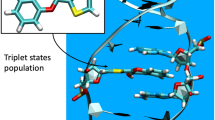
Abstract
We report spectroscopic characterization of two emissive 2′-deoxycytidine analogues: 5-(5-phenylfuran-2-yl)-2′-deoxycytidine and 5-(1-phenyl-1H-pyrazol-3-yl)-2′-deoxycytidine. Their fluorescent properties were examined using a combined experimental and theory/simulation approach, where the latter was based on Born–Oppenheimer molecular dynamics and time-dependent density functional theory. The analogues were found to exhibit unusually large Stokes shifts in polar media (>100 nm), moderate fluorescence quantum yields, and their emissions were found to be very sensitive to the local dielectric environment. These two analogues of 2′-deoxycytidine thus hold a promising potential as probes in chemical biology. In addition, the accuracy of the theoretical models for determining the optical properties is validated, which opens up for a convenient way of assessing the potential of future probes.
Similar content being viewed by others
References
K. Nakatani and Y. Tor, Modified Nucleic Acids, Springer, 2016, vol. 31.
M. Wilhelmsson and Y. Tor, Fluorescent Analogs of Biomolecular Building Blocks: Design and Applications, John Wiley & Sons, 2016.
R. W. Sinkeldam, N. J. Greco and Y. Tor, Fluorescent analogs of biomolecular building blocks: design, properties, and applications, Chem. Rev., 2010, 110, 2579–2619.
G. Mata and N. W. Luedtke, Fluorescent probe for protoncoupled DNA folding revealing slow exchange of i-motif and duplex structures, J. Am. Chem. Soc., 2015, 137, 699–707.
S. Manna and S. G. Srivatsan, Fluorescence-based tools to probe G-quadruplexes in cell-free and cellular environments, RSC Adv., 2018, 8, 25673–25694.
M. E. Østergaard, D. C. Guenther, P. Kumar, B. Baral, L. Deobald, A. J. Paszczynski, P. K. Sharma and P. J. Hrdlicka, Pyrene-functionalized triazole-linked 2′-deoxyuridines – probes for discrimination of single nucleotide polymorphisms (SNPs), Chem. Commun., 2010, 46, 4929–4931.
N. J. Greco and Y. Tor, Simple fluorescent pyrimidine analogues detect the presence of DNA abasic sites, J. Am. Chem. Soc., 2005, 127, 10784–10785.
M. Hattori, T. Ohki, E. Yanase and Y. Ueno, Fluorescence detection of single nucleotide polymorphisms using nucleic acid probe containing tricyclic base-linked acyclonucleoside, Bioorg. Med. Chem. Lett., 2012, 22, 253–257.
A. A. Beharry, S. Lacoste, T. R. O’Connor and E. T. Kool, Fluorescence monitoring of the oxidative repair of DNA alkylation damage by ALKBH3, a prostate cancer marker, J. Am. Chem. Soc., 2016, 138, 3647–3650.
M. S. Noé, Y. Xie and Y. Tor, Methods for Studying Nucleic Acid/Drug Interactions, CRC Press, 2016, pp. 178–201.
Y. Xie, A. V. Dix and Y. Tor, FRET enabled real time detection of RNA-small molecule binding, J. Am. Chem. Soc., 2009, 131, 17605–17614.
W. Xu, K. M. Chan and E. T. Kool, Fluorescent nucleobases as tools for studying DNA and RNA, Nat. Chem., 2017, 9, 1043.
L. M. Wilhelmsson, Fluorescent nucleic acid base analogues, Q. Rev. Biophys., 2010, 43, 159–183.
J. M. Prober, G. L. Trainor, R. J. Dam, F. W. Hobbs, C. W. Robertson, R. J. Zagursky, A. J. Cocuzza, M. A. Jensen and K. Baumeister, A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides, Science, 1987, 238, 336–341.
D. D. Burns, K. L. Teppang, R. W. Lee, M. E. Lokensgard and B. W. Purse, Fluorescence turn-on sensing of DNA duplex formation by a tricyclic cytidine analogue, J. Am. Chem. Soc., 2017, 139, 1372–1375.
P. Sandin, L. M. Wilhelmsson, P. Lincoln, V. E. Powers, T. Brown and B. Albinsson, Fluorescent properties of DNA base analogue tC upon incorporation into DNA – negligible influence of neighbouring bases on fluorescence quantum yield, Nucleic Acids Res., 2005, 33, 5019–5025.
S. Preus, K. Kilså, L. M. Wilhelmsson and B. Albinsson, Photophysical and structural properties of the fluorescent nucleobase analogues of the tricyclic cytosine (tC) family, Phys. Chem. Chem. Phys., 2010, 12, 8881–8892.
L. M. Wilhelmsson, A. Holmén, P. Lincoln, P. E. Nielsen and B. Nordén, A Highly Fluorescent DNA Base Analogue that Forms Watson-Crick Base Pairs with Guanine, J. Am. Chem. Soc., 2001, 123, 2434–2435.
B. Dumat, M. Bood, M. S. Wranne, C. P. Lawson, A. F. Larsen, S. Preus, J. Streling, H. Gradén, E. Wellner, M. Grøtli, et al., Second-Generation Fluorescent Quadracyclic Adenine Analogues: Environment-Responsive Probes with Enhanced Brightness, Chem. –, Eur. J., 2015, 21, 4039–4048.
A. F. Larsen, B. Dumat, M. S. Wranne, C. P. Lawson, S. Preus, M. Bood, H. Gradén, L. M. Wilhelmsson and M. Grøtli, Development of bright fluorescent quadracyclic adenine analogues: TDDFT-calculation supported rational design, Sci. Rep., 2015, 5, 12653.
D. A. Berry, K.-Y. Jung, D. S. Wise, A. D. Sercel, W. H. Pearson, H. Mackie, J. B. Randolph and R. L. Somers, Pyrrolo-dC and pyrrolo-C: fluorescent analogs of cytidine and 2′-deoxycytidine for the study of oligonucleotides, Tetrahedron Lett., 2004, 45, 2457–2461.
F. Wojciechowski and R. H. Hudson, Fluorescence and hybridization properties of peptide nucleic acid containing a substituted phenylpyrrolocytosine designed to engage guanine with an additional H-bond, J. Am. Chem. Soc., 2008, 130, 12574–12575.
A. Dumas and N. W. Luedtke, Highly fluorescent guanosine mimics for folding and energy transfer studies, Nucleic Acids Res., 2011, 39, 6825–6834.
L. Zilbershtein-Shklanovsky, M. Weitman, D. T. Major and B. Fischer, Rules for the design of highly fluorescent nucleoside probes: 8-(substituted cinnamyl)-adenosine analogues, J. Org. Chem., 2013, 78, 11999–12008.
S. De Ornellas, J. M. Slattery, R. M. Edkins, A. Beeby, C. G. Baumann and I. J. Fairlamb, Design and synthesis of fluorescent 7-deazaadenosine nucleosides containing π-extended diarylacetylene motifs, Org. Biomol. Chem., 2015, 13, 68–72.
P. Herdewijn, Modified Nucleosides: in Biochemistry, Biotechnology and Medicine, John Wiley & Sons, 2008.
P. A. Hopkins, R. W. Sinkeldam and Y. Tor, Visibly emissive and responsive extended 6-aza-uridines, Org. Lett., 2014, 16, 5290–5293.
A. Fin, A. R. Rovira, P. A. Hopkins and Y. Tor, Emissive 5-Substituted Uridine Analogues, Springer, 2016, vol. 31, pp. 1–26.
A. A. Tanpure and S. G. Srivatsan, Conformation-sensitive nucleoside analogues as topology-specific fluorescence turn-on probes for DNA and RNA G-quadruplexes, Nucleic Acids Res., 2015, 43, e149–e149.
A. A. Tanpure, M. G. Pawar and S. G. Srivatsan, Fluorescent nucleoside analogs: probes for investigating nucleic acid structure and function, Isr. J. Chem., 2013, 53, 366–378.
P. Kumar, M. Hornum, L. J. Nielsen, G. Enderlin, N. K. Andersen, C. Len, G. Hervé, G. Sartori and P. Nielsen, High-affinity RNA targeting by oligonucleotides displaying aromatic stacking and amino groups in the major groove. Comparison of triazoles and phenyl substituents, J. Org. Chem., 2014, 79, 2854–2863.
A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents, Chem. Soc. Rev., 2018, 47, 8842–8880.
F. Zhou, J. Shao, Y. Yang, J. Zhao, H. Guo, X. Li, S. Ji and Z. Zhang, Molecular rotors as fluorescent viscosity sensors: molecular design, polarity sensitivity, dipole moments changes, screening solvents, and deactivation channel of the excited states, Eur. J. Org. Chem., 2011, 4773–4787.
S.-C. Lee, J. Heo, H. C. Woo, J.-A. Lee, Y. H. Seo, C.-L. Lee, S. Kim and O.-P. Kwon, Fluorescent molecular rotors for viscosity sensors, Chem. –, Eur. J., 2018, 24, 13706–13718.
S. Sasaki, G. P. Drummen and G.-I. Konishi, Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry, J. Mater. Chem. C, 2016, 4, 2731–2743.
R. W. Sinkeldam, A. J. Wheat, H. Boyaci and Y. Tor, Emissive nucleosides as molecular rotors, ChemPhysChem, 2011, 12, 567–570.
M. Hornum, P. Kumar, P. Podsiadly and P. Nielsen, Increasing the stability of DNA: RNA duplexes by introducing stacking phenyl-substituted pyrazole, furan, and triazole moieties in the major groove, J. Org. Chem., 2015, 80, 9592–9602.
A. T. R. Williams, S. A. Winfield and J. N. Miller, Relative fluorescence quantum yields using a computercontrolled luminescence spectrometer, Analyst, 1983, 108, 1067–1071.
I. Berlman, Handbook of Fluorescence Spectra of Aromatic Molecules, Elsevier, 2012.
D. A. Case, V. Babin, J. T. Berryman, R. M. Betz, Q. Cai, D. S. Cerutti, T. E. Cheatham, T. A. Darden, R. E. Duke, H. Gohlke, A. W. Goetz, S. Gusarov, N. Homeyer, P. Janowski, J. Kaus, I. Kolossváry, A. Kovalenko, T. S. Lee, S. LeGrand, T. Luchko, R. Luo, B. Madej, K. M. Merz, F. Paesani, D. R. Roe, A. Roitberg, C. Sagui, R. Salomon-Ferrer, G. Seabra, C. L. Simmerling, W. Smith, J. Swails, R. C. Walker, J. Wang, R. M. Wolf, X. Wu and P. A. Kollman, Amber14, University of California, San Francisco, 2014.
J. A. Maier, C. Martinez, K. Kasavajhala, L. Wickstrom, K. E. Hauser and C. Simmerling, ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB, J. Chem. Theory Comput., 2015, 11, 3696–3713.
F. Paesani, W. Zhang, D. A. Case, T. E. Cheatham III and G. A. Voth, An accurate and simple quantum model for liquid water, J. Chem. Phys., 2006, 125, 184507.
J.-P. Ryckaert, G. Ciccotti and H. J. Berendsen, Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes, J. Comput. Phys., 1977, 23, 327–341.
S. Hirata and M. Head-Gordon, Time-dependent density functional theory within the Tamm–Dancoff approximation, Chem. Phys. Lett., 1999, 314, 291–299.
T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulombattenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57.
W. J. Hehre, R. Ditchfield and J. A. Pople, Self Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian–Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules, J. Chem. Phys., 1972, 56, 2257–2261.
P. C. Hariharan and J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theor. Chem. Acc., 1973, 28, 213–222.
W. L. Jorgensen, Quantum and statistical mechanical studies of liquids. 10. Transferable intermolecular potential functions for water, alcohols, and ethers. Application to liquid water, J. Am. Chem. Soc., 1981, 103, 335–340.
W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, Comparison of simple potential functions for simulating liquid water, J. Chem. Phys., 1983, 79, 926–935.
A. Klamt and G. Schüürmann, COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805.
TeraChem v1.9, PetaChem, LLC (2009, 2015). See http://www.petachem.com.
I. S. Ufimtsev and T. J. Martinez, Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics, J. Chem. Theory Comput., 2009, 5, 2619–2628.
C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, John Wiley & Sons, 2011.
N. J. Greco and Y. Tor, Furan decorated nucleoside analogues as fluorescent probes: synthesis, photophysical evaluation, and site-specific incorporation, Tetrahedron, 2007, 63, 3515–3527.
C. Liu and C. T. Martin, Fluorescence characterization of the transcription bubble in elongation complexes of T7 RNA polymerase, J. Mol. Biol., 2001, 308, 465–475.
D. Dziuba, P. Pospíšil, J. Matyašovský, J. Brynda, D. Nachtigallová, L. Rulíšek, R. Pohl, M. Hof and M. Hocek, Solvatochromic fluorene-linked nucleoside and DNA as color-changing fluorescent probes for sensing interactions, Chem. Sci., 2016, 7, 5775–5785.
D. Dziuba, P. Jurkiewicz, M. Cebecauer, M. Hof and M. Hocek, A Rotational BODIPY Nucleotide: An Environment-Sensitive Fluorescence-Lifetime Probe for DNA Interactions and Applications in Live-Cell Microscopy, Angew. Chem., Int. Ed., 2015, 55, 174–178.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Modelling data, absorption spectra & additional charts. See DOI: 10.1039/C9PP00172G
Rights and permissions
About this article
Cite this article
Stendevad, J., Hornum, M., Wüstner, D. et al. Photophysical investigation of two emissive nucleosides exhibiting gigantic stokes shifts. Photochem Photobiol Sci 18, 1858–1865 (2019). https://doi.org/10.1039/c9pp00172g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00172g