Abstract
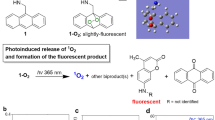
A new type of porphyrin photosensitizer capable of generating singlet oxygen upon irradiation, storing it through binding to pyridone subunits, followed by slow release at 20–40 °C, is reported. The timescale of singlet oxygen release can be varied depending on the pyridone group substitution pattern, forming endoperoxides of different stabilities. Modified tetra- and octa-substituted pyridone-porphyrins showed solubility in water, allowing for straightforward delivery into cells. The effect of delayed singlet oxygen formation due to endoperoxide decomposition was demonstrated on cancer cells in vitro.
Similar content being viewed by others
References
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and Q. Peng, Photodynamic Therapy, J. Natl. Cancer Inst., 1998, 90, 889–905.
S. B. Brown, E. A. Brown, and I. Walker, The present and future role of photodynamic therapy in cancer treatment, Lancet Oncol., 2004, 5, 497–508.
A. P. Castano, T. N. Demidova, and M. R. Hamblin, Mechanisms in photodynamic therapy: Part three–Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction, Photodiagn. Photodyn. Ther., 2005, 2, 91–106.
M. O. Senge, mTHPC–A Drug on its Way from Second to Third Generation Photosensitizer?, Photodiagn. Photodyn. Ther., 2012, 9, 170–179.
J. F. Lovell, T. W. Liu, J. Chen, and G. Zheng, Activatable Photosensitizers for Imaging and Therapy, Chem. Rev., 2010, 110, 2839–2857.
J. Moan, and K. Berg, The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen, Photochem. Photobiol., 1991, 53, 549–553.
M. K. Kuimova, G. Yahioglu, and P. R. Ogilby, Singlet oxygen in a cell: Spatially dependent lifetimes and quenching rate constants, J. Am. Chem. Soc., 2009, 131, 332–340.
L. Yang, L. Zhu, W. Dong, Y. Cao, and Z. Rong, Oxygen-generating scaffolds: A new strategy for bone tissue engineering, Bone., 2013, 57, 322–323.
A. Northup, and D. Cassidy, Calcium peroxide (CaO2) for use in modified Fenton chemistry, J. Hazard. Mater., 2008, 152, 1164–1170.
C. Moureu, C. Dufraisse, and P. M. Dean, Un peroxyde organique dissociable: Le peroxyde de rubrène, C. R. Hebd. Seances Acad. Sci., 1926, 182, 1584–1587.
J.-M. Aubry, C. Pierlot, J. Rigaudy, and R. Schmidt, Reversible binding of oxygen to aromatic compounds, Acc. Chem. Res., 2003, 36, 668–675.
M. A. Filatov, and M. O. Senge, Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications, Mol. Syst. Des. Eng., 2016, 1, 258–272.
F. Käsermann, and C. Kempf, Inactivation of enveloped viruses by singlet oxygen thermally generated from a polymeric naphthalene derivative, Antiviral Res., 1998, 38, 55–62.
D. Posavec, M. Zabel, U. Bogner, G. Bernhardt, and G. Knör, Functionalized derivatives of 1,4-dimethylnaphthalene as precursors for biomedical applications: Synthesis, structures, spectroscopy and photochemical activation in the presence of dioxygen, Org. Biomol. Chem., 2012, 10, 7062–7069.
M. A. Filatov, E. Heinrich, D. Busko, I. Z. Ilieva, K. Landfester, and S. Baluschev, Reversible oxygen addition on a triplet sensitizer molecule: Protection from excited state depopulation, Phys. Chem. Chem. Phys., 2015, 17, 6501–6510.
M. Matsumoto, M. Yamada, and N. Watanabe, Reversible 1,4-cycloaddition of singlet oxygen to N-substituted 2-pyridones: 1,4-Endoperoxide as a versatile chemical source of singlet oxygen, Chem. Commun., 2005, 483–485.
S. Benz, S. Nötzli, J. S. Siegel, D. Eberli, and H. J. Jessen, Controlled oxygen release from pyridone endoperoxides promotes cell survival under anoxic conditions, J. Med. Chem., 2013, 56, 10171–10182.
I. S. Turan, D. Yildiz, A. Turksoy, G. Gunaydin, and E. U. Akkaya, A bifunctional photosensitizer for enhanced fractional photodynamic therapy: Singlet oxygen generation in the presence and absence of light, Angew. Chem., Int. Ed., 2016, 55, 2875–2878.
S. Kolemen, T. Ozdemir, D. Lee, G. Mi Kim, T. Karatas, J. Yoon, and E. U. Akkaya, Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods: Towards a paradigm change in photodynamic therapy, Angew. Chem., Int. Ed., 2016, 55, 3606–3610.
C. C. W. Changtong, D. W. Carney, L. Luo, C. A. Zoto, J. L. Lombardi, and R. E. Connors, A porphyrin molecule that generates, traps, stores, and releases singlet oxygen, J. Photochem. Photobiol., A., 2013, 260, 9–13.
S. Kim, M. Fujitsuka, and T. Majima, Photochemistry of Singlet Oxygen Sensor Green, J. Phys. Chem. B., 2013, 117, 13985–13992.
M. A. Filatov, S. Karuthedath, P. M. Polestshuk, H. Savoie, K. J. Flanagan, C. Sy, E. Sitte, M. Telitchko, F. Laquai, R. W. Boyle, and M. O. Senge, Generation of triplet excited states via photoinduced electron transfer in meso-anthra-BODIPY: Fluorogenic response toward singlet oxygen in solution and in vitro, J. Am. Chem. Soc., 2017, 139, 6282–6285.
T. Mossman, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods., 1983, 65, 55–63.
Acknowledgments
This work was supported by grants from the Science Foundation Ireland (IvP 13/IA/1894), the European Commission (CONSORT, Grant No. 655142) and the Irish Research Council (GOIPG/2016/1250).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Callaghan, S., Filatov, M.A., Sitte, E. et al. Delayed release singlet oxygen sensitizers based on pyridone-appended porphyrins. Photochem Photobiol Sci 16, 1371–1374 (2017). https://doi.org/10.1039/c7pp00244k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00244k