibodies, whereas the same polyclonal antibodies inhibited only 50% of the fibrinogen adherence of LS-1 (data not shown)
ClfA would be a suitable component in a vaccine against S.aureus infection, because almost all S. aureus strains are believed to express ClfA, given their clumping phenotype [27, 28]. As the number of S. aureus isolates resistant to methicillin continues to increase, it is important to note that MRSA strains generally carry the clfA gene [29]. Diversity between the ClfA sequences of different strains of S. aureus might have to be taken into consideration. Alignment of the A region of ClfA from the 6 available S. aureus genome sequences indicated 91.0%–99.8% identity with the strain Newman sequence (datanot shown). rClfA from several relevant strains could be included in a vaccine. Finally, to further enhance the vaccination efficiency, ClfA could be combined with other S. aureus components that protect against S. aureus infections in active or passiveimmunization studies [30] (e.g., fibronectin-binding proteins, collagen adhesin, and polysaccharides) [12–15, 31]
To summarize, ClfA is a virulence determinant of staphylococcal arthritis and can be used as a vaccine against septic arthritis. An effective vaccine or antibody therapy against S. aureus would be of importance for immunosuppressed subjects such as persons with rheumatic diseases
Acknowledgments
We gratefully acknowledge the skillful technical assistance of Ing-Marie Nilsson, Lena Svensson, and Margareta Verdrengh
References
Presented in part: 9th International Symposium on Staphylococci and Staphylococcal Infections, Kolding, Denmark, June 2000 (abstract 170); 14th European Immunology Meeting of the European Federation of Immunological Societies 2000, Poznan, Poland, September 2000 (abstract 324)
All mice were maintained according to National Institutes of Health animal husbandry standards
Financial support: King Gustaf V’s 80 Years Foundation; Swedish Rheumatism Association; Göteborg Medical Society; Nanna Svartz Foundation; Swedish Medical Society; Knut and Alice Wallenberg Foundation; Wellcome Trust (grants 05230 and 061617)
J.M.P. has a commercial association and a financial interest in the project