Solvation free energies of alcohols in water: temperature and pressure dependences
Abstract
Solvation free energies μ* of amphiphilic species, methanol and 1,2-hexanediol, are obtained as a function of temperature or pressure based on molecular dynamics simulations combined with efficient free-energy calculation methods. In general, μ* of an amphiphile can be divided into 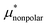 and
and 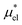 , the nonpolar and electrostatic contributions, and the former is further divided into
, the nonpolar and electrostatic contributions, and the former is further divided into 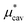 and
and 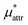 which are the work of cavity formation process and the free energy change due to weak, attractive interactions between the solute molecule and surrounding solvent molecules. We demonstrate that μ* of the two amphiphilic solutes can be obtained accurately using a perturbation combining method, which relies on the exact expressions for
which are the work of cavity formation process and the free energy change due to weak, attractive interactions between the solute molecule and surrounding solvent molecules. We demonstrate that μ* of the two amphiphilic solutes can be obtained accurately using a perturbation combining method, which relies on the exact expressions for 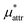 and
and 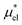 and requires no simulations of intermediate systems between the solute with strong, repulsive interactions and the solute with the van der Waals and electrostatic interactions. The decomposition of μ* gives us several physical insights including that μ* is an increasing function of T due to
and requires no simulations of intermediate systems between the solute with strong, repulsive interactions and the solute with the van der Waals and electrostatic interactions. The decomposition of μ* gives us several physical insights including that μ* is an increasing function of T due to 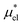 , that the contributions of hydrophilic groups to the temperature dependence of μ* are additive, and that the contribution of the van der Waals attraction to the solvation volume is greater than that of the electrostatic interactions.
, that the contributions of hydrophilic groups to the temperature dependence of μ* are additive, and that the contribution of the van der Waals attraction to the solvation volume is greater than that of the electrostatic interactions.



 Please wait while we load your content...
Please wait while we load your content...