Abstract
Depression is a highly heterogeneous disorder. Accumulating evidence suggests biological and genetic differences between subtypes of depression that are homogeneous in symptom presentation. We aimed to evaluate differences in serum protein profiles between persons with atypical and melancholic depressive subtypes, and compare these profiles with serum protein levels of healthy controls. We used the baseline data from the Netherlands Study of Depression and Anxiety on 414 controls, 231 persons with a melancholic depressive subtype and 128 persons with an atypical depressive subtype for whom the proteomic data were available. Depressive subtypes were previously established using a data-driven analysis, and 171 serum proteins were measured on a multi-analyte profiling platform. Linear regression models were adjusted for several covariates and corrected for multiple testing using false discovery rate q-values. We observed differences in analytes between the atypical and melancholic subtypes (9 analytes, q<0.05) and between atypical depression and controls (23 analytes, q<0.05). Eight of the nine markers differing between the atypical and melancholic subtype overlapped with markers from the comparison between atypical subtype and controls (mesothelin, leptin, IGFBP1, IGFBP2, FABPa, insulin, C3 and B2M), and were mainly involved in cellular communication and signal transduction, and immune response. No markers differed significantly between the melancholic subtype and controls. To conclude, although some uncertainties exist in our results as a result of missing data imputation and lack of proteomic replication samples, many of the identified analytes are inflammatory or metabolic markers, which supports the notion of atypical depression as a syndrome characterized by metabolic disturbances and inflammation, and underline the importance and relevance of subtypes of depression in biological and genetic research, and potentially in the treatment of depression.
Similar content being viewed by others
Introduction
Despite major efforts to unravel the pathophysiological mechanisms of major depressive disorder (MDD) through the analysis of genome-wide association study data1 and RNA gene expression studies,2, 3 little insight has been gained and it was only recently that a first genetic hit for MDD was found in a female sample of cases of recurrent depression.4 With the availability of new proteomic techniques that allow for the simultaneous quantitative measurement of a wide array of proteins, it is now possible to study the actual functional profiles of proteins, protein–protein interaction and pathways. Proteins are the end products of RNA and DNA, and often the functional and modifiable units in disease mechanisms. Consequently, a proteomic approach may provide a more complete and useful representation of pathophysiological processes potentially involved in MDD.
Several studies have applied proteomic approaches in MDD populations in the past few years5, 6, 7, 8, 9, 10 (2–7 years) most of them with a relatively small sample size (range N=12–49) and four studies with larger sample sizes11, 12, 13, 14, 15 (range N=102–1589), of which one was conducted by our group.11 Proteins differentially expressed in these studies were mostly involved in inflammation, insulin-related pathways and metalloproteinases.
A major factor hindering the further identification of endophenotypes, reproducible biomarkers and genes related to depression is the large heterogeneity in the presentation of depression. Consequently, an increasing number of studies now attempt to unravel pathophysiological mechanisms by distinguishing more homogeneous depressive subtypes. Two previous proteomics studies have distinguished between MDD subtypes.6, 7 One study found different protein profiles between MDD patients with and without psychosis7 and the other study differentiated between MDD with and without childhood trauma.6 Two other relevant subtypes, suggested in a comprehensive overview of subtype differences by Gold and Chrousos,16 include melancholic and atypical depression. Recent studies that distinguished atypical depression and melancholic depression using either DSM-IV criteria or data-driven classifiers, have shown that especially atypical depression is associated with obesity, metabolic syndrome and inflammation, whereas it is melancholic depression that is most strongly associated with hyperactivity of the HPA-axis.17, 18, 19, 20
To our knowledge, no study has investigated whether atypical and melancholic subtypes have different molecular signatures using a proteomic approach, and we therefore set out to evaluate this using the data from the Netherlands Study of Depression and Anxiety (NESDA). A previous analysis of all current (diagnosis in past 6 months) and remitted cases of MDD and healthy controls from the NESDA sample11 suggested associations of 33 analytes with MDD, of which seven analytes (out of 16 of the 33 available) were also associated with MDD in validation cohorts. Application of proteomic approaches to differentiate atypical and melancholic depression could increase our understanding of subtype-specific pathophysiological processes. The aim of the current study was therefore to evaluate differences in serum protein profiles between persons with atypical and melancholic depressive subtypes, and to compare these profiles with serum protein levels of controls.
Materials and methods
Sample
NESDA is an ongoing naturalistic longitudinal cohort study (N=2981) of persons with depressive and/or anxiety disorders and healthy controls, recruited from the community, primary care and secondary care.21 The study was approved by the Ethical Review Board of the VU University Medical Centre and subsequently by local review boards of each participating centre. At baseline, participants visited the study site for an extensive assessment including a psychiatric diagnostic interview, medical assessment, blood draw and self-reported questionnaires. Proteomic analytes were determined in the subset of NESDA participants who participated in both baseline and 2-year follow-up assessments and for whom sufficient serum (~1 ml) from the baseline assessment was available (n=1837).11 In the current analysis, we included persons with proteomic data for whom we had previously established as either an atypical (n=128) or melancholic (n=231) depressive subtype11, 22 (see below), and controls without lifetime depressive and/or anxiety disorder according to the CIDI diagnostic interview (n=414), giving a total sample of 773 persons.
Assessment of depressive subtypes
In all participants, the presence of depression was determined by the CIDI diagnostic interview (version 2.1),23 administered by specially trained staff, using DSM-IV algorithms. The depressive subtypes of the current study were previously determined in 818 NESDA participants within a 1-month diagnosis of MDD (n=743) or minor depression (n=75) using the baseline data.11, 22 In short, depression items from the CIDI diagnostic interview and a selection of items from the Inventory of Depressive Symptomatology-self report (IDS-SR30)24 were input variables for a latent class analysis, which was used to cluster persons with similar symptom profiles. A three-class model was found to fit the data best. On the basis of symptom probabilities, the subtypes were labeled as ‘severe melancholic’ (prevalence 46.3%), characterized mainly by decreased appetite and weight loss. This subtype also had the highest probabilities of suicidal thoughts, psychomotor changes and lack of responsiveness. The second subtype was labeled as ‘severe atypical’ (24.6%). This severe atypical subtype was mainly characterized by overeating and weight gain, and had the highest probabilities of leaden paralysis and interpersonal sensitivity. The stability over time of both the severe melancholic and the severe atypical depression subtypes was found to be high.25 The last subtype was labeled as ‘moderate’ (29.1%) and was associated with lower symptom probabilities and overall lower severity. Mean posterior probabilities were 0.80 for the melancholic class, 0.88 for the atypical class and 0.84 for the moderate class showing acceptable qualification quality. In the current study, we included only the severe melancholic and the severe atypical subtypes as they are both recognized in the literature and DSM-5. Importantly, these subtypes were of a similar severity, which reduces the risk of any observed effects resulting from severity differences.
It should be noted that our latent class analysis-based subtypes of melancholic and atypical depression do not literally resemble DSM classification, in the sense that mood reactivity in atypical depression was not a cardinal item and that the number of subtype-specific symptoms does not follow DSM classification. However, the robustness of the identified subtypes is shown by other latent modeling studies finding similar symptom patterns with appetite and weight being the most discriminating symptoms.26, 27, 28, 29, 30 In this paper, we will use the terms ‘atypical’ and ‘melancholic’ when referring to our latent class analysis-based subtypes. A more thorough description of subtypes and their correlates has been previously published.22, 31
Proteomics
Blood samples were taken after an overnight fast and stored at −80 °C in five different labs throughout the Netherlands, using standardized protocols. A panel of 243 analytes involved in various hormonal, immunological, metabolic and neurotrophic pathways were assessed in serum using multiplexed immunoassays in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory (Myriad Rules Based Medicine; Austin, TX, USA; DiscoveryMap 250+). Of the 243 analytes assessed, 171 analytes could be included in the analysis (see Statistics section). A full list of the 243 analytes can be found in Supplementary Table 1. Six quality controls (with low, medium and high concentrations of the analyte) were included on each plate. Mean inter-assay variability was 10.6% (range 5.5–32.5%) and mean intra-assay variability was 5.6% (range 2.5–15.8%). A more detailed description can be found elsewhere.11
Covariates
Sex and age information was collected using standard questions. Lab was also included as covariate. Weekly alcohol intake was derived from the Alcohol Use Disorders Identification Test (AUDIT).32 Smoking was defined as current smoking (Y/N). Cardiovascular disease, including cerebrovascular disease, myocardial infarction, angina pectoris and coronary heart disease, was based on self-report information or use of beta blocking agents, calcium channel blocking agents or nitrate vasodilators (ATC codes: C07, C08 and C01DA). Diabetes mellitus (DM) was based on medication use (ATC codes: A10) or fasting glucose levels ⩾7 mmol l−1.
In addition, the following covariates were considered for sensitivity analyses: any anxiety disorder (1-month CIDI diagnosis of social phobia, panic disorder, agoraphobia, or generalized anxiety disorder), antidepressant use including selective serotonin reuptake inhibitors (SSRIs; ATC code N06AB), tricyclic antidepressants (TCAs; ATC codes N06AA) and other antidepressants (ATC codes N06AF, N06AG, N06AX), corticosteroid use (ATC codes H02, R03BA, R03AK, D07) and anti-inflammatory medication use (ATC codes M01A, M01B, A07EB, A07EC), and body mass index (BMI; weight in kg/m2).
Statistics
Of the 243 analytes assessed, 171 analytes with <30% missing values (mostly due to levels outside the limits of detection) were included in the analysis. Missing values that were below and above the limits of detection were imputed with the values of the lower and upper limit of detection, respectively. Other missing values (one missing on average per analyte) were imputed by the median value of the analyte. These imputation methods are similar to methods used in other studies.12, 33 Data were then log10-transformed to stabilize the variance distributions. The percentage of missing values for each analyte is listed in Supplementary Table 1. We applied the ComBat procedure34 to the proteomic data to remove any potential plate effects prior to analysis.
Baseline characteristics were compared using χ2-tests, analysis of variance and Kruskal–Wallis tests, where appropriate. Separate linear models were run with each analyte as the dependent variable. Besides a group variable (melancholic subtype, atypical subtype or control), models contained the following covariates: age; sex; lab; smoking; alcohol intake; cardiovascular disease; and diabetes. Comparisons between subtypes, and between subtypes and controls were performed. To account for multiple testing, a false discovery rate (FDR) was calculated.35 All analyses were conducted in R software (version 3.0.2)36 and two-sided tests were used.
To evaluate the robustness of findings, we performed additional analyses by running three models additionally corrected for potential confounding variables. In one model, we added antidepressant use, in the second we added use of anti-inflammatory agents and corticosteroids, and in the third model we added comorbid anxiety to the standard set of covariates (as listed above). To evaluate the potential mediating effect of BMI, we also repeated the analyses with BMI as covariate. Only analytes that were significant (FDR-controlled q<0.05) in at least one of the comparisons from the main analysis were included in these additional models. We did not apply FDR correction in these additionally corrected models.
To aid interpretation of findings, we looked up the biological processes in the Human Protein Reference Database by uploading SwissProt accession numbers of proteins. We tested for statistical overrepresentation of biological processes using PANTHER tools,37, 38 using the 171 tested markers as reference set and applying a Bonferroni correction. Finally, a literature search was performed in PubMed to describe the identified markers and their previously observed associations with major depression, MDD subtypes or depressive symptoms. We structured our narrative review of the literature for each of the main biological processes.
Results
Sample description
Persons with atypical and melancholic depression were slightly older, had more chronic diseases, and were more often using medication than controls (Table 1). Persons with atypical depression were more often female, had a higher BMI and a lower alcohol intake than controls and persons with melancholic depression. Persons with melancholic depression were most often smokers compared with the other two groups. Severity of depression was not different between melancholic and atypical subtypes (group comparison P=0.59).
Differences between atypical depression, melancholic depression and controls
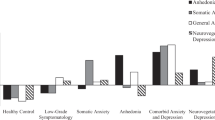
From the 171 analytes measured, we found 9 analytes to be differentially expressed between atypical and melancholic depressive subtypes (q<0.05; Table 2). Compared with melancholic depression, persons with atypical depression had higher levels of leptin, angiotensin-converting enzyme (ACE), fatty-acid-binding protein–adipocyte (FABPa), complement C3, insulin and beta-2-microglobulin (B2M), while having lower levels of insulin-like growth factor-binding protein 1 (IGFBP1), IGFBP2 and mesothelin (MSLN).
Except for ACE, all analytes significantly differing between atypical and melancholic subtypes also differed significantly between those with the atypical subtype and controls. The direction of effects for these eight analytes was similar to that in the comparison of atypical versus melancholic (that is, analytes being increased in atypical vs controls were also increased in atypicals vs melancholic; Figure 1; Supplementary Figure 1). In addition, 15 more analytes were significantly different between persons with atypical depression and controls. Levels of phosphoserine aminotransferase, alpha-1-microglobulin, serum amyloid P-component, tissue-type plasminogen activator, glutathione S-transferase alpha (GSTa), C-reactive protein (CRP), Cathepsin D (CathD), von Willebrand factor (VWF), C-peptide, macrophage migration inhibitory factor (MIF) and alpha-1-antichymotrypsin (AACT) were higher, whereas levels of adiponectin (APN), growth hormone (GH), sex hormone-binding globulin (SHBG) and angiopoietin-2 (ANG2) were lower in persons with atypical depression versus controls. Although these 15 analytes did not reach significance (q<0.05) in the comparison atypical versus melancholic depression, 9 of the 15 analytes (60%) had P-values <0.05.
Proteins differentiating between (a) atypical vs melancholic depression, (b) between atypical depression vs controls and (c) between Melancholic depression vs Controls. *q<0.05, adjusted regression coefficients and error bars (s.e.). Analytes ordered based on size-adjusted regression coefficient of atypical vs melancholic depression comparison. A1Micr, alpha-1-microglobulin; AACT, alpha-1-antichymotrypsin; ACE, angiotensin-converting enzyme; ANG2, angiopoetin-2; APN, adiponectin; B2M, beta-2-microglobulin; C3, Complement C3; CathD, Cathepsin D; Cpep, C-peptide; CRP, C-reactive protein; FABPA, fatty-acid-binding protein, adipocyte; GH, human growth hormone; GSTa, glutathione S-transferase alpha; IGFBP1, insulin-like growth factor-binding protein 1; IGFBP2, insulin-like growth factor-binding protein 2; INS, insulin; MIF, macrophage migration inhbitory factor; MSLN, mesothelin; PSAT, phosphoserine aminotransferase; SAP, serum amyloid P-component; SHBG, sex hormone-binding globulin; TPA, tissue-type plasminogen activator; VWF, von Willebrand factor.
There were no significant differences in levels of analytes when comparing persons with melancholic depression and controls after FDR correction. However, for three analytes (ACE, ANG2 and AACT), a P-value <0.05 was observed (Table 2). Supplementary Table 2 contains the regression coefficients for all group comparisons and all 171 examined analytes.
Sensitivity analyses with additional correction for antidepressant use, and corticosteroid or anti-inflammatory medication use yielded very similar results (data not shown) with all markers remaining significantly related to atypical depression (P<0.05) after taking these factors into account. In models with correction for comorbid anxiety, all other markers remained significant (P<0.05) with the exception of MSLN and ANG2 in the atypical versus control comparison (P<0.10). When evaluating the mediating influence of BMI, we found that significance for most markers was lost when correcting for BMI, pointing to a mediating effect of BMI. Insulin growth factor-binding protein 1, angiotensin-converting enzyme and B2M remained significant (P<0.05) in the comparison atypical versus melancholic depressive subtypes, whereas B2M, angiopoietin-2 and VWF remained significant in the comparison atypical depression versus control when adding BMI to the model.
Biological processes
To identify biological processes involved in the analytes associated with MDD, we entered all 24 analytes with a significant q-value in any of the pairwise comparisons simultaneously in the enrichment analysis. We used the 24 analytes because we did not find differences between the melancholic subtype and controls, and because of the large overlap in analytes differing between atypical and controls and those differing between atypical and melancholic depression. We did not observe statistical overrepresentation of any biological process. The biological processes to which the largest number of analytes were linked were cell communication and signal transduction (nine analytes), protein metabolism (six analytes), immune response (five analytes), metabolism (three analytes), and energy pathways (three analytes; Table 2).
Post hoc evaluation of homogeneous versus heterogeneous phenotype use
Because we suspected that ignoring heterogeneity in the symptom presentation of MDD would dilute associations with analytes, we repeated the analysis for the 171 markers, this time combining the atypical and melancholic depressive subtypes into one heterogeneous depression group and comparing them with controls, while using the same covariates as in the main analysis. We then evaluated the fold change in the estimate (calculated as estimate for the homogeneous atypical subtype versus control divided by the estimate for the heterogeneous depression (atypical+melancholic combined) versus control). The fold change in estimate ranged from −14.7 to 256.0 (median 1.45 (interquartile range 0.47–2.34)), demonstrating that associations are generally diluted when a more heterogeneous phenotype is used. Also, for 31 analytes, the direction of the effect changed. When looking at the 24 markers that were significantly different between atypical depression and controls, 9 (37.5%) did no longer reach significance at the P<0.05 level when comparing heterogeneous depression vs controls (that is, ACE, IGFBP2, IGFBP1, MSLN, SHBG, GSTa, CRP, CPep and APN).
Identified analytes: roles and previous findings
An overview of previously observed associations of the analytes with depression or with other psychiatric disorders and other relevant studies is given below, ordering analytes by biological process (as listed in Table 2). For a complete overview of biological functions of the analytes, see Supplementary Table 3.
Cell communication and signal transduction
Consistent with the present study, insulin was found to be increased in MDD in one proteomic study,13 but not in our previous proteomic analysis including all MDD cases in the cohort.11 Our finding that leptin was increased in atypical depression is consistent with a previous study in atypical depression39 and more recently was confirmed in the NESDA sample that also confirmed the absence of an association with melancholic depression.40 It implies leptin resistance—which is thought to contribute to depressive symptomatology41—as an underlying mechanism in atypical depression. FABPs have received attention from the depression field because fatty acid metabolism regulates neurotransmitter signaling, inflammation and thrombosis and could be a possible mechanism for the association between depression and cardiovascular disease.42 No differences in IGFBP levels in MDD compared with controls were found in two previous studies,43, 44 but one of these studies found lower IGFBP2 expression levels in brain tissue of persons with bipolar disorder compared with controls.43 Decreased GH levels in MDD were also previously found in two studies,13, 14 whereas another study found increased GH levels in elderly MDD patients only.44 Gold and Chrousos16 posed that the GH axis in both melancholic and atypical depression may be suppressed, however, we only observed this in atypical depression. MIF is a proinflammatory cytokine that has been linked to hippocampal neurogenesis in animal models.45 Two studies also found increased levels of MIF in depressed cases compared with controls,14, 46 and MIF is now increasingly gaining attention as a potential depression marker.47 Interestingly, increased MIF has been associated with lower morning cortisol levels,48 which fits in with a previous finding of lower cortisol on awakening in atypical depression compared with melancholic depression and controls.49 ANG2 levels were lower in atypical depression versus controls, which is in line with a previous analysis of all MDD cases within the same cohort.11
Protein metabolism
One marker may possibly be considered as a melancholic depression-specific marker. ACE was significantly lower in melancholic depression than in atypical depression and also lower in melancholic depression than in controls (albeit with a non-significant q-value; P=0.0001, q=0.19). This finding is in line with results from a previous study on melancholic depression.50 Aldosterone and the renin–angiotensin system have been proposed as potential biomarker and/or mediator in the pathophysiology of MDD.51
AACT, an acute phase protein, has been previously found to be increased in MDD and is correlated to depression severity.52 Serum amyloid P-component, increased in atypical versus controls, has previously been found to be increased in MDD and schizophrenia compared with controls.9 Higher levels of AACT and lower levels of serum amyloid P-component have been associated with cognitive decline and with Alzheimer’s disease.53, 54
Two markers involved in blood coagulation processes were increased in persons with atypical depression compared with controls, VWF and tissue plasminogen activator. The VWF finding is in line with a previous analysis of all MDD cases within the same cohort,11 and likewise, the increased cathepsin D levels in the atypical subtype were observed before in the entire MDD group.11
Immune response
As in a previous enzyme-linked immunosorbent assay of high-sensitivity CRP in this data,49 we found that CRP was significantly increased in atypical depression versus controls (q=0.02). Increased levels of complement C3 in MDD have been found previously by some,55, 56 but not all studies.57 B2M was previously found to be increased in MDD compared to controls.13 Mesothelin is a glycoprotein that is highly expressed in certain cancers and is thought to have a role in cellular adhesion. No studies have previously linked mesothelin to depressive disorders.
Metabolism and energy pathways
Adiponectin has a role in metabolic and immune processes and is negatively associated with obesity and insulin resistance.58 We found that adiponectin was lower in atypical depression versus controls. Previous studies have provided inconsistent findings with some studies finding decreased adiponectin levels in MDD59, 60, 61, 62 others found no difference,58 and one study reporting increased levels in MDD versus controls.63 One study found lower adiponectin levels in MDD cases with metabolic syndrome versus MDD cases without metabolic syndrome.64 This latter finding supports the suggestion that decreased adiponectin levels have a role in atypical depression only, as atypical depression has been found to be associated with metabolic syndrome.49, 65 Gluthathione S-transferase alpha 1 was not associated with MDD in a previous proteomic study;12 phosphoserine aminotransferase has not been investigated before, to our knowledge.
Transport
Levels of SHBG can be decreased by high levels of insulin and are also decreased in diabetes.66 SHBG was not associated with depressive symptoms in a sample of persons with and without diabetes and insulin resistance.66
Discussion
To our knowledge, this study is the first to identify serum proteins that are differentially expressed in atypical and melancholic depression subtypes versus controls using a proteomics approach. We observed differences in analytes between atypical depression and melancholic depression (9 analytes) and between atypical depression and controls (23 analytes), but not between melancholic depression and controls. There was overlap in the analytes identified in each of the three pairwise comparisons; of the 24 analytes, 8 (33%) emerged both in the comparison of atypical versus melancholic depression and in the comparison atypical depression versus control, indicating that these 8 markers could be specific to the atypical depressive subtype.
Many of the identified analytes are inflammatory markers or are involved in metabolism. This fits in with our previous findings of increased BMI, increased prevalence of metabolic syndrome and increased inflammation (CRP, interleukin-6 and tumor necrosis factor-α) in the atypical subtype,22, 49 and these findings support the idea that the atypical depressive subtype may in fact be a metabolic/inflammatory type of depression. In the past years, anti-inflammatory treatment has been increasingly investigated in trials with a recent meta-analysis showing a medium effect size in depression.67 However, this estimate was associated with considerable heterogeneity, and the authors recommended identification of subgroups that may benefit more from anti-inflammatory treatment. We hypothesize that the atypical subtype is such a subgroup.
Markers that may be of particular interest for future studies are the eight markers that seem specific to the atypical depressive subtype, namely leptin, IGFBP1 and IGFBP2, FABPa, insulin, mesothelin, C3 and B2M, all involved in either cell communication and signal transduction or immune responses. One other marker of interest is the ACE, which may be a marker that is specific to melancholic depression. As part of the renin–angiotensin system, ACE may be of interest because the renin–angiotensin system also partially regulates aldosterone secretion from the HPA-axis. Aldosterone and the renin–angiotensin system have been considered as potential biomarker and/or mediator in the pathophysiology of MDD.51
Although we controlled for various potential confounders, we did not control for BMI in the main analysis. It is known from the literature that there is a bidirectional relationship between BMI and depressive symptoms.68 This may be particularly the case for atypical depression as characterized by increased appetite and weight gain among other.69, 70 Atypical depression and increased BMI may also be a part of the same syndrome, as has been implied by the recent findings that atypical depression—but not melancholic depression—was associated with a variant of the obesity-related FTO gene and with a genomic profile risk scores for BMI.71, 72 Because of this evidence of (causal) associations between atypical depression and BMI, we consider BMI to be a mediator (rather than a confounder) and correction for BMI as an overcorrection. When we ran models for the 24 markers from Table 2 with additional correction for BMI to evaluate the mediating effects of BMI, results suggested mediation for most analytes (non-significant P-values and large changes in estimates). No mediation was found for IGFBP1, ACE and B2M (comparison atypical and melancholic depression) and B2M, ANG2 and VWF (comparison atypical depression and controls).
This paper follows a study evaluating changes in protein levels in all 6-month MDD cases and controls (N=1598) from the same cohort by Bot and colleagues. Only 5 of 33 markers previously found to be associated with MDD in the study by Bot et al.11 were also found to differ between controls and atypical depression in the present study (FABPa, ANG2, CathD, vWF and MIF), the observed directions of effect being consistent with the analysis by Bot et al. Notably, the markers in Bot et al. were derived before FDR correction and FDR adjustment showed that these markers were related to MDD only at a relatively high FDR level (range q-values 0.09–0.30). In contrast, in our study—with about half the number of MDD cases compared with the previous paper—we found that after FDR correction atypical depressed cases had significantly different levels of markers compared with controls and melancholic depression. Although differences in samples and analysis could explain some of the discrepancies, our results could be interpreted as proof of the usefulness of more homogenous subgroups of MDD for the identification of pathophysiological mechanisms. A post hoc comparison of estimates for the 171 analytes from the atypical depression versus controls model versus estimates from a model of all MDD cases (melancholic and atypical combined) versus controls showed a median fold-change of 1.45 for the estimates, demonstrating that associations get diluted when a more heterogeneous phenotype is used.
Strengths of this study include the relatively large sample size, with cases from the community, primary care and specialized care. Limitations include the fact that some analytes could not be evaluated because of levels below the level of detection. Also, the subtypes used were not based on stringent DSM-criteria, but are based on a data-driven analysis. However, the DSM definition of atypical depression has been criticized,73, 74 and other studies applying data-driven analysis of subtypes have yielded similar types26, 27, 29, 30 supporting the validity of these subtypes. Because assignment of persons to latent classes can be somewhat inaccurate for some persons,11, 22 we repeated analyses restricted to persons with a high classification accuracy (a posterior probability >0.80, that is, 65% of depressed cases). This showed highly comparable results, with overall stronger effects indicating that classification inaccuracy had little effect on overall conclusions. Furthermore, the decision to include markers with <30% missing values may be lenient and could have introduced error. However, using only markers with <20% missing values did not lead to different results, other than omission of two markers from Table 2 that had >20% missing values (INS and GSTa). Also, no proteomics replication samples were available. Although for some analytes we have found similar results using different assays (CRP49 and leptin 40) or have been found by others (CRP18 and leptin39), our proteomics results need to be replicated in independent samples. Unfortunately, independent replication of the identified changes is at present not possible for the following reasons: first, there is a lack of large cohorts and lack of detailed proteomic data for existing cohorts. Second, there are shortcomings in the assessment protocols for MDD in other studies; often, only change in appetite, weight and sleep are being recorded, rather than the direction of change. It would be recommendable to include direction of change in future data-collection protocols.
To conclude, we found no differences between the melancholic depressive subtype and controls, although the role of low levels of ACE in melancholic depression requires more research. We did find that the atypical depressive subtype had a differential protein profile compared to controls and the melancholic subtype. Identified proteins were mainly involved in cell communication and signal transduction, protein metabolism, immune response and metabolism and energy pathways, the latter two underlining the inflammatory and metabolic character of the atypical depressive subtype, and most of these associations seemed mediated by BMI. Although some uncertainties exist in our results, as a result of the missing data imputation and lack of proteomic replication samples, the presented results support the notion of atypical depression as a syndrome of metabolic disturbances that may benefit from add-on anti-inflammatory treatment. This study also suggests that depressive subtypes display distinct molecular profiles. Importantly, despite the need for additional validation studies, these results may provide the initial groundwork towards future patient stratification strategies that have more power to elucidate pathophysiological mechanisms of depression in research on biomarkers and pathophysiology.
References
Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013; 18: 497–511.
Jansen R, Penninx BW, Madar V, Xia K, Milaneschi Y, Hottenga JJ et al. Gene expression in major depressive disorder. Mol Psychiatry 2015; 21: 444.
Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J et al. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry 2014; 19: 1267–1274.
CONVERGE consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 2015; 523: 588–591.
Ditzen C, Tang N, Jastorff AM, Teplytska L, Yassouridis A, Maccarrone G et al. Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology 2012; 37: 1013–1025.
Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: A cytokine antibody array analysis. Compr Psychiatry 2013; 54: 953–961.
Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, Rahmoune H et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2012; 2: e87.
Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol 2008; 18: 230–233.
Xu HB, Fang L, Hu ZC, Chen YC, Chen JJ, Li FF et al. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res 2012; 200: 1054–1057.
Xu HB, Zhang RF, Luo D, Zhou Y, Wang Y, Fang L et al. Comparative proteomic analysis of plasma from major depressive patients: identification of proteins associated with lipid metabolism and immunoregulation. Int J Neuropsychopharmacol 2012; 15: 1413–1425.
Bot M, Chan MK, Jansen R, Lamers F, Vogelzangs N, Steiner J et al. Serum proteomic profiling of major depressive disorder. Transl Psychiatry 2015; 5: e599.
Arnold SE, Xie SX, Leung YY, Wang LS, Kling MA, Han X et al. Plasma biomarkers of depressive symptoms in older adults. Transl Psychiatry 2012; 2: e65.
Domenici E, Wille DR, Tozzi F, Prokopenko I, Miller S, McKeown A et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE 2010; 5: e9166.
Stelzhammer V, Haenisch F, Chan MK, Cooper JD, Steiner J, Steeb H et al. Proteomic changes in serum of first onset, antidepressant drug-naive major depression patients. Int J Neuropsychopharmacol 2014; 17: 1599–1608.
Zheng P, Wang Y, Chen L, Yang DY, Meng HQ, Zhou DZ et al. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics 2013; 12: 207–214.
Gold PW, Chrousos GP . Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 2002; 7: 254–275.
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N . Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11: 129.
Hickman RJ, Khambaty T, Stewart JC . C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination Survey (NHANES) 1999-2004. J Behav Med 2014; 37: 621–629.
Rudolf S, Greggersen W, Kahl KG, Huppe M, Schweiger U . Elevated IL-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res 2014; 217: 34–38.
Glaus J, Vandeleur C, Gholam-Rezaee M, Castelao E, Perrin M, Rothen S et al. Atypical depression and alcohol misuse are related to the cardiovascular risk in the general population. Acta Psychiatr Scand 2013; 128: 282–293.
Penninx BWJH Beekman ATF, Smit JH, Zitman FG, Nolen WA, Spinhoven P et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res 2008; 17: 121–140.
Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, Beekman AT et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 2010; 71: 1582–1589.
World Health Organization Composite International Diagnostic Interview, Core Version 21: Interviewer's Manual. World Health Organization: Sydney, Australia, 1997.
Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH . The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26: 477–486.
Lamers F, Rhebergen D, Merikangas KR, de JP, Beekman AT, Penninx BW . Stability and transitions of depressive subtypes over a 2-year follow-up. Psychol Med 2012; 42: 2083–2093.
Kendler KS, Eaves LJ, Walters EE, Neale MC, Heath AC, Kessler RC . The identification and validation of distinct depressive syndromes in a population-based sample of female twins. Arch Gen Psychiatry 1996; 53: 391–399.
Sullivan PF, Kessler RC, Kendler KS . Latent class analysis of lifetime depressive symptoms in the national comorbidity survey. Am J Psychiatry 1998; 155: 1398–1406.
Sullivan PF, Prescott CA, Kendler KS . The subtypes of major depression in a twin registry. J Affect Disord 2002; 68: 273–284.
Rodgers S, Ajdacic-Gross V, Muller M, Hengartner MP, Grosse HM, Angst J et al. The role of sex on stability and change of depression symptom subtypes over 20 years: a latent transition analysis. Eur Arch Psychiatry Clin Neurosci 2014; 264: 577–588.
Lamers F, Burstein M, He JP, Avenevoli S, Angst J, Merikangas KR . Structure of major depressive disorder in adolescents and adults in the US general population. Br J Psychiatry 2012; 201: 143–150.
Lamers F, Beekman ATF, van Hemert AM, Schoevers RA, Penninx BWJH . Six-year longitudinal course and outcomes of subtypes of depression. Br J Psychiatry 2016; 208: 62–68.
Babor TF, JRDI Fuente, Saunders J, Grant M . The Alcohol Use Disorders Identification Test: Guidelines for Use In Primary Health Care. World Health Organization: Geneva, Switzerland, 1992.
Bot M, Chan MK, Jansen R, Lamers F, Vogelzangs N, Steiner J et al. Serum proteomic profiling of major depressive disorder. Transl Psychiatry 2015; 5: e599.
Johnson WE, Li C, Rabinovic A . Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–127.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57: 289–300.
R Core TeamR: a language and environment for statistical computing. R Foundation for Statistical Computing Available at http://www.R-project.org/2014.
Mi H, Thomas P . PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Methods Mol Biol 2009; 563: 123–140.
Mi H, Muruganujan A, Thomas PD . PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 2013; 41 (Database issue): D377–D386.
Gecici O, Kuloglu M, Atmaca M, Tezcan AE, Tunckol H, Emul HM et al. High serum leptin levels in depressive disorders with atypical features. Psychiatry Clin Neurosci 2005; 59: 736–738.
Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW, Leptin Dysregulation Is . Specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biol Psychiatry 2015; doi:10.1016/j.biopsych.2015.10.023.
Lu XY . The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 2007; 7: 648–652.
Mocking RJ, Lok A, Assies J, Koeter MW, Visser I, Ruhe HG et al. Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism in recurrent depression: associations with fatty acid concentrations and waist circumference. PLoS ONE 2013; 8: e82980.
Bezchlibnyk YB, Xu L, Wang JF, Young LT . Decreased expression of insulin-like growth factor binding protein 2 in the prefrontal cortex of subjects with bipolar disorder and its regulation by lithium treatment. Brain Res 2007; 1147: 213–217.
Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Korner A et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology 1997; 22: 493–503.
Conboy L, Varea E, Castro JE, Sakouhi-Ouertatani H, Calandra T, Lashuel HA et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry 2011; 16: 533–547.
Musil R, Schwarz MJ, Riedel M, Dehning S, Cerovecki A, Spellmann I et al. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression—no influence of celecoxib treatment. J Affect Disord 2011; 134: 217–225.
Bloom J, Al-Abed Y . MIF: mood improving/inhibiting factor? J Neuroinflamm 2014; 11: 11.
Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT . Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain Behav Immun 2010; 24: 1202–1208.
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW . Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013; 18: 692–699.
Maes M, Scharpe S, Meltzer HY, Suy E, Cosyns P, Calabrese J . Lower angiotensin I converting enzyme activity in melancholic subjects: a pilot study. Biol Psychiatry 1992; 32: 621–624.
Murck H, Schussler P, Steiger A . Renin-angiotensin-aldosterone system: the forgotten stress hormone system: relationship to depression and sleep. Pharmacopsychiatry 2012; 45: 83–95.
Joyce PR, Hawes CR, Mulder RT, Sellman JD, Wilson DA, Boswell DR . Elevated levels of acute phase plasma proteins in major depression. Biol Psychiatry 1992; 32: 1035–1041.
Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P . Serum inflammatory proteins and cognitive decline in older persons. Neurology 2005; 64: 1371–1377.
Verwey NA, Schuitemaker A, van der Flier WM, Mulder SD, Mulder C, Hack CE et al. Serum amyloid p component as a biomarker in mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord 2008; 26: 522–527.
Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res 1997; 66: 1–11.
Song C, Dinan T, Leonard BE . Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Disord 1994; 30: 283–288.
Berk M, Wadee AA, Kuschke RH, O'Neill-Kerr A . Acute phase proteins in major depression. J Psychosom Res 1997; 43: 529–534.
Taylor VH, Macqueen GM . The role of adipokines in understanding the associations between obesity and depression. J Obes 2010; 2010: 6; doi:10.1155/2010/748048.
Cizza G, Nguyen VT, Eskandari F, Duan Z, Wright EC, Reynolds JC et al. Low 24- hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry 2010; 71: 1079–1087.
Diniz BS, Teixeira AL, Campos AC, Miranda AS, Rocha NP, Talib LL et al. Reduced serum levels of adiponectin in elderly patients with major depression. J Psychiatr Res 2012; 46: 1081–1085.
Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K et al. Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatr Scand 2010; 121: 209–215.
Leo R, Di LG, Tesauro M, Cola C, Fortuna E, Zanasi M et al. Decreased plasma adiponectin concentration in major depression. Neurosci Lett 2006; 407: 211–213.
Narita K, Murata T, Takahashi T, Kosaka H, Omata N, Wada Y . Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1159–1162.
Zeugmann S, Quante A, Heuser I, Schwarzer R, Anghelescu I . Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. J Clin Psychiatry 2010; 71: 1007–1016.
Takeuchi T, Nakao M, Kachi Y, Yano E . Association of metabolic syndrome with atypical features of depression in Japanese people. Psychiatry Clin Neurosci 2013; 67: 532–539.
Mantyselka P, Korniloff K, Saaristo T, Koponen H, Eriksson J, Puolijoki H et al. Association of depressive symptoms with impaired glucose regulation, screen-detected, and previously known type 2 diabetes: findings from the Finnish D2D Survey. Diabetes Care 2011; 34: 71–76.
Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O et al. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2014; 17: 1381–1391.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67: 220–229.
Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D et al. The associations between psychopathology and being overweight: a 20-year prospective study. Psychol Med 2004; 34: 1047–1057.
Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F et al. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a Prospective, Population-Based Study. JAMA Psychiatry 2014; 71: 880–888.
Milaneschi Y, Lamers F, Mbarek H, Hottenga JJ, Boomsma DI, Penninx BW . The effect of FTO rs9939609 on major depression differs across MDD subtypes. Mol Psychiatry 2014; 19: 960–962.
Milaneschi Y, Lamers F, Peyrot WJ, Abdellaoui A, Willemsen G, Hottenga JJ et al. Polygenic dissection of major depression clinical heterogeneity. Mol Psychiatry 2015; 21: 516–522.
Angst J, Gamma A, Benazzi F, Silverstein B, jdacic-Gross V, Eich D et al. Atypical depressive syndromes in varying definitions. Eur Arch Psychiatry Clin Neurosci 2006; 256: 44–54.
Parker G, Roy K, Mitchell P, Wilhelm K, Malhi G, Hadzi-Pavlovic D . Atypical depression: a reappraisal. Am J Psychiatry 2002; 159: 1470–1479.
Acknowledgements
The infrastructure for the NESDA study (www.nesda.nl) has been funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (Zon-Mw, grant number 10-000-1002) and participating universities (VU University Medical Center, Leiden University Medical Center, University Medical Center Groningen). SB, MKC and JC acknowledge funding through the Stanley Medical Research Institute (SMRI), the Dutch Fund for Economic Structure Reinforcement ((No. 0908) the NeuroBasic PharmaPhenomics project) and through Myriad Genetics. FL has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no PCIG12-GA-2012-334065.
Data Availability
We confirm that all the data underlying the findings are fully available without restriction. Because of ethical and legal restrictions, our data are not freely available in the manuscript, Supplementary Files, or in a public repository. However, the data are available upon request via the NESDA Data Access Committee (nesda@ggzingeest.nl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JDC is the consultant for Myriad Genetics. SB is the consultant for Myriad Genetics and the director of Psynova Neurotech. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lamers, F., Bot, M., Jansen, R. et al. Serum proteomic profiles of depressive subtypes. Transl Psychiatry 6, e851 (2016). https://doi.org/10.1038/tp.2016.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.115
This article is cited by
-
Protease-activated receptor 2 activation induces behavioural changes associated with depression-like behaviour through microglial-independent modulation of inflammatory cytokines
Psychopharmacology (2022)
-
Reduced plasma Fetuin-A is a promising biomarker of depression in the elderly
European Archives of Psychiatry and Clinical Neuroscience (2020)
-
Involvement of inflammatory gene expression pathways in depressed patients with hyperphagia
Translational Psychiatry (2019)
-
Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping
Molecular Psychiatry (2019)
-
Prognosis and improved outcomes in major depression: a review
Translational Psychiatry (2019)