Molecular chemisorption on passivated and defective boron doped silicon surfaces: a “forced” dative bond
Abstract
We investigate the adsorption mechanism of a single trans 4-pyridylazobenzene molecule (denoted by PAB) on a doped boron 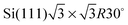 surface (denoted by SiB) with or without boron-defects, by means of density functional theory calculations. The semiempirical approach proposed by Grimme allows us to take the dispersion correction into account. The role of the van der Waals correction in the adsorption geometries and energies is presented. In particular, two adsorption configurations are electronically studied. In the first one, the molecule is parallel to the surface and interacts with the SiB surface via the –N
surface (denoted by SiB) with or without boron-defects, by means of density functional theory calculations. The semiempirical approach proposed by Grimme allows us to take the dispersion correction into account. The role of the van der Waals correction in the adsorption geometries and energies is presented. In particular, two adsorption configurations are electronically studied. In the first one, the molecule is parallel to the surface and interacts with the SiB surface via the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond. In the presence of a boron-defect, a Si–N chemical bond between the molecule and the surface is then formed, while electrostatic or/and van der Waals interactions are observed in the defectless surface. In the second adsorption configuration, the molecule presents different orientations with respect to the surface and interacts via the nitrogen atom of the pyridyl part of the PAB molecule. If the molecule is perpendicular to the perfect SiB surface, the lone-pair electrons associated with the heterocyclic nitrogen atom fill the empty dangling bond of a silicon adatom via a dative bond. Finally, in the presence of one boron-defect, the possibility of a “forced” dative bond, corresponding to a chemical bond formation between the PAB molecule and the silicon electron occupied dangling bond, is emphasized.
N– bond. In the presence of a boron-defect, a Si–N chemical bond between the molecule and the surface is then formed, while electrostatic or/and van der Waals interactions are observed in the defectless surface. In the second adsorption configuration, the molecule presents different orientations with respect to the surface and interacts via the nitrogen atom of the pyridyl part of the PAB molecule. If the molecule is perpendicular to the perfect SiB surface, the lone-pair electrons associated with the heterocyclic nitrogen atom fill the empty dangling bond of a silicon adatom via a dative bond. Finally, in the presence of one boron-defect, the possibility of a “forced” dative bond, corresponding to a chemical bond formation between the PAB molecule and the silicon electron occupied dangling bond, is emphasized.


 Please wait while we load your content...
Please wait while we load your content...