A novel potentiometric self-plasticizing polypyrrole sensor based on a bidentate bis-NHC ligand for determination of Hg(ii) cation†
Abstract
In this approach, a new potentiometric self-plasticizing polypyrrole sensor was constructed based on a bidentate bis-NHC ligand for the purpose of Hg2+ cation determination. An ionophore, namely bis[1-benzyl-benzimidazoliumethyl]-4-methylbenzenesulfonamide bromide (NHCL) was successfully synthesized and characterized using FT-IR, 1H NMR, 13C NMR spectroscopy, CHN elemental analysis, X-ray single crystal diffraction and UV-Vis spectroscopy. The electrode with a membrane optimum composition demonstrated a good Nernstian response to Hg2+ cations ranging from 1.0 × 10−6 to 1.0 × 10−2 M with a detection limit of 2.5 × 10−7 M and a Nernstian slope of 28.10 ± 0.29 mV per decade over a pH range between 4.5–7.0 with a response time of about 20 seconds at room temperature. The electrode exhibited better selectivity towards Hg2+ ions in comparison with some soft and hard metals; most of these metal ions do not show significant interference 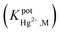 . The proposed electrode also has applications in the direct determination of Hg2+ cations in aqueous solutions with sensible accuracy and precision.
. The proposed electrode also has applications in the direct determination of Hg2+ cations in aqueous solutions with sensible accuracy and precision.


 Please wait while we load your content...
Please wait while we load your content...