Molecular structure of clonidine: gas-phase electron diffraction, single-crystal X-ray diffraction and quantum chemical studies†
Abstract
This study presents the first determination of the molecular structure of the antihypertensive drug clonidine in the gas phase using gas electron diffraction (GED). The refinement was supported by quantum chemical calculations (QCs). The tautomeric and conformational distribution was investigated theoretically, providing an explanation for the presence of the single conformer in the gas phase. The molecular conformation of clonidine has been shown to have a nearly perpendicular arrangement of the phenyl and imidazolidine rings as described by the torsion angle C2–N6–C7–C8 = −72(6)°. The following structural parameters were obtained (bond lengths in Angstroms and bond angles in degrees with 3σ in parentheses): r(CHH–CHH) = 1.549(7), r(CHH–NH)av = 1.470(7), r(NH–C)av = 1.388(2), r(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) = 1.286(7), r(C–N) = 1.388(2), r(C
N) = 1.286(7), r(C–N) = 1.388(2), r(C![[partial double bond, bottom dashed]](https://www.rsc.org/images/entities/char_e00f.gif) C)av = 1.403(2), r(C–Cl)av = 1.737(2); ∠(NH–C–NH) = 108.1(11), ∠(CHH–NH–C)av = 109.7(12), ∠(CHH–CHH–NH)av = 100.9(12), ∠(C–N
C)av = 1.403(2), r(C–Cl)av = 1.737(2); ∠(NH–C–NH) = 108.1(11), ∠(CHH–NH–C)av = 109.7(12), ∠(CHH–CHH–NH)av = 100.9(12), ∠(C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) = 122.5(12), ∠(CCl
C) = 122.5(12), ∠(CCl![[partial double bond, bottom dashed]](https://www.rsc.org/images/entities/char_e00f.gif) C
C![[partial double bond, bottom dashed]](https://www.rsc.org/images/entities/char_e00f.gif) CCl) = 114.9(2), and ∠(CH
CCl) = 114.9(2), and ∠(CH![[partial double bond, bottom dashed]](https://www.rsc.org/images/entities/char_e00f.gif) CCl
CCl![[partial double bond, bottom dashed]](https://www.rsc.org/images/entities/char_e00f.gif) C)av = 123.1(2). The standard enthalpy of formation of clonidine in the gas phase was calculated using G4 theory with both atomisation and isodesmic reaction approaches, yielding the corresponding value of
C)av = 123.1(2). The standard enthalpy of formation of clonidine in the gas phase was calculated using G4 theory with both atomisation and isodesmic reaction approaches, yielding the corresponding value of 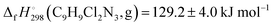 . The molecular structure of clonidine in the solid phase was determined using X-ray diffraction (XRD). Clonidine crystallizes in the monoclinic space group P21/c as a twinned crystal. The imino-tautomer, as an equimolar mixture of the two conformers with geometries close to the enantiomeric pair, is present in the solid phase. The identical conformers are linked into centrosymmetric dimers by paired N–H⋯N hydrogen bonds. The geometries of gaseous and solid clonidine differ especially in the immediate vicinity of the intermolecular hydrogen bonds formed in the crystal.
. The molecular structure of clonidine in the solid phase was determined using X-ray diffraction (XRD). Clonidine crystallizes in the monoclinic space group P21/c as a twinned crystal. The imino-tautomer, as an equimolar mixture of the two conformers with geometries close to the enantiomeric pair, is present in the solid phase. The identical conformers are linked into centrosymmetric dimers by paired N–H⋯N hydrogen bonds. The geometries of gaseous and solid clonidine differ especially in the immediate vicinity of the intermolecular hydrogen bonds formed in the crystal.



 Please wait while we load your content...
Please wait while we load your content...