Abstract
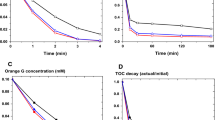
The degradation of a model mixture composed of Acid Yellow 36 (AY36) and Methyl Orange (MO) azo dyes was performed using the photo-Fenton process (PFP). The performance of this process conducted under artificial UV light (365 nm) was compared with the Fenton reaction. Some important operating parameters that affect the degradation of azo dyes, such as initial Fe2+ and H2O2 concentrations and the presence or absence of chloride ions, were investigated. Decolorisation of the dye mixture sample was achieved in 70 min with the photo-Fenton reaction, while the complete mineralization evaluated by TOC abatement was completed in 180 min. These results provide important knowledge for the treatment of wastewater containing azo dye mixtures by Fenton and photo-Fenton oxidation processes.
Similar content being viewed by others
References
J. M. Peralta-Hernández, C. A. Martínez-Huitle, J. L. Guzmán-Mar and A. Hernández-Ramírez, Recent advances in the application of electro-Fenton and photoelectro-Fenton process for removal of synthetic dyes in wastewater treatment, J. Environ. Eng. Manage., 2009, 19, 257.
Official Mexican Law NMX-AA-045-SCFI-2001. Water analysis - determination of color platinum cobalt in natural, wastewaters and treated wastewaters - test method. 2001.
Official Mexican Law NOM-127-SSA1-1994. Potabilization of water usage and human consumption, quality permissible limits and types of treatment, 2000.
H. Music, N. Koprivanac and L. Srsan, Azo dye degradation using Fenton type processes assisted by UV irradiation: A kinetic study, J. Photochem. Photobiol., A, 2006, 181, 195.
R. Andreozzi, V. Caprio, A. Insola and R. Marotta, Advanced oxidation processes (AOP) for water purification and recovery, Catal. Today, 1999, 53, 51.
M. Perez, F. Torrades, X. Domenech and J. Peral, Fenton and photo-Fenton oxidation of textile effluents, Water Res., 2002, 36, 2703.
N. Clarke and G. Knowles, High purity water using H2O2 and UV radiation, Effluent Water Treat. J., 1982, 23, 335.
F. Torrades, J. A. García-Hortal and L. Núñnez, Fenton and photo- Fenton oxidation of a model mixture of dyes - overall kinetic analysis, Coloration Technol., 2009, 124, 370.
D. Melgoza, A. Hernández-Ramírez and J. M. Peralta-Hernández, Comparative efficiencies of the decolorisation ofMethylene Blue using Fentons and photo-Fenton’s reactions, Photochem. Photobiol. Sci., 2009, 8, 596.
M. D. Nikolaki, K. N. Zerva and C. J. Philippopoulos, Photo-Fenton Treatment of 1,3-dichloro-2-Propanol Aqueous Solutions using UV Radiation and H2O2—A Kinetic Study, Int. J. Chem. Biomol. Eng., 2008, 1, 3.
J. M. Chacon, M. T. Leal, M. Sanchez and E. R. Bandala, Solar photocatalytic degradation of azo dyes by photo-Fenton process. Dyes and Pigments, Dyes Pigm., 2006, 69, 144.
C. Sirtori, A. Zapata, S. Malato, W. Gernjak, A. R. Fernández-Alba and A. Agüera, Solar photocatalytic treatment of quinolones: intermediates and toxicity evaluation, Photochem. Photobiol. Sci., 2009, 8, 644.
P. N. Tantak and S. Chaudhari, Degradation of azo dyes by sequential Fenton’s oxidation and aerobic biological treatment, J. Hazard.Mater., 2006, 136, 698.
S. Papic, D. Vujevic, N. Koprivanac and D. Sinko, Decolourization and mineralization of commercial reactive dyes by using homogeneous and heterogeneous Fenton and UV/Fenton processes, J. Hazard. Mater., 2009, 164, 1137.
F. Torrades, J. Garcia-Montano, J. A. Garcıa-Hortal, X. Domenech and J. Peral, Decolorization and mineralization of commercial reactive dyes under solar light assisted photo-Fenton conditions, Sol. Energy, 2004, 77, 573.
R. F. P. Nogueira, M. R. A. Silva and A. G. Trovo, Influence of the iron source on the solar photo-Fenton degradation of different classes of organic compounds, Sol. Energy, 2005, 79, 384.
L. Gomathi-Devi, S. Girish-Kumar, K. Mohan-Reddy and C. Munikrishnappa, Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism, J. Hazard. Mater., 2009, 164, 459.
L. M. Saragiotto-Colpini, H. J. Alves, O. A. Apreciada dos Santos and C. M. Macedo-Costa, Discoloration and degradation of textile dye aqueous solutions with titanium oxide catalysts obtained by the sol–gel method, Dyes Pigm., 2008, 76, 525.
T. N. Nagaraja and T. Desiraju, Effects of chronic consumption of metanil yellow by developing and adult rats on brain regional levels of noradrenaline, dopamine and serotonin, on acetylcholine esterase activity and on operant conditioning, Food Chem. Toxicol., 1993, 31, 41.
MSDS, Methyl Orange, CAS #:547-58-0.
J. Macías-Sánchez, L. Hinojosa-Reyes, J. L. Guzmán-Mar, J. M. Peralta-Hernández and A. Hernández-Ramirez, Degradation of acid yellow 36 and methyl orange azo dyes by photo-Fenton process, Proceedings of 2nd Mexico YoungWater Professional Conference, 2010.
P. K. Malik and S. K. Saha, Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst, Sep. Purif. Technol., 2003, 31, 241.
M. Muruganandham and M. Swaminatham, Advanced oxidative decolorisation of Reactive Yellow 14 azo dye by UV/TiO2, UV/H2O2, UV/H2O2/Fe2+ processes - a comparative study, Sep. Purif. Technol., 2006, 48, 297.
J. J. Pignatello, E. OLiveros and A. MacKay, Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1.
H. Kusic, A. Loncaric-Bozic and N. Koprivanac, Fenton type processes for minimization of organic content in coloured wastewaters: Part I: Processes optimization, Dyes Pigm., 2007, 74, 380.
I. I. Arslan-Alaton, G. Tureli and T. Olmez-Hanci, Optimization of the photo-Fenton-like process for real and synthetic azo dye production wastewater treatment using response surface methodology, Photochem. Photobiol. Sci., 2009, 8, 628.
S. Malato, P. Fernandez-Ibañnez and M. I. Maldonado, Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends, Catal. Today, 2009, 147, 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is published as part of the themed issue of contributions from the 6th European Meeting on Solar Chemistry and Photocatalysis: Environmental Applications held in Prague, Czech Republic, June 2010.
Rights and permissions
About this article
Cite this article
Macías-Sánchez, J., Hinojosa-Reyes, L., Guzmán-Mar, J.L. et al. Performance of the photo-Fenton process in the degradation of a model azo dye mixture. Photochem Photobiol Sci 10, 332–337 (2011). https://doi.org/10.1039/c0pp00158a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00158a